Presynaptic Ca2+ entry occurs through voltage-gated Ca2+ (CaV) channels which are activated by membrane depolarization. Depolarization accompanies neuronal firing and elevation of Ca2+ triggers neurotransmitter release from synaptic vesicles. For synchronization of efficient neurotransmitter release, synaptic vesicles are targeted by presynaptic Ca2+ channels forming a large signaling complex in the active zone. The presynaptic CaV2 channel gene family (comprising CaV2.1, CaV2.2, and CaV2.3 isoforms) encode the pore-forming α1 subunit. The cytoplasmic regions are responsible for channel modulation by interacting with regulatory proteins.
- Ca2+ channels
- synaptic transmission
- G-proteins
- synaptic proteins
- Ca2+ binding proteins
1. Introduction
Presynaptic Ca
2+
entry into the active zone (AZ) occurs through voltage-gated Ca
2+
(Ca
V
) channels which are activated membrane depolarization and triggers synchronous neurotransmitter release from synaptic vesicles (SVs). Multiple mechanisms regulate the function of presynaptic Ca
2+ channels [1][2][3][4]. The channel activity for opening, closing, or inactivation in response to membrane depolarization changes every few milliseconds during and after neuronal firing, resulting in control of synaptic strength [3][4]. Following a brief overview of Ca
channels [1,2,3,4]. The channel activity for opening, closing, or inactivation in response to membrane depolarization changes every few milliseconds during and after neuronal firing, resulting in control of synaptic strength [3,4]. Following a brief overview of Ca
2+
channel structure/function, this article reviews the molecular and cellular mechanisms that modulate the activity of presynaptic Ca
2+
channels in the regulation of neurotransmitter release and in the induction of short-term synaptic plasticity. To understand the physiological role of Ca
2+
channel modulation in the regulation of synaptic transmission, a model synapse formed between sympathetic, superior cervical ganglion (SCG) neurons in culture was employed for functional study of channel interaction with G proteins, SNARE proteins, and Ca
2+
-binding proteins which sense residual Ca
2+
in the AZ after the arrival of an action potential (AP).
2. Presynaptic Ca
2+
Channels
Ca
2+
currents have diverse physiological roles and different pharmacological properties. Early investigations revealed distinct classes of Ca
2+
currents which were identified with an alphabetical nomenclature [5]. P/Q-type, N-type, and R-type Ca
2+
currents are observed primarily in neurons, require strong depolarization for activation [6], and are blocked by specific polypeptide toxins from snail and spider venoms [7]. P/Q-type and N-type Ca
2+ currents initiate neurotransmitter release at most fast synapses [1][8][9]. The Ca
currents initiate neurotransmitter release at most fast synapses [1,8,9]. The Ca
2+
channels are composed of four or five distinct subunits (
Figure 1a) [8][10]. The α1 subunit incorporates the conduction pore, the voltage sensors and gating apparatus, and target sites of toxins and intracellular regulators. The α1 subunit is composed of about 2000 amino acid residues and is organized in four homologous domains (I–IV) (
a) [8,10]. The α1 subunit incorporates the conduction pore, the voltage sensors and gating apparatus, and target sites of toxins and intracellular regulators. The α1 subunit is composed of about 2000 amino acid residues and is organized in four homologous domains (I–IV) (
b). Each domain consists of six transmembrane α helices (S1 through S6) and a membrane-associated P loop between S5 and S6. The S1 through S4 segments serve as the voltage sensor module, whereas transmembrane segments S5 and S6 in each domain and the P loop between them form the pore module [11]. The intracellular segments serve as a signaling platform for Ca
2+
-dependent regulation of neurotransmission, as discussed below.
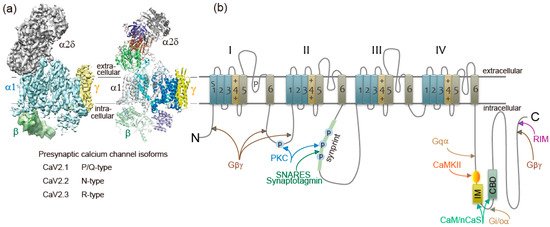
Figure 1.
