Cardiac calcifications are generally asymptomatic findings frequently encountered on routine echocardiographic examination or CT scanning. The sites affected most often are the aortic valve (prevalence about 24%) and the mitral annulus (prevalence 8- 15%). The recognition of even small calcium deposits on valves and other cardiac structures is clinically relevant, both as a marker of systemic atherosclerosis and as a predictor of future cardiovascular events. The detection of cardiac calcifications by ultrasound is a promising tool for identifying subclinical atherosclerosis and thus improving risk stratification in asymptomatic subjects.
- subclinical atherosclerosis
- cardiac calcification
- risk reclassification
1. Introduction
Cardiovascular (CV) diseases, primarily ischemic heart disease (IHD) and stroke, are still the leading cause of global mortality and a major contributor to disability. Indeed, prevalent cases of total CV diseases nearly doubled from 271 million in 1990 to 523 million in 2019, and the number of CV deaths steadily increased from 12.1 million in 1990, reaching 18.6 million in 2019 [1]. Because CV risk is the result of the interaction of different traditional risk factors (TRFs), in terms of severity and time to exposure, risk prediction algorithms combining multiple TRFs have gained a central role in CV disease prevention [2].
1.1. Current Cardiovascular Risk Prediction: Algorithms and Limitations
The European Society of Cardiology (ESC) SCORE charts [3], the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease (ACC/AHA ASCVD) Risk Calculator [4] and the Framingham Risk Score (FRS) [5] are the main currently used risk algorithms, and all of them estimate the absolute risk of CV events over 10 years. They identify subjects at very high risks if the calculated SCORE risk is ≥10%, subjects at high CV risk if the SCORE risk is between 5% and 10%, the FRS risk is ≥20% or the ASCVD is ≥20%, subjects at moderate risk if the SCORE is between 1% and 5%, the FRS is between 10% and 20% or the ASCVD is between 7.5% and 19.9% [6][7][6,7].
Although algorithms are aimed to customize risk, they have at least three well-acknowledged limitations. First, they are primarily influenced by age; therefore, an asymptomatic young person with multiple TRFs is likely to be identified as low risk, while, conversely, an elderly person is likely to be identified as high risk regardless of actual risk [4][6][4,6]. Second, risk charts do not take into account either the time of risk exposure or some important TRFs such as family history for CV disease, obesity and glucose intolerance. They also miss important nontraditional risk factors, such as cumulative exposure to air pollution [8]. Finally, as postulated by the epidemiologist Geoffrey Rose, most CV events will develop in low-risk subjects, simply because they are much more numerous than those at high risk [9]. As a consequence, people with low estimated risk but who already have subclinical atherosclerosis could be underrecognized and undertreated. Therefore, it is recommended that asymptomatic people with calculated CV risks near the decisional thresholds (between two risk classes) undergo noninvasive testing (looking for subclinical atherosclerosis) for further risk stratification and possible reclassification [6]. For example, a recent manuscript suggests implementing the screening for subclinical atherosclerosis in subjects with a calculated SCORE ≥ 3 or a calculated Framingham ≥ 10 [10].
Reclassification means changing the risk class of a subject after complementing risk algorithms with other data; for example, an imaging technique [11]. Obviously, reclassification to a higher risk class can entail the adoption of preventive strategies, such as pharmacological treatment (i.e., statins); vice versa, a subject reclassified as at low risk of CV events does not require such preventive strategies. Anyway, reclassification can be considered appropriate (when “moving” individuals who will develop future CV events into higher estimated-risk levels) or not appropriate (when “moving” individuals who will develop future CV events into lower estimated-risk levels) [2]. Generally, a new risk prediction model is considered useful if it leads to a “net reclassification improvement (NRI)” of at least 10%, i.e., at least 10% of people are more appropriately reclassified with the new method compared with the old one [12].
1.2. The Role of Imaging in Detecting Subclinical Atherosclerosis and Reclassify Patients Risk
It is increasingly clear that the use of preventive models based on the detection of subclinical atherosclerosis and organ damage is useful [2][13][2,13]. Indeed, as Shah [14] emphasized, despite the lack of randomized clinical trial evidence, the totality of observational evidence supports “imaging-guided prediction and management” because:
-
Detecting atherosclerosis (the consequences of which we aim to avoid) is better than simply identifying TRF exposure;
-
Reclassification of low-risk subjects into higher-risk strata may guide appropriate therapy;
-
Disease visualization might improve adherence to risk-modifying interventions by increasing awareness.
Several imaging modalities have been evaluated and proposed for the identification of preclinical atherosclerosis and phenotypic evidence of disease [15]. There are currently two primary imaging techniques used to reclassify low-intermediate cardiovascular risk subjects.
