Pluripotent stem cells (PSC) such as embryonic stem cells (ESC) and induced PSCs (iPSC) are originated from embryos and induced from adult tissue cells, respectively. PSCs are capable of proliferating almost indefinitely, and differentiating into all somatic cells, through processes that mimic early embryogenesis. The resulting cells tend to carry embryonic characteristics.
- pluripotent stem cell
- cartilage
- regeneration
1. Introduction
Joint articular cartilage lacks spontaneous repair activity in adult humans and large animals [1][2][1,2]. In contrast to adult joint cartilage, embryonic/fetal joint cartilage possesses spontaneous scar-less repair activity for a chondral (partial-thickness) defect [3][72]. In small animals, such activity continues to the postnatal infant stage. Therefore, the embryonic and infant chondroprogenitors and chondrocytes are interesting cell-types to test for their capability of long-term hyaline cartilage repair in adult articular cartilage, but these cells are not easily obtained from humans in a clinical quantity. In contrast, pluripotent stem cells (PSC) such as embryonic stem cells (ESC) and induced PSCs (iPSC) are capable of differentiating into all somatic cells, through processes that mimic early embryogenesis, and the resulting cells tend to carry embryonic characteristics. PSCs can be expanded in culture almost indefinitely, too. Therefore, for humans, PSCs are the only practical source for obtaining large numbers of embryonic/fetal cell-types. Methods to generate embryonic chondrocytes as well as embryonic chondroprogenitors from mouse (m) and human (h)PSCs have been established by many groups, which have been reviewed previously [4][5][97,98]. We have also previously established and refined signaling requirements for the differentiation of PSCs to three embryonic precursors of chondrocytes, namely lateral plate mesoderm, paraxial mesoderm, and (cranial) neural crest [6][7][8][9][10][11][12][13][14][99,100,101,102,103,104,105,106,107]. Interestingly, we and others have also shown that hPSC-derived chondrogenic cells of the mesodermal origin gave rise to hyaline cartilage pellets in vitro [11][104], which were maintained to some extent as an unmineralized state in vivo, especially when BMP signaling was limited in a late stage of the in vitro chondrogenesis culture [13][15][16][106,108,109]. These observations suggest that PSC-derived chondrogenic mesodermal cells may contain progeny that are committed to generate permanent chondrocytes: i.e., chondrocytes that resist endochondral ossification, the process which stimulates chondrocyte hypertrophy, terminal maturation, and mineralization to form bone as in the growth plate.
The PSC-derived chondrogenic mesenchymal cells can be expanded in a serum-containing medium or in a specialized serum-free medium (e.g., FGF2 + TGF-β receptor inhibitor) [5][98]. When expanded under the serum-free condition, hPSC-derived chondroprogenitors well maintain their hyaline chondrogenic activity for over 15 passages [13][106], but chondrocytes developed from such expanded cells acquire tendency to commit themselves to the endochondral ossification process: i.e., cartilage pellets developed with them express signs of hypertrophic differentiation (e.g., transcripts of the type X collagen and alkaline phosphatase genes) in vitro and readily form a bony tissue in vivo, similar to adult MSC-derived cartilage pellets [17][18][19][70,110,111]. These observation suggest that chondroprogenitors, generated in culture from mesodermal progeny of hPSCs and expanded in a way to maintain long-term their hyaline chondrogenic activity, somehow lose their capacity to form permanent chondrocytes.
2. Development and Isolation of Chondrogenic Cells from Pluripotent Stem Cells
There is a report that full-thickness defects of sheep articular cartilage were successfully repaired by providing undifferentiated sheep ES-like cells in a fibrin glue [20][112]. However, PSCs are tumorigenic, i.e., teratoma-forming cells, and the teratoma-forming activity has been the definition of pluripotency for hPSCs [21][22][23][113,114,115]. Therefore, lineage-restricted progenitor cells differentiated from PSCs are considered more suitable for therapeutic purposes than PSC themselves, but risk of contamination of tumor forming, undifferentiated PSCs in the differentiated PSC population remains [24][25][116,117]. In fact, when hPSCs, especially hiPSCs, are differentiated into chondrocytes or chondroprogenitors that are used without a step to purify them or their precursors by physical methods: e.g., FACS and magnetic-activated cell sorting (MACS), or by biological methods: e.g., expanding specifically the differentiated cell-type of interest in culture, immature teratoma-like tumor is developed in cartilage mass generated from them in vitro [26][118], and in an immunodeficient mouse knee after transplantation of them for 16 weeks [27][119]. Therefore, the safest way to regenerate cartilage using hPSCs is to include a step in the protocol to physically or biologically eliminate the tumor forming, undifferentiated PSCs, prior to transplantation.
