Titin, also called connectin, is a giant sarcomere protein, which functions as a spring for muscle extension and elasticity. Titin interconnects the contraction of actin-containing thin filaments and myosin-containing thick filaments. Recently, the N-terminal fragment of titin, which is the breakdown product of titin, has become measurable using an enzyme-linked immunosorbent assay kit (27900 titin N-fragment Assay Kit; Immuno-Biological Laboratories, Fujioka, Japan). This kit has been used to evaluate muscle breakdown in muscle dystrophy, in which the level of urinary titin N-fragment was 700-times above the normal level.
- titin
- muscle
1. Titin
Titin, initially known as connectin, was discovered in 1976 by Maruyama et al. [1]. Being the largest protein in the human body, it was renamed titin after the giant god, Titan, from Greek mythology. Titin is the largest protein in humans, and is 3.0–3.7 MDa. This protein is located in the muscle sarcomere, and interconnects the contraction of actin-containing thin filaments and myosin-containing thick filaments. Passive tension and elasticity during muscle contraction develops as a result of the titin protein by Ca
-dependent stiffening (
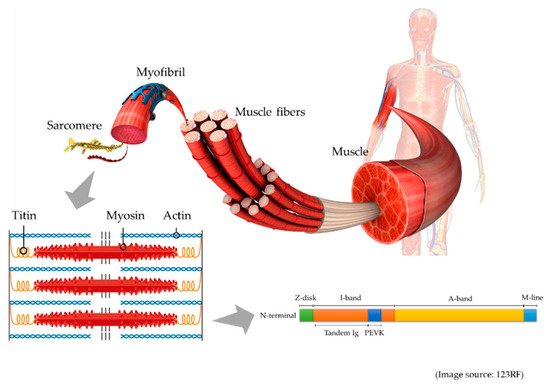
This is a schematic illustration of titin in the muscle. Muscle comprises of muscle fibers, myofibrils, and the smallest units, known as sarcomeres. In the sarcomere (lower left), titin connects actin-containing thin filaments and myosin-containing thick filaments. In the schematic illustration of titin structure (lower right), titin is composed of Z-disk, I-band, A-band, and M-line regions, and the I-band includes tandem Ig and PEVK (Pro-Glu-Val-Lys) domains.
During muscle degradation, titin is broken down into small fragments, and several different fragments are measurable. In serum, the metalloproteinase (MMP) 2-cleaved titin fragment and MMP 12-cleaved titin fragment are measurable. MMP 2-cleaved titin reflected muscle atrophy in a human bed rest study [4]. On the other hand, MMP 12-cleaved titin fragment could be used to assess cardiac infarction, because the level of the fragment increased after an acute myocardial infarction [5].
Recently, the N-terminal fragment of titin, which is 25 kDa, and is known as the urinary titin N-fragment, has become measurable in urine. Unlike serum, this urinary biomarker is noninvasive, but requires correction by urinary creatinine to adjust for the kidney function. The standard value of urinary titin N-fragment was 2.1 (1.2–2.6) pmol/mg Cr in healthy adult volunteers [6].
2. Muscle Atrophy
2.1. Limb and Trunk Muscle Atrophy
Muscle atrophy is a serious problem in critically ill patients [7]. After one week of ICU admission, critically ill patients exhibited 13.2–16.9% of muscle atrophy in the upper limbs, and 18.8–20.7% in the lower limbs [8]. These muscle atrophies are associated with impaired physical function and mortality [9][10].
Muscle atrophy is caused by an increase in protein degradation or decrease in protein synthesis. Increased protein degradation occurs mainly due to inflammation and immobilization. Calpain, caspase, ubiquitin-proteasome, and autophagy-lysosome have been implicated in this protein degradation pathway [11][12][13]. Decreased protein synthesis is caused by suppressed insulin-like growth factor-1 and inactivated myogenesis [14].
In critically ill patients, inflammation is an important cause of muscle atrophy [15], and various inflammatory cytokines are associated with muscle atrophy [16]. Immobilization is frequently observed during critical illness, and causes muscle atrophy [17]. Malnutrition causes a decrease in protein synthesis [18]. In critically ill patients, recommended protein intake is 1.2–2.0 g/kg/day [19], but this level of intake is often not achieved in the ICU [20], resulting in decreased protein synthesis.