1.2.1. Two-Dimensional (2D) Ultrasound of Carotid Arteries
This imaging modality allows the physician to detect both the presence of increased carotid intima-media thickness (CIMT) and carotid plaques [16]. Conflicting results have been published on the added value of CIMT measurements in cardiovascular risk prediction [17][18][17,18]. In fact, while Framingham investigators [19] showed that the maximal CIMT of the internal carotid artery added a modest value (NRI: 7.6%) in risk prediction, a meta-analysis [20] of about 46,000 patients showed that the addition of common CIMT measurements to the FRS was associated only with a small improvement (NRI 0.8%) in the 10-year risk prediction of myocardial infarction or stroke. However, carotid ultrasound can give information besides CIMT. The presence of carotid plaques conferred a two-fold increase in the risk of future CV adverse events (hazard ratio (HR): 2.3) in a population free of overt CV disease [21]. A meta-analysis by Peters et al. [22] also noted that the presence of carotid plaques added value for screening asymptomatic subjects at intermediate risk, improving the NRI from 8 to 11%. Therefore, maximum carotid plaque thickness seems to be a simple useful measure to enhance the prediction (NRI: 17.8%) of future cardiovascular disease events [23]. Furthermore, the morphological characteristics of carotid plaques are associated with future cerebrovascular ischemic events as well [24]. Indeed, a recent study conducted with magnetic resonance showed that in patients with carotid plaque the presence of intraplaque hemorrhage is a stronger predictor of stroke than any known clinical risk factors [25].
1.2.2. Coronary Artery Calcium Score (CACs)
Coronary calcium detection by computed tomography (CT) is the most commonly used technique for the detection of subclinical disease, prognostic stratification of asymptomatic individuals and implementation of preventive strategies [26]. It is possible to quantitatively assess coronary calcium using Agatston CACs, a surrogate for plaque burden that has been shown to provide powerful prognostic information in multiple studies involving both sexes and multiple ethnic groups [14][27][14,27]. The body of evidence on the predictive and risk-reclassification role of CACs is large and founded on several well-designed prospective studies. It is now supported by a large amount of data the fact that the presence of coronary artery calcium provides independent incremental information in addition to TRF to predict all-cause mortality, whereas its absence (CACs = 0) identifies a group of asymptomatic subjects at very low CV risk (regardless of the presence of underlying risk factors) with a consequent reduced need for aggressive therapy or further diagnostic tests [22][28][29][22,28,29]. The risk class in which this technique has proved most useful is the intermediate one, in which adding CACs to TRFs resulted in a NRI of 55% [30]. Indeed, in the 6814 participants from the Multiethnic Study of Atherosclerosis (MESA) [30], CACs assessment allowed the reclassification of 292 subjects (16%) from moderate to high risk and of 712 (39%) from moderate to low risk. Furthermore, a recent analysis of the MESA data found that among persons with LDL-C ≥ 190 mg/dL (therefore at increased CV risk), a CAC of 0 was associated with a low risk of cardiovascular events, suggesting the utility of CACs for redefining risk in this patient group as well [31]. In support of this imaging technique, a brilliant Danish study [32] recently showed that coronary plaque burden, not stenosis per se, is the main predictor of CV events and death. Thus, patients with a comparable calcium burden measured as CACs generally have a similar risk for CV events regardless of whether they have nonobstructive or obstructive coronary artery disease. It should be emphasized that coronary CT angiography (CCTA), an imaging technique currently not recommended for reclassifying asymptomatic subjects, is, however, capable of detecting “soft” noncalcified plaque in patients who have CACs = 0 [33].
However, both of these techniques have limitations to consider. Regarding the 2D ultrasound imaging of the carotid arteries, despite providing a simple noninvasive (and relatively inexpensive) modality for detecting subclinical atherosclerosis, results show more subjects moving from intermediate-risk to lower-risk categories than to higher-risk categories. This implies the possibility of not undertaking preventive interventions in subjects who could benefit from them [34]. The major drawback of CT imaging is the exposure to ionizing radiation, which is particularly undesirable in young subjects, especially women. Its use is also limited by cost, by the impossibility of being performed “bedside” and by the limited availability in nonspecialized centers. CT also requires interpretation by a physician with specialized training (e.g., radiologist) who is often not the clinician taking care of the patient. This fact inevitably entails greater time, organizational and bureaucratic cumbersome, with the possibility that both the clinician and the subject/patient are discouraged from carrying out an in-depth diagnostic pathway to reclassify the CV risk.
