Osteoporosis results from excessive bone resorption and reduced bone formation, triggered by sex hormone deficiency, oxidative stress and inflammation. Tanshinones are a class of lipophilic phenanthrene compounds found in the roots of Salvia miltiorrhiza with antioxidant and anti-inflammatory activities, which contribute to its anti-osteoporosis effects.
- tanshinones
- osteoclastogenesis
1. Introduction
Osteoporosis is a condition that increases the fracture risk of patients due to decreased bone strength, which is a result of bone microstructure and declining bone mass [1][2]. Osteoporosis can occur in both sexes, but it is more common in postmenopausal women than their male counterparts. This sex distinction occurs because of lower peak bone mass in women and the rapid decline of bone mass due to sudden cessation of oestrogen production in the body. Oestrogen deficiency leads to increased bone turnover, subsequently bone loss and osteoporosis [3]. Osteoporosis frequently remains undiagnosed due to its asymptomatic nature until it presents as low-trauma hip, spine, proximal humerus, pelvis and/or wrist fractures [4][5]. In 2010, it was estimated that 158 million individuals globally, aged 50 years or older, were at high risk of osteoporotic fracture and this number is expected to double by 2040 [6]. The cost of fractures in the United States is estimated to reach $25 billion annually by 2025 corresponding to three million projected fractures [7].
Pharmacotherapeutics for osteoporosis could be divided into anti-resorptive (i.e., bisphosphonates, oestrogen receptors modulators, oestrogen replacement and denosumab) or anabolic (i.e., teriparatide) medications. Anti-resorptive osteoporotic drugs reduce the rate of bone resorption by inhibiting osteoclasts [8]. Although they manage osteoporosis effectively, bone resorption inhibition is often associated with reduced bone formation since both processes are coupled. Coupling is modulated by osteogenic variables produced by osteoblasts. This event will prevent the repair of bone micro-fractures and jeopardise skeletal microarchitecture [9][10]. Cathepsin K (CatK) inhibitors allow inhibition of bone resorption without disturbing bone formation [11], but most agents are still in development [12][13][14]. Odonacatib is the only CatK inhibitor that had entered phase III clinical trial but was terminated due to cardio-cerebrovascular adverse effects [15][16]. On the other hand, anabolic agents increase bone formation rate more than bone resorption [8], but they are offered to patients with high fracture risk and have failed other therapies [11]. Other preventive agents like calcium and vitamin D are available, but their effectiveness is inconsistent. Ensuring adequate dietary calcium and vitamin D is compulsory in stopping the progression of osteoporosis [17]. However, vitamin D and calcium supplementation alone might not be sufficient to stop osteoporosis from occurring [18].
Salva miltiorrhiza. They are the major lipid-soluble pharmacological constituents of Danshen and give the root its reddish-brown colour [19]. The major tanshinones isolated from Danshen included 15,16-dihydrotanshinone (D-T), tanshinone I (T-I), cryptotanshinone (C-T) and tanshinone IIA (T-IIA) [20][21]. A previous study indicated that D-T could selectively block the collagenase activity of CatK without affecting protease activity and osteoclastogenesis [22]. In vitro studies have also reported that T-IIA and C-T possess anti-inflammatory effect by inhibiting the activation of nuclear factor kappa B (NF-κB) pathway [23][24][25][26]. These studies suggest the potential of tanshinones as an anti-osteoporotic agent. Therefore, this article aims to review the effects of tanshinones on bone based on evidence from in vitro and in vivo studies.
2. Selection of Articles
n
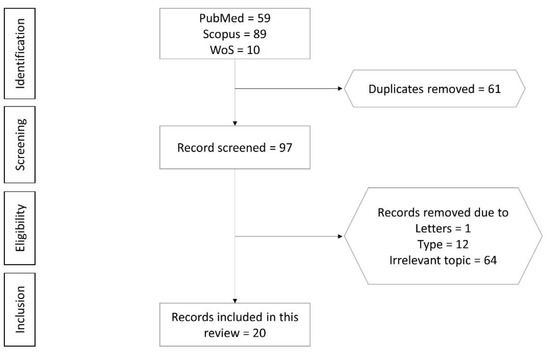
Figure 1.
