Exhaustive exercise can induce excessive generation of reactive oxygen species (ROS), which may enhance oxidative stress levels. Besides single dosages of antioxidants, whole diets rich in antioxidants are gaining more attention due to their practicality and multicomponent ingredients.
- diet
- antioxidants
- exercise
- oxidative stress
- reactive oxygen species
1. Introduction
The term oxidative stress is defined as a disturbance in the homeostatic balance between pro-oxidants and antioxidants with a subsequent excessive generation of free radicals
. Free radicals are highly reactive compounds that contain one or more unpaired electrons in their outer atomic or molecular orbital
, and thus readily react with various organic substrates in order to make themselves more stable
[3]
. Species derived from oxygen are generally referred to as reactive oxygen species (ROS) and are naturally occurring byproducts of the human metabolism. Thereby, redox reactions represent fundamental components of organic and biological chemistry
[5]
. While low to moderate ROS concentrations seem to be involved in cell signaling and muscle remodulation
, prolonged exposure to high doses of ROS induces oxidative damage
[3]
.
As depicted in
, high-intensity exercise might induce mitochondrial stress, leading the mitochondria to emit ROS in order to facilitate adaptations and thus protect against subsequent cellular stress
[8]
. In case of excessive ROS production, this might lead to oxidative damage.
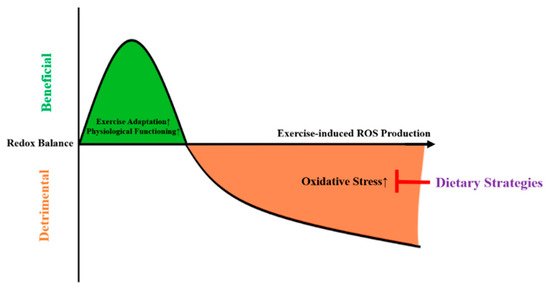
Figure 1.
The mitohormesis-based model to explain the effects of dietary strategies on exercise-induced oxidative stress. ROS = reactive oxygen species.
As a potential countermeasure against excessive oxidative stress during exercise, antioxidative supplementations, which aim to protect against muscle damage and thus improve exercise performance, have been frequently discussed
. Nonetheless, many studies have indicated that large-dose antioxidant supplementation can interfere with intrinsic adaptive responses and may abolish the benefit of exercise
. These highly purified antioxidants can negatively affect ROS-mediated physiological processes through prooxidant mechanisms
[9]
.
Along with their high antioxidant content, specific diets, including products such as oatmeal, dark chocolate, and mixed fruit beverages may also contain additional bioactive compounds which are not found in single-dose pharmacological antioxidant supplements but can act synergistically to reveal more beneficial effects than a single dose of antioxidant supplements
. Additionally, these compounds are more accessible than specific isolated antioxidants. Until now, few studies have investigated the clinical effects on exercise-induced oxidative stress by using a whole dietary strategy and consistent evidence from human study remains scarce.
2. Dietary Strategies
The majority of currently available studies addressed the effects of phenol-rich foods on exercise-induced oxidative stress, including dark chocolate
, high-flavanol cocoa drink
[17]
, green tea
[18]
, mate tea
[19]
, New Zealand blueberry smoothie
[20]
, blueberries
, grape juice
, Montmorency cherry juice
[25]
, tart cherry juice
[26]
, oatmeal
[27]
, avenanthramides (AVA)-rich cookie
, juçara juice
[30]
, Sanguinello cultivar red orange juice
[31]
, and purple sweet potato leaves
[32]
. Frequently, the effects of dietary strategies on exercise-induced stress are evaluated within short-term
, as well as long-term interventions
[15][16][18][19][22][24][25][26][28][29][31][32]
. Across all studies, there is a compelling amount of evidence suggesting that different dietary regimens are viable tools for decreasing exercise-induced oxidative stress. However, the different biomarkers of oxidative stress do not allow a direct comparison between studies.
3. Effects on Biomarkers of Exercise-Induced Oxidative Stress
3.1. Effects of Dietary Interventions on Direct ROS Generation
Zeng et al.
[27]
revealed that consumption of AVA-rich oatmeal before high-intensity interval training (HIIT) significantly mitigates exercise-induced ROS generation compared to the control group, by using the EPR method. AVA, as one of the major components of polyphenolic amides (nonflavonoids), is considered the most important antioxidant found in oats
. Therefore, it can be speculated that the hydroxyl groups of AVA contribute to antioxidant defense through their ability to trap ROS in vitro
[35]
.