In early studies, biological methods: e.g., selective expansion culture, were mainly employed for enriching or purifying chondrogenic mesenchymal cells or MSCs [5][98]. PSCs were differentiated by way of forming embryoid bodies (EB) in vitro. Then, mesenchymal cells growing out of EBs, called EB outgrowth cells, were selectively expanded in media similar to those developed for expanding bone marrow MSCs, prior to induction of chondrogenesis and use for cartilage repair analyses [5][98].
Recent studies tend to make use of antibody-based physical separation methods to enrich PSC-derived chondroprogenitor cells or their precursors such as PSC-derived mesoderm or neural crest (Figure 2). For example, FACS-isolated VEGF receptor 2 (FLK1/KDR)− platelet-derived growth factor receptor alpha (PDGFRα)+ EB cells are chondrogenic mesodermal progeny of PSCs [6][7][11][12][99,100,104,105]. The hPSC-derived mesodermal progeny, enriched by FACS-isolation of KDR−CD146+CD166+ BMP receptor 1B (BMPR1B)−/lo cells, or by MACS-depletion of contaminated epithelial endodermal, cardiovascular, and hematoendothelial mesodermal cells, as well as undifferentiated hPSCs, are chondrogenic [28][29][120,121]. Furthermore, FACS-isolated green fluorescence protein (GFP)+ cells from the type II collagen gene (Col2a1) promoter-GFP knocked-in PSCs are enriched in chondrogenic progeny [30][31][122,123], and FACS/MACS-purified CD271+ hPSC-derived neural crest cells generate chondrogenic ectomesenchymal cells [13][32][33][106,124,125].
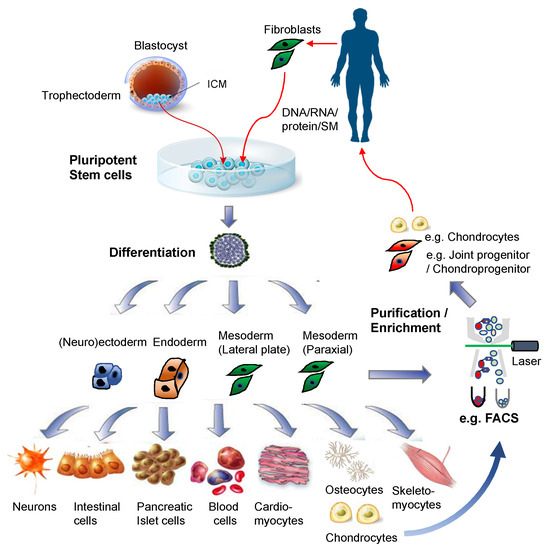
Figure 2. Pluripotent stem cells for regenerative medicine. Illustrations for the blastocyst, pluripotent stem cells, and differentiated cells were purchased from Dreamstime.com.
As for hPSC-derived MSCs, surface markers such as CD73, CD24, CD105, and CD90 have been used for detecting and isolating them by FACS, as reviewed in [5][98]. However, since MSCs can be relatively easily generated via spontaneous differentiation of hPSCs, and enriched by expansion culture in the standard, serum-containing MSC medium, FACS/MACS is not widely employed for purifying or enriching PSC-derived MSCs. However, the developmental process of mesodermal MSCs from hPSCs was first defined by Slukvin’s group using FACS isolation of mesodermal progeny [34][126]. Their method generates Apelin receptor+ mesoderm (that is PDGFRα+KDR+ and Lin- [VE-cadherin-CD31−CD73−CD43−CD45−], and expresses T, MIXL1, and FOXF1: i.e., primitive streak and lateral plate mesoderm transcripts) from hPSCs, isolates them by MACS, and subjects them to mesenchymal colony forming culture to generate PDGFRβ+ CD271+Delta-like1(DLK1)+CD73− primitive mesenchymal cells (expressing PRRX1: i.e., limb bud mesenchyme transcript). Then, PDGFRβ+CD73+CD90+ MSCs are generated from them in the presence of FGF2 in a serum-free medium [35][127].