Muscle mass can be assessed using computed tomography, bioelectrical impedance analysis, and ultrasound [21]. Computed tomography is accurate, but exposes patients to radiation, whereas bioelectrical impedance analysis is influenced by fluid changes in critically ill patients [22]. Ultrasound can be used to monitor muscle atrophy at the bedside, but it requires a skilled and experienced operator [23][24]. Thus, a biomarker to assess muscle atrophy is urgently needed. In a rat study, Udaka et al. found that six weeks of immobilization caused titin loss in the soleus muscle, which was associated with muscle dysfunction [25]. Thus, it is theoretically reasonable to expect levels of urinary titin to be elevated in the urine of patients with muscle atrophy.
In muscular dystrophy, the urinary titin N-fragment reflects the disease severity. Patients with Duchenne muscular dystrophy had a higher concentration of the urinary titin N-fragment than those with Becker muscular dystrophy (965.8 vs. 171.2 pmol/mg Cr,
< 0.01) [26]. This result possibly reflects the amount of muscle breakdown in these conditions. Recently, two studies have reported the usefulness of the urinary titin N-fragment in the evaluation of muscle atrophy in critically ill patients. Furthermore, Nakano et al. reported that urinary titin N-fragment could be used to evaluate muscle atrophy in critically ill patients [27]. In their study, investigating four critically ill patients, there was a negative correlation between mean urinary titin level during the first seven days of ICU admission and femoral muscle volume measured using computed tomography (
= −0.729). Furthermore, Nakanishi et al. reported that in 56 nonsurgical critically ill patients, the cumulative urinary titin concentration on days 3, 5, and 7 was significantly higher in the prominent muscle atrophy group (
≤ 0.03), suggesting that urinary titin reflects muscle atrophy in nonsurgical critically ill patients [28]. However, in their study, the correlation between muscle atrophy and urinary titin was limited to
= 0.29–0.54 (
≤ 0.03), suggesting that urinary titin levels are affected by various physiologic conditions. As inflammation is an important cause of muscle atrophy, the peak urinary titin N-fragment level was higher in patients with sepsis (93.0 vs. 57.9 pmol/mg Cr,
= 0.02). Moreover, the high levels of urinary titin N-fragment were associated with increased mortality. Although further studies are required, it is clear that a relationship exists between muscle atrophy and urinary titin N-fragment.
The molecular mechanism underlying titin breakdown remains unclear. Several pathways, activated by inflammation and immobilization, are involved in the breakdown of titin. Calpain contributes to the cleavage of titin because titin has calpain-binding sites [29]. Lang et al. investigated the ubiquitination of titin in denervated mouse, and found that levels of ubiquitinated titin gradually increased in denervation-induced muscle atrophy [30]. Consistent with this finding, Swist et al. found increased levels of ubiquitinated titin in patients with critical illness [31]. In their study, the markers of the autophagy-lysosome pathway were also upregulated. Thus, the autophagy-lysosome pathway may be involved in the breakdown of titin.
Unlike other promising biomarkers of muscle atrophy, urinary titin N-fragment is noninvasive and reliable. Creatinine kinase and BUN/Cr are possible biomarkers for muscle atrophy [32], but these biomarkers require blood tests. Furthermore, creatinine kinase is derived from various tissues [33], and BUN/Cr is influenced by various conditions including dehydration [32]. Urinary creatinine has also been suggested to be a biomarker of muscle atrophy, but it does not consider kidney function [34]. Thus, urinary titin N-fragment, corrected by urinary creatinine, is a reliable biomarker because it does not depend on kidney function [28][35].
2.2. Diaphragm Muscle Atrophy
Diaphragm atrophy is observed in 60% of mechanically ventilated critically ill patients [36], and it is a serious problem because of its association with prolonged mechanical ventilation and prolonged ICU stay [37]. As with limb muscle atrophy, diaphragm atrophy is caused by the calpain, caspase, ubiquitin-proteasome, and autophagy-lysosome pathways [38][39][40][41]. As reported in limb muscles, inflammation and immobilization are important causes of diaphragm atrophy. Sepsis is a cause of diaphragm atrophy [42], and deep sedation causes immobilization of the diaphragm [43]. Most importantly, ventilator settings strongly influence diaphragm atrophy and subsequent diaphragm dysfunction. Thus, diaphragm dysfunction in such cases is termed as ventilator induced diaphragm dysfunction [44].