2+
a
2+
2+
b
2+
Ca
2+ channel α1 subunits are encoded by ten distinct genes in mammals, which are divided into three subfamilies by sequence similarity [2][8][13]. The Ca
channel α1 subunits are encoded by ten distinct genes in mammals, which are divided into three subfamilies by sequence similarity [2,8,13]. The Ca
V
2 subfamily members Ca
V
2.1, Ca
V
2.2, and Ca
V
2.3 channels conduct P/Q-type, N-type, and R-type Ca
2+ currents, respectively [2][8][9][13].
currents, respectively [2,8,9,13].
Ca
V
channels are complexes of a pore-forming α1 subunit and auxiliary subunits. Skeletal muscle Ca
V
channels have three distinct auxiliary protein subunits [8] (
a), the intracellular β subunit, the disulfide-linked α2δ subunit complex, and the γ subunit having four transmembrane segments. In contrast, brain neuron Ca
V
2 channels are composed of the pore-forming α1 and the auxiliary β subunit [14]. The auxiliary subunits of Ca
2+ channels have an important influence on their function [15][16]. The Ca
channels have an important influence on their function [15,16]. The Ca
Vβ subunit shifts their kinetics and voltage dependence of activation and inactivation [15][16]. Cell surface expression of the α1 subunits is enhanced by the Ca
β subunit shifts their kinetics and voltage dependence of activation and inactivation [15,16]. Cell surface expression of the α1 subunits is enhanced by the Ca
Vβ subunit [15][16]. The α2δ subunits are potent modulators of synaptic transmission. The α2δ subunits increase not only Ca
β subunit [15,16]. The α2δ subunits are potent modulators of synaptic transmission. The α2δ subunits increase not only Ca
v
1.2 but also Ca
v
2.2, Ca
v
2.1 currents, suggesting that the α2δ subunits enhance trafficking of the Ca
V
channel complex [17]. Expression of α2δ subunits also appears to play a role in setting release probability [18]. Further details of these regulatory interactions are discussed below.
3. Intracellular Molecules Modulate Presynaptic Ca
2+
Channels Activity
3.1. G Proteins
Presynaptic Ca2+ currents are reduced in magnitude by activation of G protein-coupled receptors for neurotransmitters at nerve terminals [19][20]. Gβγ subunits released from heterotrimeric G proteins of the Gi/Go class [19][20] bind directly to α1 subunits of the N-type Ca
currents are reduced in magnitude by activation of G protein-coupled receptors for neurotransmitters at nerve terminals [19,20]. Gβγ subunits released from heterotrimeric G proteins of the Gi/Go class [19,20] bind directly to α1 subunits of the N-type Ca2+ channel [21][22] at the N terminus [23], the intracellular loop connecting domains I and II [21][24], and at the C terminus [25] (
channel [21,22] at the N terminus [23], the intracellular loop connecting domains I and II [21,24], and at the C terminus [25] (b). Gβγ causes a positive shift in the voltage dependence of activation of the Ca2+ current [26][27][28]. The Gβγ-induced reduction of Ca
current [26,27,28]. The Gβγ-induced reduction of Ca2+ currents can be reversed by strong positive depolarization [26][27][28]. Reversal of this inhibition by depolarization provides a point of intersection between chemical and electrical signal transduction at the synapse and can potentially provide novel forms of short-term synaptic plasticity that do not rely on residual Ca
currents can be reversed by strong positive depolarization [26,27,28]. Reversal of this inhibition by depolarization provides a point of intersection between chemical and electrical signal transduction at the synapse and can potentially provide novel forms of short-term synaptic plasticity that do not rely on residual Ca2+