3. The Skeletal Effects of Tanshinones
Osteoblasts are mesenchymal cells that produce and mineralise the bone matrix [27]. Osteoblast differentiation is regulated by Runx2 phosphorylation and transcription, which is mediated by the MAPK cascades. MAPK pathways and its components, JNK, ERK and p38, form the non-canonical BMP2 signal transduction pathways that regulate osteoblastogenesis [28][29][30][31]. T-IIA increases the activation of osteogenic genes which indicate its stimulatory effect on osteogenic differentiation [32][33][34]. Wang et al. pinpointed that the effects of T-IIA were mediated through JNK as inhibition of this pathway negated its effects on osteoblast differentiation and mineralisation [35].
Excessive ROS production could overwhelm intracellular antioxidant defence, causing oxidative stress and osteoporosis [36][37]. Apart from inhibiting osteoblast proliferation [38] and differentiation [39], oxidative stress also induces osteoblast apoptosis [40][41], thereby jeopardising bone formation [42]. Ameliorating oxidative stress could decrease osteoblast apoptosis and increase its differentiation [43]. Hydrogen peroxide, an end-product of Nox4, is one of the major ROS predominately present in mitochondria [44][45]. Blocking Nox4 activation may prevent osteoblast apoptosis. T-IIA was reported to inhibit Nox4 expression, which in turn decreased osteoblast apoptosis [46]. NF-κB activation negatively regulates bone formation by suppressing osteoblast differentiation [47]. T-IIA increased osteoblast differentiation via suppressing NF-κB activation and translation of its target genes (TNF-α, iNOS and COX2). This event is achieved by preventing IKK-β and IκBα degradation and p65 nuclear translocation. These effects translated to improved bone microstructure and biomechanical properties in animals treated with T-IIA [43].
Osteoclasts are derived from hematopoietic lineage cells and are capable of bone resorption [48]. It is necessary for normal bone homeostasis, but excessive resorption can induce pathological bone loss. A variety of hormones and cytokines regulate osteoclast differentiation and activation. Particularly, RANKL and M-CSF are the essential cytokines for osteoclastic differentiation [49]. M-CSF binds to colony-stimulating factor 1 receptor, and RANKL binds to RANK to promote osteoclast differentiation and survival, as well as bone resorptive activity [50][51]. RANK-RANKL binding leads to the recruitment of TRAF factors like TRAF 6 [52], resulting in the activation of NF-kB, Akt and MAPKs (ERK/p38/JNK) pathways. Besides, RANKL signalling activates c-Fos and subsequently NFATc1, a master switch for controlling osteoclast terminal differentiation [53][54][55]. All these pathways act in concert to initiate osteoclast differentiation and bone resorption by inducing transcription and expression of osteoclast-specific genes, such as TRAP, cathepsin K, matrix metalloproteinase 9 (MMP-9) and C-Src [56]. Exogenous factors like LPS can alter RANKL signalling and influence osteoclastogenesis. LPS induces production of proinflammatory cytokines of osteoblasts and precursor cells via COX-2, especially TNF-α, which subsequently augment RANKL signalling in osteoclasts [57][58]. Blocking of COX-2 is reported to inhibit osteoclastogenesis in vitro [59][60].
T-IIA treatment is reported to suppress RANKL and M-CSF-induced osteoclastogenesis from precursor cells and osteoclast function [61][62]. Similar effects were observed with T06, T-1, C-T and D-T treatment, indicating all tanshinones share similar properties [63][64][65]. T-IIA prevents RANKL-induced activation of TRAF 6, which in turn reduces activation of NF-κB, MAPK, Akt and c-Src pathways, and inhibits osteoclast formation and activity, marked by reduced TRAP and MMP9 expression [61][62]. T-IIA also inhibits the expression of RANKL-induced c-Fos and NFATc1, which suppress osteoclast differentiation [66]. Besides, T-IIA is reported to inhibit LPS-mediated COX-2 expression in bone marrow and calvarial osteoblast cells [67]. This action could reduce osteoclast formation and bone loss prevention in mice administered with LPS [67].
RANK-RANKL signalling also regulates CatK expression [68]. CatK represents a potential target of anti-osteoporosis therapy. CatK inhibition does not affect bone formation [69][70], suggesting that bone formation and resorption are uncoupled [16]. Furthermore, osteoclast formation and survival required for osteoblastic bone formation response during remodelling are not affected by CatK inhibition [71]. In contrast, increased bone formation was observed in CatK deficient mice [72]. The different forms of tanshinones (T-IIA and T06) exert distinct effects in CatK-associated bone remodelling. T-IIA suppressed RANKL-induced expression of CatK in RAW264.7 cells and BMMCs, but reduced the function and survival of osteoclasts [62]. This observation suggests that T-IIA may not be a selective inhibitor of CatK. On the other hand, T06 inhibited the activity of CatK but did not alter CatK-positive osteoclast number in mice with OVX-induced osteoporosis [64], which suggests that T06 may be a selective inhibitor of CatK. The selectivity ensures normal osteoblast-osteoclast crosstalk and bone remodelling are not interrupted. In comparison, bisphosphonates, an antiresorptive agent commonly used as the first-line treatment for osteoporosis, also inhibit bone formation [73].