3.2. Effects of Dietary Interventions on ROS-Induced Macromolecule Damage
In the majority of studies, F2-isoprostanes, 8-isoprostanes, lipid hydroperoxides (LH), thiobarbituric acid-reactive substances (TBARS) and malondialdehydes (MDA) were used as the oxidative markers, which result from lipoperoxidation by oxidative damage. Similarly, protein carbonylation (PC) was used as a marker of protein damage, and 8-Hydroxydeoxyguanosine (8-oxodG) as a specific marker of 20-deoxyguanosine damage after ROS attack to DNA.
Davison et al. [14] and Wiswedel et al. [17] confirmed the acute antioxidant effects of dark chocolate by detecting the plasma levels of F2-isoprostane due to its polyphenolic properties,
Allgrove et al.
[15]
and Taub et al.
[16]
showed that these beneficial effects can also be seen following long-term dietary interventions. The derivatives of catechin and epicatechin, which can both be defined as monomeric flavanols, are the major antioxidant components in cacao beans (chocolate)
[36]
.
In addition to cocoa, other phenol-rich fruits also exhibited antioxidant effects during exercise by detecting oxidative stress markers, including blueberry
[22]
, cherry
[25]
and red orange
[31]
.
In the study by McAnulty et al. [22], participants consumed 150g of blueberries in a milkshake every day for one week prior to one session of high-intensity training in hyperthermic conditions. The results showed that the blueberry diet attenuated an increase in LH concentration caused by exercise stress but not F2-isoprostane levels, compared with a blueberry-flavored shake as a placebo.
Purple sweet potato leaves (PSPL), as another phenol-rich diet, showed decreases in oxidative stress markers in an exercise trial
[32]
.
Chang et al. [32] investigated the effects of a 7-day PSPL-diet on running exercise-induced oxidative stress in a nontrained, young male population. PSPL consumption significantly increased total polyphenols concentrations, and significantly decreased plasma PC and TBARS in the PSPL group [32].
Exercise-induced oxidative stress can activate a range of transcription factors that contribute to the differential expression of certain genes involved in inflammatory pathways [37] . In this review, diets with antioxidant effects have demonstrated to reduce inflammatory markers including neutrophil respiratory burst (NRB), interleukin-6 (IL-6), nuclear factor-kappa B (NF-κB), granulocyte-colony stimulating factor (G-CSF), interleukin-1 receptor antagonist (IL-1Ra), soluble vascular cell adhesion molecule-1 (sVCAM-1). Koenig et al. [28] and Zhang et al. [29] investigated the eight-week effects of AVArich cookies on exercise-induced oxidative stress by detecting the inflammatory markers. Both found that this AVA-rich diet decreased ROS production from the NRB after high intensity downhill training when compared to control group. In concert with alterations affecting levels of oxidative stress markers and inflammatory markers, exercise-induced oxidative stress could attenuate the endogenous antioxidant defense including enzymatic antioxidant activity (catalase (CAT), SOD, GPx, cyclooxygenase-2 (COX-2)) and nonenzymatic antioxidant activity (GSH, oxygen radical absorbance capacity (ORAC), total antioxidant capacity (TAC), total antioxidant status (TAS), ferric reducing antioxidant power (FRAP), vitamins C and E, and reduced glutathione content). Two studies by Panza et al. [18][19] investigated the antioxidant activity following the consumption of green tea or mate tea for one week in young men undergoing resistance exercise. Green tea increased the values of total polyphenols, GSH, FRAP and diminished the plasma levels of LH after a bench press exercise [18]. Similarly, mate tea increased the concentration of total polyphonic compounds at all time points and the levels of GSH after twenty maximal eccentric elbow flexion exercises [19]. McLeay et al. [20] and Park et al. [21] demonstrated the short-term effects of blueberries by detecting antioxidant activity. Integral grape juice was used as the dietary strategy against exercise-induced oxidative stress in an acute study [23] and a 28-day study [24] by Toscano et al. Tart cherry juice showed subchronic positive effects on antioxidant activity caused by high-intensity exercises in the study of Howatson et al. [26]. Copetti et al. [30] evaluated the acute antioxidant effect of juçara juice during HIIT by observing antioxidant status. In this narrative review, most studies found positive effects of dietary strategies on exercise-induced ROS generation. Especially, phenol-rich diets showed effects in combating exercise-induced oxidative stress in the greater proportion of the articles. Accordingly, while dietary strategies might help to keep ROS generation in a physiological range during exercise, the use of the antioxidant-rich diets may upregulate the endogenous antioxidants' defense system, which may have important implications for preventing excessive damage and facilitating recovery. Nevertheless, consistent evidence is still lacking, and the underlying mechanisms in human trials are not well understood. 3.3. Effects of Dietary Interventions on Inflammatory Markers
3.4. Effects of Dietary Interventions on Antioxidant Activity
References
- Halliwell, B. Free Radicals, Antioxidants, and Human Disease: Curiosity, Cause, or Consequence? Lancet 1994, 344, 721–724.