. The subtype of CaVβ can influence the extent and kinetics of Gβγ mediated inhibition and this regulation also depends on the subtype of Gβ involved [29][30]. Gβγ interacts with multiple sites on the N-terminus, I–II linker, and the C-terminus of the α1 subunit. Binding of Gβγ causes a conformational shift that promotes interaction of the N-terminus “inhibitory module” with the initial one-third of the I–II-linker. Strong membrane depolarization leads to unbinding of Gβγ and loss of interaction between the N-terminus and the I–II linker. This depends upon binding of Ca
β can influence the extent and kinetics of Gβγ mediated inhibition and this regulation also depends on the subtype of Gβ involved [29,30]. Gβγ interacts with multiple sites on the N-terminus, I–II linker, and the C-terminus of the α1 subunit. Binding of Gβγ causes a conformational shift that promotes interaction of the N-terminus “inhibitory module” with the initial one-third of the I–II-linker. Strong membrane depolarization leads to unbinding of Gβγ and loss of interaction between the N-terminus and the I–II linker. This depends upon binding of CaV
β subunit to the α interaction domain (AID) on the I–II linker. In the absence of CaV
β1 subunit binding with tryptophan mutation in the AID (W391) of the CaV
2.2 α1 subunit, Ca2+
channel inhibition still occurs but cannot be reversed by strong depolarization. CaV
β2a, that is palmitoylated at two N-terminal cysteine residues, can still bind to the α1 subunit and permit voltage-dependent relief of the inhibition [31]. It is possible that binding of CaV
β1 to the AID induces a rigid α-helical link with domain IS6, and this transmits the movement of the voltage-sensor and activation gate to the I–II linker to alter the Gβγ binding pocket at depolarized potentials [32]. Specific Gβ subunits have been shown to be responsible for the CaV
2 channel modulation in different neurons. In rat SCG neurons CaV
2.2 channels are differentially modulated by different types of Gβ subunits, with Gβ1
and Gβ2
being most effective, Gβ5
showing weaker modulation, and Gβ3
and Gβ4 being ineffective [33][34][35]. In contrast, in rat stellate ganglion neurons, Gβ
being ineffective [33,34,35]. In contrast, in rat stellate ganglion neurons, Gβ2
and Gβ4
but not Gβ1
subunit are responsible for the coupling of CaV
2.2 channels with noradrenaline receptors [36]. In the transfected human embryonic kidney tsA-201 cell line, CaV
2.2 channel inhibition, with Gβ1
and Gβ3
being more effective than Gβ4
and Gβ2
, and no significant modulation being induced by Gβ5
[37]. Gβ subunit-induced inhibition of CaV
2.1 channel differed from those observed with the CaV
2.2 channel. CaV
2.1 channels exhibited more rapid rates of recovery from inhibition than those observed with CaV
2.2 channels, on average, twice as rapidly for the CaV
2.1 channels, indicating that Gβ binding to this channel subtype is less stable [37]. Regulation of the CaV
2.2 channels also involves the interplay between Ca2+
channels and G protein interaction. Syntaxin-1A, a presynaptic plasma membrane protein, is required for G protein inhibition of presynaptic Ca2+
channels [38]. Physical interaction between syntaxin-1A and Ca2+
channels is a prerequisite for tonic Gβγ modulation of CaV
2.2 channels, suggesting that syntaxin-1A mediates a colocalization of Gβγ subunits and CaV
2.2 channels, thus resulting in a more effective G protein coupling to, and regulation of, the channel. The interactions between syntaxin, G proteins, and CaV
2.2 channels are part of the structural specialization of the presynaptic terminal [39]. G proteins also induce voltage-independent inhibition of CaV2 channels through intracellular signaling pathways [1][19][40]. This often involves the Gq family of G proteins, which regulate the levels of phosphatidylinositide lipids by inducing hydrolysis of phosphatidylinositol bisphosphate via activation of phospholipase C enzymes [41]. Acetylcholine release from rat sympathetic neurons is reduced through this pathway via presynaptic muscarinic receptors activation [42].