Titin plays an important role in the diaphragm contractile force [45], and titin loss has been associated with diaphragm dysfunction in rats [46][47]. In the diaphragm biopsy of human subjects, Hussain et al. found that prolonged controlled mechanical ventilation decreased titin levels and impaired the diaphragm myofibrillar force [48]. Furthermore, Lindqvist et al. suggested that the positive-end expiratory pressure (PEEP) during ventilation led to the breakdown of the diaphragm titin, because the PEEP stretched out the sarcomere of the diaphragm muscle fibers [49]. Excessive extension may be detrimental to diaphragm titin.
Although titin is associated with diaphragm atrophy and dysfunction, urinary titin N-fragment is not useful for detecting diaphragm atrophy. Our previous study investigated the change of diaphragm thickness in 50 critically ill patients using ultrasound. Diaphragm atrophy, defined by a >10% decrease of diaphragm thickness, was observed in 32 patients (64%), and the mean diaphragm thickness decreased by 4.9% ± 15.8%, 8.0% ± 16.9%, and 15.4% ± 10.2% on days 3, 5, and 7, respectively. On comparing the diaphragm atrophy and unchanged groups, the levels of urinary titin N-fragment were not higher in the diaphragm atrophy group than those in the unchanged group (147.9 vs. 192.4 pmol/mg in the unchanged vs. atrophy group,
= 0.33) [28]. The urinary titin N-fragment can measure the titin breakdown product in all muscles, and is not specific to the diaphragm. To quantify the diaphragm atrophy, a diaphragm-specific titin kit is necessary. This may be theoretically possible, because a cardiac-specific titin kit has been developed in another study [50].
Interestingly, several studies have reported that, as well as diaphragm atrophy, increased diaphragm thickness has worsened clinical outcomes [36][37][51]. Insufficient ventilatory support leads to excessive respiratory effort in mechanically ventilated patients. This condition increases the diaphragm thickness. Since the increased muscle thickness has worsened outcomes, the hypertrophied diaphragm may not have sufficient functional titin to function appropriately. In a previous study on urinary titin N-fragment, there was no significant difference in the cumulative urinary titin N-fragment between the unchanged diaphragm thickness and increased diaphragm thickness groups (147.9 (79.0–257.8) vs. 426.1 (140.8–578.2) pmol/mg Cr in unchanged vs. increased,
= 0.45) [28]. The combination of increased diaphragm thickness and atrophy also did not have a significant difference in terms of the cumulative level of urinary titin N-fragment (147.9 (79.0–257.8) vs. 206.5 (99.3–440.8) pmol/mg Cr in unchanged vs. combination,
= 0.31). The mechanism underlying the increase in diaphragm thickness remains to be elucidated.
Diaphragm dysfunction is preventable and reversible. Therefore, it is important to maintain spontaneous breathing and avoid excessive ventilatory support during mechanical ventilation, which is called diaphragm protective ventilation [37]. Diaphragm protective ventilation can prevent diaphragm atrophy, compared with lung protective ventilation [52]. Furthermore, O’ Rourke et al. reported that percutaneous electrical phrenic nerve stimulation increased diaphragm thickness by 15.1% within 48 h [53]. Extracorporeal support is also considered to prevent diaphragm injury [54], and in a case report, the early initiation of extracorporeal support prevented diaphragm atrophy, with a relatively suppressed level of urinary titin N-fragment of 24.1–38.4 pmol/mg Cr [55].
2.3. Other Respiratory Muscle Atrophy
In addition to the diaphragm muscle, intercostal muscle atrophy is also observed in patients with mechanical ventilation [56], and is associated with prolonged mechanical ventilation and prolonged ICU stay [36]. Moreover, muscle atrophy occurs in other expiratory muscles, including the obliquus interna, obliquus externa, transversus abdominis, and rectus abdominis muscles [57]. In the case report of a mechanically ventilated patient, intercostal muscle biopsy showed the loss of myosin-containing thick filaments, with the possible detachment of titin [58]. Titin loss may be an important cause of other respiratory muscle dysfunctions, as well as that of the diaphragm. Jonkman et al. reported that breath-synchronized electrical stimulation increased the thickness of abdominal expiratory muscles (1.76 mm vs. −0.50 mm in intervention vs. control, respectively,
= 0.02) [59]. Thus, titin loss may be reversible by active rehabilitation.
References
- Maruyama, K.; Natori, R.; Nonomura, Y. New elastic protein from muscle. Nat. Cell Biol. 1976, 262, 58–60.