2. Definition and Epidemiology of Cardiac Calcifications
Cardiac calcifications are frequently encountered on routine echocardiographic examination or CT scanning. They are generally asymptomatic, and their prevalence varies according to the site evaluated, age and presence of cardiovascular risk factors (including chronic kidney disease [35] or diabetes [36]). The sites affected most often are the aortic valve (prevalence about 24%) and the mitral annulus (prevalence 8% and up to 15% with increasing age and number of risk factors) [37][38][37,38]. Furthermore, calcifications can often involve both the aortic and mitral valves simultaneously, especially in diabetic patients. Rossi et al. [39] observed that approximately 45% of 900 type-2 diabetic subjects had aortic valve sclerosis (AVS, a precursor of calcification), mitral annulus calcification (MAC) or both.
Generally, aortic valve sclerosis (AVS)/calcification (AVC) is defined as the presence of sclerotic and calcific lesions that reside within the aortic valve leaflets, not involving the aortic annulus (or coronary artery ostia. Valve sclerosis cannot always be differentiated by ultrasound from calcification except that the latter tends to be whiter in appearance. Specifically, AVC is diagnosed by ultrasound if there is hyperreflectivity of the valve cusps (usually the presence of nodular brightness), with or without obstruction to the outflow (Figure 1). Detection by CT is more precise; indeed, calcification is diagnosed [40] if there are at least 3 contiguous pixels of at least 130 Hounsfield units of brightness. This allows accurate quantitation, typically employing the Agatston score technique [41].
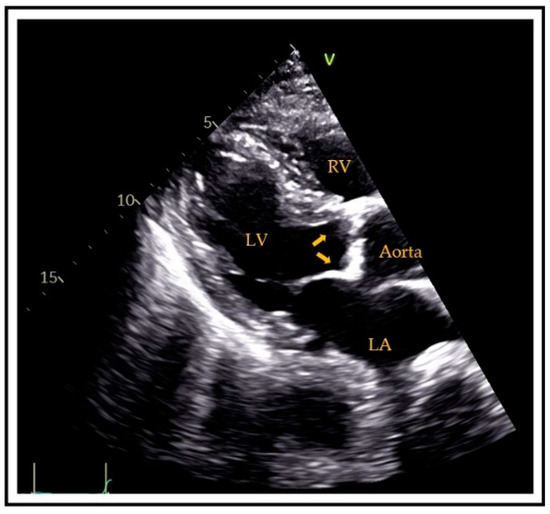
Figure 1. Transthoracic parasternal long-axis view showing aortic valve calcifications (arrows). RV = right ventricle, LV = left ventricle, LA = left atrium.
MAC is a chronic, degenerative process of the fibrous support structure of the mitral valve, which most often affects the posterior annulus [42]. MAC is visualized by echocardiography as an echo-dense structure with an irregular, lumpy appearance and associated acoustic shadowing [43] (Figure 2). MAC can also be diagnosed and quantified by CT using the Agatston score technique.
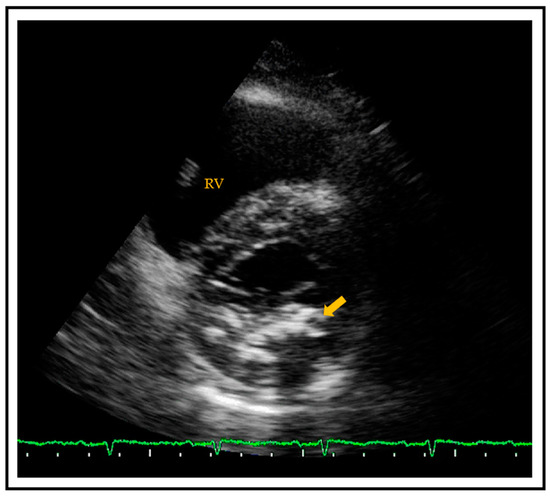
Figure 2. Transthoracic parasternal short axis view showing a calcified mitral posterior annulus (arrow). RV = right ventricle.
The question of whether MAC and AVC are expressions of atherosclerotic disease or simply reflect a primary degenerative process progressing with advancing age was partially clarified in the late 1980s and 1990s. Several authors [44][45][44,45] reported that cardiac calcification and vascular atherosclerosis have many shared risk factors, and thus, the former should be regarded as a manifestation of generalized atherosclerosis. Like atherosclerosis, cardiac calcifications progress over time. Some studies describe an increase in the extent of MAC [46] and AVC [47], which can lead to clinically relevant mitral regurgitation [48], nonrheumatic mitral stenosis [49] and aortic valve regurgitation/stenosis [47]. It is therefore not surprising that more than one author has proposed that AVC and MAC could represent a surrogate marker for underlying atherosclerotic disease and an “easy-to-see” ultrasound window of what is occurring in the arterial beds [50][51][50,51].