2 channels through intracellular signaling pathways [1,19,40]. This often involves the Gq family of G proteins, which regulate the levels of phosphatidylinositide lipids by inducing hydrolysis of phosphatidylinositol bisphosphate via activation of phospholipase C enzymes [41]. Acetylcholine release from rat sympathetic neurons is reduced through this pathway via presynaptic muscarinic receptors activation [42].3.2. Active Zone Proteins
Rab-interacting molecule (RIM), an AZ protein required for SVs docking and priming [43][44][45][46][47][48], and synaptic plasticity [49], interacts with the C-terminal cytoplasmic tails of Ca
Rab-interacting molecule (RIM), an AZ protein required for SVs docking and priming [43,44,45,46,47,48], and synaptic plasticity [49], interacts with the C-terminal cytoplasmic tails of CaV
2.1 and CaV2.2 channels [46][48][50][51] (
2.2 channels [46,48,50,51] (b). The interaction is essential for recruiting Ca2+
channels to the presynaptic AZ [46] and determines channel density and SVs docking at the presynaptic AZ [48]. RIM-binding proteins, RIM-BPs, also interact with CaV
2.1 and CaV
2.2 channels [51], and are selectively required for high-fidelity coupling of AP-induced Ca2+
influx to Ca2+
-stimulated SVs exocytosis [52]. The tripartite complex of RIM, RIM-BPs, and C-terminal tails of the CaV
2 channels regulate the recruitment of CaV
2 channels to AZs. Interaction of RIM with CaV
β subunits shifts the voltage dependence of inactivation to more positive membrane potentials, increasing Ca2+
channel activity [53]. In contrast, CaV
β subunits interaction with CAST/ERC2 shifts the voltage dependence of activation to more negative membrane potentials [54]. Positive regulation of presynaptic Ca2+
channel activity by RIM and CAST/ERC2, in addition to their function in SVs docking, increase the release probability of SVs docked close to CaV
2 channels. Furthermore, Munc13, required for SVs priming, controls CaV
2 channels shortly after AP firing to guarantee transmitter release for continuous neural activity [55].3.3. t-SNAREs and Synaptotagmin-1
SV (v)-SNARE synaptobrevin 2 and presynaptic plasma membrane (t)-SNAREs syntaxin-1 and SNAP-25 are required for fusion of SVs with a plasma membrane to release neurotransmitters [56]. Both CaV
2.1 and CaV2.2 channels at the presynaptic nerve terminals colocalize densely with syntaxin-1A [57][58][59], and also form a complex of with SNARE proteins [60][61][62] dependent on Ca
2.2 channels at the presynaptic nerve terminals colocalize densely with syntaxin-1A [57,58,59], and also form a complex of with SNARE proteins [60,61,62] dependent on Ca2+
with maximal binding at 20 μM and reduced binding at lower or higher concentrations of Ca2+
[63]. The t-SNARE proteins syntaxin-1A and SNAP-25, but not the v-SNARE synaptobrevin, bind to the intracellular loop between domains II and III of the α1
subunit of CaV
2.2 (amino acid residues 718-963) named as the synprint site (b) [64,65]. CaV2.1 channels have an analogous synprint site, and different channel isoforms have distinct interactions with syntaxin and SNAP-25 [66][67], suggesting specialized regulatory properties for synaptic modulation.
2.1 channels have an analogous synprint site, and different channel isoforms have distinct interactions with syntaxin and SNAP-25 [66,67], suggesting specialized regulatory properties for synaptic modulation. t-SNAREs interacting with presynaptic CaV
2.1 and CaV2.2 channels regulate channel activity (Figure 3a). Syntaxin-1A or SNAP-25 shifts the voltage dependence of inactivation toward more negative membrane potentials and reduces the availability of the channels to open [68][69][70]. Coexpression of SNAP-25 can reverse the inhibitory effects of syntaxin-1A [69][71]. The transmembrane region of syntaxin-1A and only a short segment within the H3 helix are critical for channel modulation [72], whereas the synprint site binds to the entire H3 helix in the cytoplasmic domain of syntaxin-1A [63][64][72]. Deletion of the synprint site weakened the modulation of the channels by syntaxin-1A, but did not abolish it, arguing that the synprint site acts as an anchor in facilitating channel modulation but is not required absolutely for modulatory action.