- Linke, W.A. Titin Gene and Protein Functions in Passive and Active Muscle. Annu. Rev. Physiol. 2018, 80, 389–411.
- Maciejewska-Skrendo, A.; Leźnicka, K.; Leońska-Duniec, A.; Wilk, M.; Filip, A.; Cięszczyk, P.; Sawczuk, M. Genetics of Muscle Stiffness, Muscle Elasticity and Explosive Strength. J. Hum. Kinet. 2020, 74, 143–159.
- Sun, S.; Henriksen, K.; Karsdal, M.A.; Armbrecht, G.; Belavy, D.L.; Felsenberg, D.; Rittweger, J.; Wang, Y.; Zheng, Q.; Nedergaard, A. Measurement of a MMP-2 degraded Titin fragment in serum reflects changes in muscle turnover induced by atrophy. Exp. Gerontol. 2014, 58, 83–89.
- Vassiliadis, E.; Rasmussen, L.M.; Byrjalsen, I.; Larsen, D.V.; Chaturvedi, R.; Hosbond, S.; Saabye, L.; Diederichsen, A.; Genovese, F.; Duffin, K.L.; et al. Clinical evaluation of a matrix metalloproteinase-12 cleaved fragment of titin as a cardiovascular serological biomarker. J. Transl. Med. 2012, 10, 140.
- Maruyama, N.; Asai, T.; Abe, C.; Inada, A.; Kawauchi, T.; Miyashita, K.; Maeda, M.; Matsuo, M.; Nabeshima, Y.-I. Establishment of a highly sensitive sandwich ELISA for the N-terminal fragment of titin in urine. Sci. Rep. 2016, 6, 39375.
- Nakanishi, N.; Takashima, T.; Oto, J. Muscle atrophy in critically ill patients: A review of its cause, evaluation, and prevention. J. Med. Investig. 2020, 67, 1–10.
- Nakanishi, N.; Oto, J.; Tsutsumi, R.; Iuchi, M.; Onodera, M.; Nishimura, M. Upper and lower limb muscle atrophy in critically ill patients: An observational ultrasonography study. Intensiv. Care Med. 2018, 44, 263–264.
- Nakanishi, N.; Oto, J.; Tsutsumi, R.; Akimoto, Y.; Nakano, Y.; Nishimura, M. Upper limb muscle atrophy associated with in-hospital mortality and physical function impairments in mechanically ventilated critically ill adults: A two-center prospective observational study. J. Intensiv. Care 2020, 8, 1–9.
- Lee, Z.-Y.; Ong, S.P.; Ng, C.C.; Yap, C.S.L.; Engkasan, J.P.; Barakatun-Nisak, M.Y.; Heyland, D.K.; Hasan, M.S. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: A single-center prospective observational study. Clin. Nutr. 2020.
- Doyle, A.; Zhang, G.; Fattah, E.A.A.; Eissa, N.T.; Li, Y.-P. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2010, 25, 99–110.
- Schiaffino, S.; Hanzlíkovávěra, V. Studies on the effect of denervation in developing muscle. II. The lysosomal system. J. Ultrastruct. Res. 1972, 39, 1–14.
- Talbert, E.E.; Smuder, A.J.; Min, K.; Kwon, O.S.; Powers, S.K. Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J. Appl. Physiol. 2013, 114, 1482–1489.
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74.
- Langhans, C.; Weber-Carstens, S.; Schmidt, F.; Hamati, J.; Kny, M.; Zhu, X.; Wollersheim, T.; Koch, S.; Krebs, M.; Schulz, H.; et al. Inflammation-Induced Acute Phase Response in Skeletal Muscle and Critical Illness Myopathy. PLoS ONE 2014, 9, e92048.
- Files, D.C.; Sanchez, M.A.; Morris, P.E. A conceptual framework: The early and late phases of skeletal muscle dysfunction in the acute respiratory distress syndrome. Crit. Care 2015, 19, 1–10.
- Sibilla, A.; Nydahl, P.; Greco, N.; Mungo, G.; Ott, N.; Unger, I.; Rezek, S.; Gemperle, S.; Needham, D.M.; Kudchadkar, S.R. Mobilization of Mechanically Ventilated Patients in Switzerland. J. Intensiv. Care Med. 2020, 35, 55–62.