The biological effects of tanshinones suggest their various potential clinical applications. Direct gingival mucosa injection of T-IIA reduced the recurrence distance and percentage of first molar tooth movement in Wistar rats by suppressing the osteoclast activity [74]. This evidence showed that T-IIA could prevent the loosening of teeth in gingival and periodontal diseases. Tanshinones enhanced skeletal health in healthy animals, by increasing BMD, femoral microstructures and strength, as well as lowering osteoclast activity [75]. Therefore, it could serve as an agent for the primary prevention of osteoporosis. Furthermore, various skeletal actions of tanshinones could be harnessed for secondary prevention of osteoporosis. T-IIA restored retinoic acid-induced decrease of cortical bone thickness and Tb.N by increasing serum oestradiol levels and preventing high bone remodelling in Wistar rats [76]. T-IIA also improved bone structural properties in mice with STZ-induced diabetic osteoporosis [77]. Additionally, T-IIA [62][78], T06 [64] and total tanshinones (T-IIA + C-T) [79] prevented OVX-induced bone loss in rats and mice by improving bone microstructures. T06 increased osteoblast number [64] while total tanshinones decreased osteoclast number [79] in vivo. Untreated osteoporosis could lead to fragility fracture. T-IIA could increase fracture healing in mice with femoral osteotomy to mimic fracture [35]. Osteolysis following joint replacement is mainly caused by the abrasive particles introduced by the prosthesis [80]. Recent studies showed that these abrasive particles could induce the release of cytokines associated with osteolysis, such as IL-6, IL-1, TNF-α and PGE
2, worsening the inflammatory response [81]. PE particles have been confirmed to induce osteolysis around artificial joints [82]. T-IIA was shown to prevent calvarial bone resorption in PE particle-induced osteolysis [80]. Hence, T-IIA could be embedded with arthroplasty materials to avoid triggering inflammation and bone resorption.
Limited studies examined the safety of tanshinones. T-IIA at high concentration (≥6 μM) caused severe growth inhibition, development malformation and cardiotoxicity in zebrafish [83]. Similarly, T-11A at a high concentration (25 μM) was toxic to human endothelial EAhy926 cells as these cells were killed after a 24 h treatment [84]. There is limited data on the pharmacokinetic properties of tanshinones. Zhang et al. reported that tanshinones have limited bioavailability when administered orally [85]. After oral administration at 100 mg/kg, the systemic bioavailability of C-T was 2.05%, suggesting poor absorption or significant metabolism in the gut or/and liver. After intraperitoneal administration at 100 mg/kg, the systemic bioavailability of C-T was 10.60%, suggesting hepatic metabolism or low solubility of C-T [86]. There is a paucity of pharmacokinetics, pharmacodynamics and safety data of tanshinones in humans. A search through
https://clinicaltrials.gov/ (accessed on 30 March 2021) using the term “tashinones” revealed four registered trials on left ventricular remodelling secondary to acute myocardial infarction (identifier: NCT02524964), pulmonary hypertension (identifier: NCT01637675), polycystic ovary syndrome (identifier: NCT01452477) and childhood acute promyeloid leukemia (identifier: NCT02200978). The last trial was recruiting subjects, while the status of the other three trials was unknown. No human clinical trial on the effects of tanshinones on skeletal diseases has been attempted, probably due to poor intestinal absorption and bioavailability [86][87]. Various methods have been developed to address the problem of low bioavailability, including designing water-soluble tanshinone derivatives, loading of tanshinones into discoidal and spherical high-density lipoproteins, liposomes, nanoparticles, microemulsions, cyclodextrin and solid dispersions [88][89][90][91][92]. However, due to the complicated manufacturing process, little progress has been made in the clinical application of these formulations [93]. Therefore, more comprehensive studies in this regard will help to establish tanshinones as one of the clinical therapeutic options for various bone conditions.