2.2 channels regulate channel activity (Figure 3a). Syntaxin-1A or SNAP-25 shifts the voltage dependence of inactivation toward more negative membrane potentials and reduces the availability of the channels to open [68,69,70]. Coexpression of SNAP-25 can reverse the inhibitory effects of syntaxin-1A [69,71]. The transmembrane region of syntaxin-1A and only a short segment within the H3 helix are critical for channel modulation [72], whereas the synprint site binds to the entire H3 helix in the cytoplasmic domain of syntaxin-1A [63,64,72]. Deletion of the synprint site weakened the modulation of the channels by syntaxin-1A, but did not abolish it, arguing that the synprint site acts as an anchor in facilitating channel modulation but is not required absolutely for modulatory action. Dependent on Ca2+
concentration, syntaxin-1 interacts with either the synprint site or synaptotagmin-1; at low Ca2+
concentrations, syntaxin-1 binds synprint, while at higher concentrations (>30 μM) it associates with synaptotagmin-1 [63]. Synaptotagmin-1, -2, and -9 serve as the Ca2+ sensors for the fast, synchronous neurotransmitter release [56][73][74]. The Ca
sensors for the fast, synchronous neurotransmitter release [56,73,74]. The Ca2+
binding site C2B domain of synaptotagmin-1 interacts with the synprint sites of both CaV
2.1 and CaV
2.2 channels (b) [75]. Synaptotagmin-1 can relieve the inhibitory effects of SNAP-25 on CaV2.1 channels [70][76]. Relief of Ca
2.1 channels [70,76]. Relief of Ca2+
channel inhibition by the formation of the synaptotagmin/SNARE complex favors Ca2+
influx. This is a potential mechanism to increase the release probability of SVs docked close to CaV
2 channels [4]. Interaction of syntaxin-1A and SNAP-25 with the synprint site is controlled by phosphorylation of the synprint site with protein kinase C (PKC) (b) [65] and Ca2+
/calmodulin-dependent protein kinase II (CaMKII) [77]. The negative shift of steady-state inactivation of CaV2.2 channels caused by syntaxin is blocked by PKC phosphorylation [65][71]. Thus, phosphorylation of the synprint site may serve as a biochemical switch controlling the SNARE-synprint interaction.
2.2 channels caused by syntaxin is blocked by PKC phosphorylation [65,71]. Thus, phosphorylation of the synprint site may serve as a biochemical switch controlling the SNARE-synprint interaction.3.4. Ca
2+
-Sensor Proteins
Ca
2+
elevation regulates Ca
V2.1 channels activity by its binding to CaM [8][78][79][80][81] and related neuron-specific Ca
2.1 channels activity by its binding to CaM [8,78,79,80,81] and related neuron-specific Ca
2+-binding proteins, CaBP1, VILIP-2 [82][83][84], and NCS-1 (frequenin) [85]. The presynaptic Ca
-binding proteins, CaBP1, VILIP-2 [82,83,84], and NCS-1 (frequenin) [85]. The presynaptic Ca
V
2.1 channel proteins consist of a pore-forming α
1
subunit associated with β, and possibly α
2
δ subunits (
a) [86]. The intracellular C terminus of the α1 subunit [81] called the IQ-like motif, which begins with the sequence isoleucine-methionine (IM) instead of isoleucine-glutamine (IQ), and the nearby downstream CaM-binding domain (CBD) are the interacting sites with these Ca
2+
-binding proteins (
b). Displacement with alanine in the IQ-like domain inhibited Ca
2+
-dependent Ca
V2.1 channels facilitation [78][81], whereas deletion of CBD inhibited Ca
2.1 channels facilitation [78,81], whereas deletion of CBD inhibited Ca
2+
-dependent Ca
V2.1 channels inactivation [79][80][81][83][84]. Ca
2.1 channels inactivation [79,80,81,83,84]. Ca
2+
/CaM-dependent inactivation of Ca
V
2.1 channels, dependent on global elevations of Ca
2+
, is observed in transfected cells overexpressing Ca
V2.1 channels [78][79][80] and in the nerve terminals of the calyx of Held [87][88] where Ca
2.1 channels [78,79,80] and in the nerve terminals of the calyx of Held [87,88] where Ca
V
2.1 channels are densely localized. In contrast, the large neuronal cell bodies of Purkinje neurons [89] or SCG neurons [90] rarely show Ca
2+
-dependent Ca
V
2.1 channels inactivation.