- Wykes, L.J.; Fiorotto, M.; Burrin, D.; Del Rosario, M.; Frazer, M.E.; Pond, W.G.; Jahoor, F. Chronic Low Protein Intake Reduces Tissue Protein Synthesis in a Pig Model of Protein Malnutrition. J. Nutr. 1996, 126, 1481–1488.
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79.
- Yatabe, T.; Egi, M.; Sakaguchi, M.; Ito, T.; Inagaki, N.; Kato, H.; Kaminohara, J.; Konishi, A.; Takahashi, M.; Tatsumi, H.; et al. Influence of Nutritional Management and Rehabilitation on Physical Outcome in Japanese Intensive Care Unit Patients: A Multicenter Observational Study. Ann. Nutr. Metab. 2019, 74, 35–43.
- Looijaard, W.G.P.M.; Molinger, J.; Weijs, P.J. Measuring and monitoring lean body mass in critical illness. Curr. Opin. Crit. Care 2018, 24, 241–247.
- Nakanishi, N.; Tsutsumi, R.; Okayama, Y.; Takashima, T.; Ueno, Y.; Itagaki, T.; Tsutsumi, Y.; Sakaue, H.; Oto, J. Monitoring of muscle mass in critically ill patients: Comparison of ultrasound and two bioelectrical impedance analysis devices. J. Intensiv. Care 2019, 7, 1–8.
- Palakshappa, J.A.; Bakhru, R.N. Bedside Ultrasonography Can and Should Be Used in the Intensive Care Unit to Evaluate Muscle Atrophy. Ann. Am. Thorac. Soc. 2019, 16, 1107–1111.
- Branea, O.-E.; Jugariu, A.R.; Budeanu, R.-G.; Copotoiu, S.M.; Copotoiu, M. Ultrasonography: New Insights in its Applicability to Explore Muscle Mass and Musculoskeletal Inflammation in Critically ill Patients. Acta Med. Marisiensis 2018, 64, 147–150.
- Udaka, J.; Ohmori, S.; Terui, T.; Ohtsuki, I.; Ishiwata, S.; Kurihara, S.; Fukuda, N. Disuse-induced Preferential Loss of the Giant Protein Titin Depresses Muscle Performance via Abnormal Sarcomeric Organization. J. Gen. Physiol. 2007, 131, 33–41.
- Rouillon, J.; Zocevic, A.; Léger, T.; Garcia, C.; Camadro, J.-M.; Udd, B.; Wong, B.; Servais, L.; Voit, T.; Svinartchouk, F. Proteomics profiling of urine reveals specific titin fragments as biomarkers of Duchenne muscular dystrophy. Neuromuscul. Disord. 2014, 24, 563–573.
- Nakano, H.; Matsubara, T.; Yamakawa, K.; Nakamura, K. Urine TITIN N-fragment as a novel biomarker for critical illness myopathy: A pilot study. Crit. Care 2020, 24, 1–3.
- Nakanishi, N.; Tsutsumi, R.; Hara, K.; Takashima, T.; Nakataki, E.; Itagaki, T.; Matsuo, M.; Oto, J.; Sakaue, H. Urinary Titin Is a Novel Biomarker for Muscle Atrophy in Nonsurgical Critically Ill Patients: A two-center, prospective observational study. Crit. Care Med. 2020, 48, 1327–1333.
- Raynaud, F.; Fernandez, É.; Coulis, G.; Aubry, L.; Vignon, X.; Bleimling, N.; Gautel, M.; Benyamin, Y.; Ouali, A. Calpain 1-titin interactions concentrate calpain 1 in the Z-band edges and in the N2-line region within the skeletal myofibril. FEBS J. 2005, 272, 2578–2590.
- Lang, F.; Aravamudhan, S.; Nolte, H.; Türk, C.; Hölper, S.; Müller, S.; Günther, S.; Blaauw, B.; Braun, T.; Krüger, M. Dynamic changes in the mouse skeletal muscle proteome during denervation-induced atrophy. Dis. Model. Mech. 2017, 10, 881–896.
- Swist, S.; Unger, A.; Li, Y.; Vöge, A.; Von Frieling-Salewsky, M.; Skärlén, Å.; Cacciani, N.; Braun, T.; Larsson, L.; Linke, W.A. Maintenance of sarcomeric integrity in adult muscle cells crucially depends on Z-disc anchored titin. Nat. Commun. 2020, 11, 1–18.