3. Cartilage Tissue Engineering Using Pluripotent Stem Cell-Derived Chondroprogenitors
Use of PSC-derived chondrogenic cells for articular cartilage repair has not been extensively performed. Many early studies employed methods to generate chondrogenic mesenchymal cells or MSCs (e.g., EB outgrowth cells) from spontaneously differentiated PSCs, expand and prime them, and then use them for repairing damaged articular cartilage. Hwang et al. [36][128] and Toh et al. [37][38][129,130] have convincingly demonstrated, using this strategy, that hESC-derived EB outgrowth cells are capable of repairing damaged articular cartilage at least up to 12 weeks, when the cells were either embedded in a hyaluronan-hydrogel followed by pre-differentiated toward chondrocytes for 4 weeks in the presence of BMP7 and TGF-β1 [37][38][129,130], or expanded in chondrocyte-conditioned medium, followed by pellet cultured for 3 days [36][128], prior to transplantation. Similarly, Gibson, et al. has demonstrated that use of MSCs, which had been generated by a 2-dimensional, spontaneous differentiation method of hESCs and pellet cultured with BMP2 for 2 days and then with WNT5a for 12 days, showed statistically significant improvements in the repair of damaged articular cartilage [39][131]. In contrast, EB outgrowth cell-derived MSCs that had been complexed with poly(lactic-co-glycolide) scaffold and transplanted to full-thickness defects of rabbit articular cartilage without any pre-treatments, such as chondrogenic differentiation or chondrocyte-conditioned medium treatment, showed only a weak repair [40][132]. Effects of various biomaterials have also been explored but mostly in vitro, which have been reviewed elsewhere [10][41][103,133].
More refined lineage-restricted (e.g., mesodermal) chondrogenic mesenchymal cells were also used for cartilage repair. Ferguson, et al. [28][120] identified cell surface markers that can be used for identifying and isolating chondrocytes from different locations in human fetal articular cartilage. The integrin alpha 4 (IGTA4)− BMPR1B+ chondrocytes that demonstrate the strongest matrix-depositing activity are form transitional zone, and the IGTA4+BMPR1B+ chondrocytes that show osteochondrogenic activity and PRG4 expression are from superficial zone. Interestingly, when mesodermal progeny of hPSCs generated based on the method of Wu, et al. [29][121] were purified by MACS-depletion of epithelial endodermal cells, cardiovascular and hematoendothelial mesodermal cells as well as undifferentiated hPSCs, and then differentiated by pellet culture for 60 days, the resulting cartilage pellets were enriched in IGTA4+BMPR1B- mesenchymal cells, with a minor population of IGTA4+BMPR1B+ superficial chondrocytes. These cartilage pellets were capable of repairing a focal lesion of rat articular cartilage in as soon as 30 days [28][120].
Similarly, Gardner, et al. [42][134] reported hPSC-derived mesodermal cartilage tissue also repair a focal osteochondral defects of articular cartilage in nude rats. They employed Craft et al.’s method of mesodermal differentiation of hPSCs [15][108], followed by EB outgrowth cell generation and expansion for 12 days in a serum-free medium to get chondrogenic mesenchymal cells. Then, these cells were subjected to TGF-β3-based micro-mass culture for 12–15 weeks to generate cartilage mass that was used to fill the osteochondral defects. The quantitative analyses of repair outcome based on the ICRSII scoring system showed statistically significant improvement 12 weeks, but not 6 weeks after transplantation of the hPSC-derived cartilage mass.
The first demonstration of significant cartilage repair by hPSC-derived chondrogenic progeny, without pre-differentiation or chondrocyte-condition medium treatment prior to transplantation, was reported by Cheng et al. [43][135]. Their method gives rise to SOX9+ chondroprogenitors and chondrocytes via mesodermal progeny of hESCs, based on Oldershaw et al.’s 2-dimmensional hESC differentiation method [44][136] that has been improved to bring the SOX9+ cell population up from 75 to 95%, by removing the day-12 obligated split during chondrogenesis stage of differentiation culture. These SOX9+ cells encapsulated in fibrin glue resulted in better repair outcome than spontaneous repair of a focal osteochondral defect of articular cartilage in nude rats from 4 to 12 weeks [43][135].
More direct roles of PSC-derived chondroprogenitors or chondrocytes on repairing a damage of articular cartilage were demonstrated by organ culture systems. Diekman, et al. [31][123] showed that Col2a1-GFP+ cells isolated from differentiating mPSCs by FACS and embedded in 1% agarose were capable of regenerating cartilage matrices within a chondral defect introduced in pig explant cartilage in 21 days of culture. In addition, Wu, et al. [29][121] demonstrated that FACS-purified CD166−/lo BMPR1B+ prechondrocytic cells, which had been generated by a 12–15-day chondrogenesis culture of CD166+CD146+KDR−/loEpCAM-BMPR1B−/lo hPSC-derived mesodermal cells in the presence of TGF-β1 and Leukemia Inhibitory Factor, contributed to repair defects introduced into a human fetal hip joint explant in 14 days of culture. These observations suggest the capacity of PSC-derived chondroprogenitors or chondrocytes to retain in defects sites of articular cartilage and regenerate cartilage matrices.
(References would be added automatically after the entry is online)