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015; ISBN 978-0198717485.
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95.
- Peternelj, T.T.; Coombes, J.S. Antioxidant Supplementation during Exercise Training: Beneficial or Detrimental? Sports Med. 2011, 41, 1043–1069.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84.
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive Oxygen Species Are Signalling Molecules for Skeletal Muscle Adaptation. Exp. Physiol. 2010, 95, 1–9.
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive Oxygen Species: Impact on Skeletal Muscle. Compr. Physiol. 2011, 1, 941–969.
- Merry, T.L.; Ristow, M. Mitohormesis in Exercise Training. Free Radic. Biol. Med. 2016, 98, 123–130.
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and Oxidative Stress: Potential Effects of Antioxidant Dietary Strategies in Sports. Nutrition 2015, 31, 916–922.
- Paulsen, G.; Hamarsland, H.; Cumming, K.T.; Johansen, R.E.; Hulmi, J.J.; Børsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E Supplementation Alters Protein Signalling after a Strength Training Session, but Not Muscle Growth during 10 Weeks of Training. J. Physiol. 2014, 592, 5391–5408.
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral Administration of Vitamin C Decreases Muscle Mitochondrial Biogenesis and Hampers Training-Induced Adaptations in Endurance Performance. Am. J. Clin. Nutr. 2008, 87, 142–149.
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants Prevent Health-Promoting Effects of Physical Exercise in Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670.
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin e and c Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452.
- Davison, G.; Callister, R.; Williamson, G.; Cooper, K.A.; Gleeson, M. The Effect of Acute Pre-Exercise Dark Chocolate Consumption on Plasma Antioxidant Status, Oxidative Stress and Immunoendocrine Responses to Prolonged Exercise. Eur. J. Nutr. 2012, 51, 69–79.
- Allgrove, J.; Farrell, E.; Gleeson, M.; Williamson, G.; Cooper, K. Regular Dark Chocolate Consumption’s Reduction of Oxidative Stress and Increase of Free-Fatty-Acid Mobilization in Response to Prolonged Cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 113–123.
- Taub, P.R.; Ramirez-Sanchez, I.; Patel, M.; Higginbotham, E.; Moreno-Ulloa, A.; Román-Pintos, L.M.; Phillips, P.; Perkins, G.; Ceballos, G.; Villarreal, F. Beneficial Effects of Dark Chocolate on Exercise Capacity in Sedentary Subjects: Underlying Mechanisms. A Double Blind, Randomized, Placebo Controlled Trial. Food Funct. 2016, 7, 3686–3693.
- Wiswedel, I.; Hirsch, D.; Kropf, S.; Gruening, M.; Pfister, E.; Schewe, T.; Sies, H. Flavanol-Rich Cocoa Drink Lowers Plasma F2-Isoprostane Concentrations in Humans. Free Radic. Biol. Med. 2004, 37, 411–421.
- Panza, V.S.P.; Wazlawik, E.; Ricardo Schütz, G.; Comin, L.; Hecht, K.C.; da Silva, E.L. Consumption of Green Tea Favorably Affects Oxidative Stress Markers in Weight-Trained Men. Nutrition 2008, 24, 433–442.
- Panza, V.P.; Diefenthaeler, F.; Tamborindeguy, A.C.; Camargo, C.D.Q.; De Moura, B.M.; Brunetta, H.S.; Sakugawa, R.L.; De Oliveira, M.V.; Puel, E.D.O.; Nunes, E.A.; et al. Effects of Mate Tea Consumption on Muscle Strength and Oxidative Stress Markers after Eccentric Exercise. Br. J. Nutr. 2016, 115, 1370–1378.
- McLeay, Y.; Barnes, M.J.; Mundel, T.; Hurst, S.M.; Hurst, R.D.; Stannard, S.R. Effect of New Zealand Blueberry Consumption on Recovery from Eccentric Exercise-Induced Muscle Damage. J. Int. Soc. Sports Nutr. 2012, 9, 19.
- Park, C.H.; Kwak, Y.S.; Seo, H.K.; Kim, H.Y. Assessing the Values of Blueberries Intake on Exercise Performance, TAS, and Inflammatory Factors. Iran J. Public Health 2018, 47, 27–32.
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Morrow, J.D.; Utter, A.C.; Henson, D.A.; Proulx, W.R.; George, G.L. Consumption of Blueberry Polyphenols Reduces Exercise-Induced Oxidative Stress Compared to Vitamin C. Nutr. Res. 2004, 24, 209–221.