- Haines, R.W.; Zolfaghari, P.; Wan, Y.; Pearse, R.M.; Puthucheary, Z.; Prowle, J.R. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensiv. Care Med. 2019, 45, 1718–1731.
- Moghadam-Kia, S.; Oddis, C.V.; Aggarwal, R. Approach to asymptomatic creatine kinase elevation. Clevel. Clin. J. Med. 2016, 83, 37–42.
- Volbeda, M.; Hessels, L.; Posma, R.; Bakker, S.; Nijsten, M.W.N. Time courses of urinary creatinine excretion, measured creatinine clearance and estimated glomerular filtration rate over 30 days of ICU admission. J. Crit. Care 2020.
- Nakano, H.; Hashimoto, H.; Mochizuki, M.; Naraba, H.; Takahashi, Y.; Sonoo, T.; Matsubara, T.; Yamakawa, K.; Nakamura, K. Urine Titin N-fragment as a Biomarker of Muscle Injury for Critical Illness Myopathy. Am. J. Respir. Crit. Care Med. 2020.
- Nakanishi, N.; Oto, J.; Ueno, Y.; Nakataki, E.; Itagaki, T.; Nishimura, M. Change in diaphragm and intercostal muscle thickness in mechanically ventilated patients: A prospective observational ultrasonography study. J. Intensiv. Care 2019, 7, 1–10.
- Goligher, E.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Vorona, S.; Sklar, M.C.; Rittayamai, N.; Lanys, A.; et al. Mechanical Ventilation–induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes. Am. J. Respir. Crit. Care Med. 2018, 197, 204–213.
- Smuder, A.J.; Sollanek, K.J.; Nelson, W.B.; Min, K.; Talbert, E.E.; Kavazis, A.N.; Hudson, M.B.; Sandri, M.; Szeto, H.H.; Powers, S.K. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic. Biol. Med. 2018, 115, 179–190.
- McClung, J.M.; Kavazis, A.N.; DeRuisseau, K.C.; Falk, D.J.; Deering, M.A.; Lee, Y.; Sugiura, T.; Powers, S.K. Caspase-3 Regulation of Diaphragm Myonuclear Domain during Mechanical Ventilation–induced Atrophy. Am. J. Respir. Crit. Care Med. 2007, 175, 150–159.
- Zhu, X.; Van Hees, H.W.H.; Heunks, L.; Wang, F.; Shao, L.; Huang, J.; Shi, L.; Ma, S. The role of calpains in ventilator-induced diaphragm atrophy. Intensiv. Care Med. Exp. 2017, 5, 1–11.
- Hooijman, P.E.; Beishuizen, A.; Witt, C.C.; De Waard, M.C.; Girbes, A.R.J.; Man, A.M.E.S.-D.; Niessen, H.W.M.; Manders, E.; Van Hees, H.W.H.; Brom, C.E.V.D.; et al. Diaphragm Muscle Fiber Weakness and Ubiquitin–Proteasome Activation in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2015, 191, 1126–1138.
- Hadda, V.; Kumar, R.; Tiwari, P.; Mittal, S.; Kalaivani, M.; Madan, K.; Mohan, A.; Guleria, R. Decline in diaphragm thickness and clinical outcomes among patients with sepsis. Hear. Lung 2021, 50, 284–291.
- Sklar, M.C.; Madotto, F.; Jonkman, A.; Rauseo, M.; Soliman, I.; Damiani, L.F.; Telias, I.; Dubo, S.; Chen, L.; Rittayamai, N.; et al. Duration of diaphragmatic inactivity after endotracheal intubation of critically ill patients. Crit. Care 2021, 25, 1–15.
- Peñuelas, Ó.; Keough, E.; López-Rodríguez, L.; Carriedo, D.; Gonçalves, G.; Barreiro, E.; Lorente, J.Á. Ventilator-induced diaphragm dysfunction: Translational mechanisms lead to therapeutical alternatives in the critically ill. Intensiv. Care Med. Exp. 2019, 7, 1–25.
- Van Der Pijl, R.J.; Granzier, H.L.; Ottenheijm, C. Diaphragm contractile weakness due to reduced mechanical loading: Role of titin. Am. J. Physiol. Cell Physiol. 2019, 317, C167–C176.