4. Synchronous Neurotransmitter Release Regulated by Ca
2+
Channel/SNARE Proteins Complex
Synprint peptides derived from Ca
V
2.2 channels reduced transmitter release from the microinjected presynaptic SCG neurons in culture, due to competitive uncoupling of the endogenous Ca
2+ channel-SNARE proteins interaction in nerve terminals [91]. Synprint peptides selectively inhibited fast synchronous synaptic transmission, while they increased late asynchronous release (
channel-SNARE proteins interaction in nerve terminals [99]. Synprint peptides selectively inhibited fast synchronous synaptic transmission, while they increased late asynchronous release (
Figure 2b). Similarly, synprint peptides reduced transmitter release from embryonic
3b). Similarly, synprint peptides reduced transmitter release from embryonic
Xenopus spinal neurons [92]. Increasing the external Ca
spinal neurons [100]. Increasing the external Ca
2+
concentration effectively rescued this inhibition, implying that synprint peptides competitively displaces docked SVs away from Ca
2+
channels, and this effect can be overcome by increasing Ca
2+ influx into presynaptic terminals [92].
influx into presynaptic terminals [100].
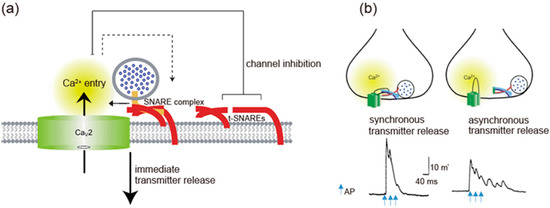
Figure 23.
a
2+
b
Figure 1b). Adapted from [91].
At the calyx of Held, presynaptic neurons express P/Q-, N- and R-type Ca
2+
currents in postnatal day 7 rats. P/Q-type Ca
2+
currents are more effective than N-type Ca
2+
currents and R-type Ca
2+ currents in eliciting neurotransmitter release [93][94][95]. The high efficiency of P/Q-type Ca
currents in eliciting neurotransmitter release [101,102,103]. The high efficiency of P/Q-type Ca
2+
currents to initiate neurotransmitter release is correlated with the close localization of Ca
V2.1 channels near docked SVs [96], as shown by immunocytochemistry [97], suggesting localization of Ca
2.1 channels near docked SVs [104], as shown by immunocytochemistry [105], suggesting localization of Ca
V
2 channels determines the efficiency of neurotransmitter release in response to neural activity.
Ca
V
2 channels interaction with SNARE proteins, that is dependent on Ca
2+
concentration [63], have two opposing effects: at the pre-firing state synaptic transmission is blocked by enhancing Ca
V
2 channels inactivation, whereas immediately after AP firing tethering SVs near the point of Ca
2+
entry enhances synaptic transmission. The overexpression of a syntaxin mutant that is unable to regulate Ca
V2.2 channels, but still binds to them [72], increased the efficiency of synaptic transmission at Xenopus neuromuscular junctions, as reflected in increased quantal content [98]. In contrast, injected synprint peptides reduced the basal efficiency of synaptic transmission, as reflected in reduced quantal content of synaptic transmission [98]. These results demonstrate a bidirectional regulation of synaptic transmission in vivo by interactions of Ca
2.2 channels, but still binds to them [72], increased the efficiency of synaptic transmission at Xenopus neuromuscular junctions, as reflected in increased quantal content [106]. In contrast, injected synprint peptides reduced the basal efficiency of synaptic transmission, as reflected in reduced quantal content of synaptic transmission [106]. These results demonstrate a bidirectional regulation of synaptic transmission in vivo by interactions of Ca
V
2.2 channels with SNARE proteins.