- de Lima Tavares Toscano, L.; Silva, A.S.; de França, A.C.L.; de Sousa, B.R.V.; de Almeida Filho, E.J.B.; da Silveira Costa, M.; Marques, A.T.B.; da Silva, D.F.; de Farias Sena, K.; Cerqueira, G.S.; et al. A Single Dose of Purple Grape Juice Improves Physical Performance and Antioxidant Activity in Runners: A Randomized, Crossover, Double-Blind, Placebo Study. Eur. J. Nutr. 2020, 59, 2997–3007.
- Toscano, L.T.; Tavares, R.L.; Toscano, L.T.; da Silva, C.S.O.; de Almeida, A.E.M.; Biasoto, A.C.T.; Gonçalves, M.d.C.R.; Silva, A.S. Potential Ergogenic Activity of Grape Juice in Runners. Appl. Physiol. Nutr. Metab. 2015, 40, 899–906.
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency Cherry Juice Reduces Muscle Damage Caused by Intensive Strength Exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551.
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Scand. J. Med. Sci. Sport. 2010, 20, 843–852.
- Zeng, Z.; Jendricke, P.; Centner, C.; Storck, H.; Gollhofer, A.; König, D. Acute Effects of Oatmeal on Exercise-Induced Reactive Oxygen Species Production Following High-Intensity Interval Training in Women: A Randomized Controlled Trial. Antioxidants 2021, 10, 3.
- Koenig, R.T.; Dickman, J.R.; Kang, C.H.; Zhang, T.; Chu, Y.F.; Ji, L.L. Avenanthramide Supplementation Attenuates Eccentric Exercise-Inflicted Blood Inflammatory Markers in Women. Eur. J. Appl. Physiol. 2016, 116, 67–76.
- Zhang, T.; Zhao, T.; Zhang, Y.; Liu, T.; Gagnon, G.; Ebrahim, J.; Johnson, J.; Chu, Y.F.; Ji, L.L. Avenanthramide Supplementation Reduces Eccentric Exercise-Induced Inflammation in Young Men and Women. J. Int. Soc. Sports Nutr. 2020, 17.
- Copetti, C.L.K.; Orssatto, L.B.R.; Diefenthaeler, F.; Silveira, T.T.; da Silva, E.L.; de Liz, S.; Mendes, B.C.; Rieger, D.K.; Vieira, F.G.K.; Hinnig, P.F.; et al. Acute Effect of Juçara Juice (Euterpe Edulis Martius) on Oxidative Stress Biomarkers and Fatigue in a High-Intensity Interval Training Session: A Single-Blind Cross-over Randomized Study. J. Funct. Foods 2020, 67, 103835.
- Pittaluga, M.; Sgadari, A.; Tavazzi, B.; Fantini, C.; Sabatini, S.; Ceci, R.; Amorini, A.M.; Parisi, P.; Caporossi, D. Exercise-Induced Oxidative Stress in Elderly Subjects: The Effect of Red Orange Supplementation on the Biochemical and Cellular Response to a Single Bout of Intense Physical Activity. Free Radic. Res. 2013, 47, 202–211.
- Chang, W.H.; Hu, S.P.; Huang, Y.F.; Yeh, T.S.; Liu, J.F. Effect of Purple Sweet Potato Leaves Consumption on Exercise-Induced Oxidative Stress and IL-6 and HSP72 Levels. J. Appl. Physiol. 2010, 109, 1710–1715.
- Collins, F.W. Oat Phenolics: Avenanthramides, Novel Substituted N-Cinnamoylanthranilate Alkaloids from Oat Groats and Hulls. J. Agric. Food Chem. 1989, 37, 60–66.
- Bratt, K.; Sunnerheim, K.; Bryngelsson, S.; Fagerlund, A.; Engman, L.; Andersson, R.E.; Dimberg, L.H. Avenanthramides in Oats (Avena Sativa L.) and Structure-Antioxidant Activity Relationships. J. Agric. Food Chem. 2003, 51, 594–600.
- Lee-Manion, A.M.; Price, R.K.; Strain, J.J.; Dimberg, L.H.; Sunnerheim, K.; Welch, R.W. In Vitro Antioxidant Activity and Antigenotoxic Effects of Avenanthramides and Related Compounds. J. Agric. Food Chem. 2009, 57, 10619–10624.
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J.L. Cocoa Polyphenols and Their Potential Benefits for Human Health. Oxid. Med. Cell. Longev. 2012, 2012, 906252.
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797.