- Van Hees, H.; Ottenheijm, C.; Granzier, H.; Dekhuijzen, P.; Heunks, L. Heart failure decreases passive tension generation of rat diaphragm fibers. Int. J. Cardiol. 2010, 141, 275–283.
- Van Hees, H.W.H.; Schellekens, W.-J.M.; Acuña, G.L.A.; Linkels, M.; Hafmans, T.; Ottenheijm, C.A.C.; Granzier, H.L.; Scheffer, G.-J.; Van Der Hoeven, J.G.; Dekhuijzen, P.N.R.; et al. Titin and diaphragm dysfunction in mechanically ventilated rats. Intensiv. Care Med. 2012, 38, 702–709.
- Hussain, S.N.A.; Cornachione, A.S.; Guichon, C.; Al Khunaizi, A.; Leite, F.D.S.; Petrof, B.J.; Mofarrahi, M.; Moroz, N.; De Varennes, B.; Goldberg, P.; et al. Prolonged controlled mechanical ventilation in humans triggers myofibrillar contractile dysfunction and myofilament protein loss in the diaphragm. Thorax 2016, 71, 436–445.
- Lindqvist, J.; Berg, M.V.D.; Van Der Pijl, R.; Hooijman, P.E.; Beishuizen, A.; Elshof, J.; De Waard, M.; Girbes, A.; Man, A.S.-D.; Shi, Z.-H.; et al. Positive End-Expiratory Pressure Ventilation Induces Longitudinal Atrophy in Diaphragm Fibers. Am. J. Respir. Crit. Care Med. 2018, 198, 472–485.
- Bogomolovas, J.; Gasch, A.; Bajoras, V.; Karčiauskaitė, D.; Šerpytis, P.; Grabauskiene, V.; Labeit, D.; Labeit, S. Cardiac specific titin N2B exon is a novel sensitive serological marker for cardiac injury. Int. J. Cardiol. 2016, 212, 232–234.
- Goligher, E.C.; Fan, E.; Herridge, M.S.; Murray, A.; Vorona, S.; Brace, D.; Rittayamai, N.; Lanys, A.; Tomlinson, G.; Singh, J.M.; et al. Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort. Am. J. Respir. Crit. Care Med. 2015, 192, 1080–1088.
- Filyk, O. Prevention of respiratory muscle dysfunction due to diaphragm atrophy in children with respiratory failure. Eureka Health Sci. 2020, 6, 40–45.
- O’Rourke, J.; Soták, M.; Curley, G.F.; Doolan, A.; Henlín, T.; Mullins, G.; Tyll, T.; Omlie, W.; Ranieri, M.V. Initial Assessment of the Percutaneous Electrical Phrenic Nerve Stimulation System in Patients on Mechanical Ventilation. Crit. Care Med. 2020, 48, e362–e370.
- Spinelli, E.; Carlesso, E.; Mauri, T. Extracorporeal support to achieve lung-protective and diaphragm-protective ventilation. Curr. Opin. Crit. Care 2020, 26, 66–72.
- Nakanishi, N.; Okamoto, Y.; Okahisa, T.; Oto, J. Early Initiation of Awake Veno-Venous Extracorporeal Membrane Oxygenation Can Attenuate Muscle Atrophy and Weakness in Acute Respiratory Distress Syndrome. Cureus 2020, 12, e9926.
- Formenti, P.; Umbrello, M.; Dres, M.; Chiumello, D. Ultrasonographic assessment of parasternal intercostal muscles during mechanical ventilation. Ann. Intensiv. Care 2020, 10, 1–9.
- Ijland, M.M.; Lemson, J.; Van Der Hoeven, H.G.; Heunks, L. The impact of critical illness on the expiratory muscles and the diaphragm assessed by ultrasound in mechanical ventilated children. Ann. Intensiv. Care 2020, 10, 1–11.
- Lopate, G.; Pestronk, A.; Yee, W.-C. N lines in a myopathy with myosin loss. Muscle Nerve 1998, 21, 1216–1219.
- Jonkman, A.H.; Frenzel, T.; McCaughey, E.J.; McLachlan, A.J.; Boswell-Ruys, C.L.; Collins, D.W.; Gandevia, S.C.; Girbes, A.R.J.; Hoiting, O.; Kox, M.; et al. Breath-synchronized electrical stimulation of the expiratory muscles in mechanically ventilated patients: A randomized controlled feasibility study and pooled analysis. Crit. Care 2020, 24, 1–11.
