Osteoarthritis (OA) is one of the most common and disabling rheumatic diseases. It is mainly characterized by articular cartilage degradation, which is fundamental for correct joint function, lubrication, and resistance to mechanical loading. However, the whole joint is normally compromised. As a result of the articular cartilage alterations, typical OA symptoms such as pain and joint failure appear, increasing population’s dependency.
OA aetiology is not fully understood. However, there are some risk factors such as certain genetic profiles, gender, age, exercise, metabolic alterations, obesity and diet habits, which could increase its prevalence. Notably, diet can play a crucial role in OA onset and evolution. Specifically, it is known that caffeine intake exerts a negative impact on articular cartilage. Overall, there is ample evidence indicating that caffeine intake has a negative impact on the physiology of articular cartilage, increasing consumers predisposition to suffer OA. Considering these results, caffeine consumption should be reduced and closely controlled. Specifically, this control should be compulsory to those people whose caffeine metabolism is reduced, such as children and pregnant women.
- osteoarthritis
- articular cartilage
- growth plate cartilage
- catabolism
- long bone growth inhibition
- growth retardation
- caffeine.
1. Osteoarthritis: Articular Cartilage Catabolism
Osteoarthritis (OA) is the most common and one of the most disabling rheumatic diseases[1][2][3][4][5][6][7][8] [1–8]. It is mainly characterized by a progressive cartilage destruction which leads to pain, joint failure and dependency[1][8][9] [1,8,9].
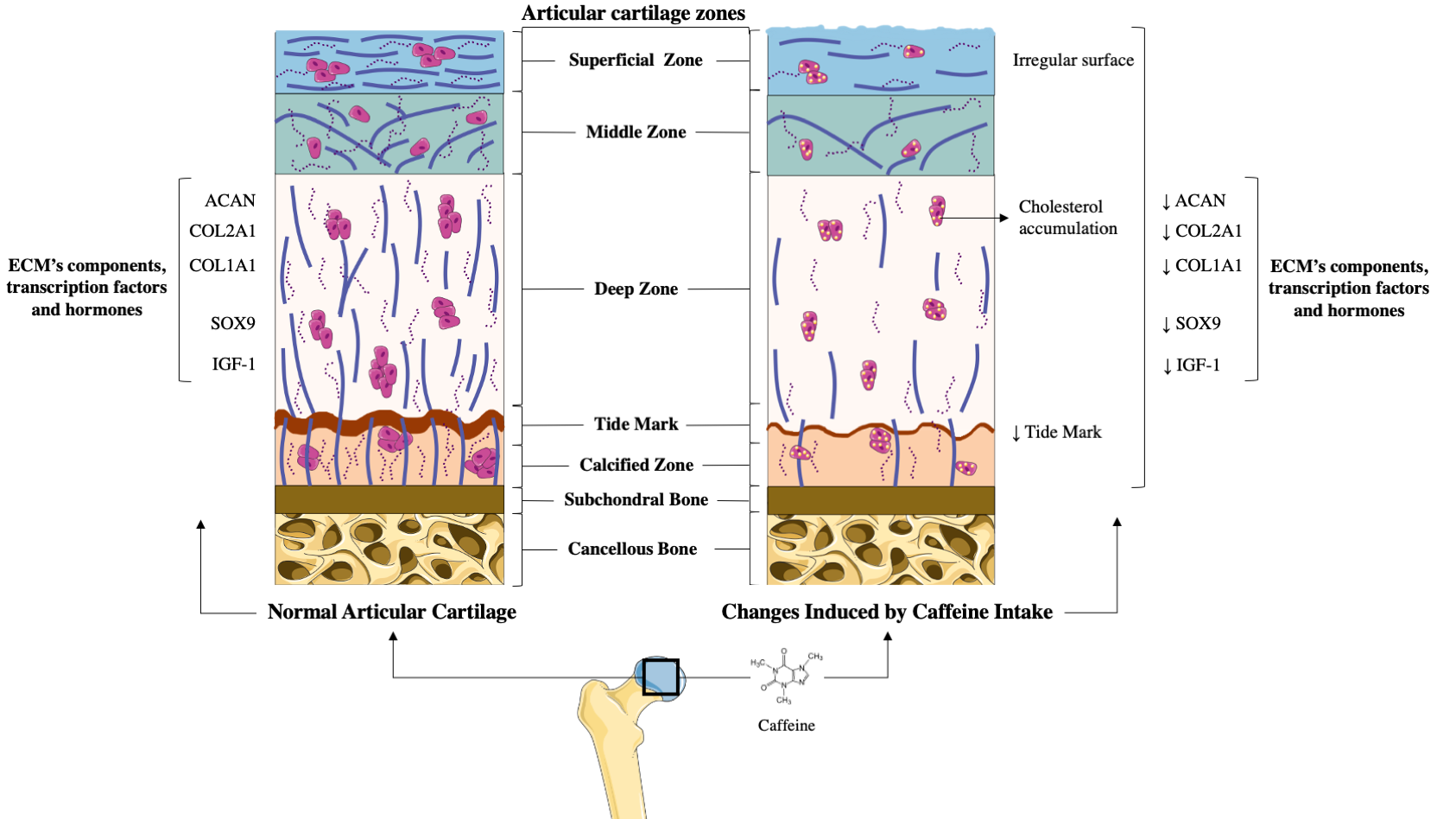
Articular cartilage is a specialized tissue that covers the surface of diarthrodial joints, providing them for a correct joint function, lubrication, and resistance to mechanical loading[5][6][7][8] [5–8]. This tissue is devoid of nerves, blood, and lympathic vessels, which hampers its capacity to be repaired[5][10] [5,10]. Articular cartilage is mainly composed by resting chondrocytes, and the extracellular matrix (ECM), which main components are water, collagens (Collagen type I alpha 1 (COL1A1), Collagen type II alpha 1 (COL2A1), etc.), and proteoglycans (agreccan (ACAN))[5][6][10] [5,6,10]. This composition is essential for the maintenance of its unique properties and is crucial for guarantying chondrocyte’s survival[5][6][10] [5,6,10] (Figure 1).
Figure 1. Comparison between healthy articular cartilage and the changes induced on it by caffeine. (A) Normal articular cartilage appearance. Articular cartilage is composed of chondrocytes and its extracellular matrix (ECM) components chondrocytes are crucial for the maintenance and repair of the ECM. They respond to a variety of stimuli, such as cytokines, mechanical loading, and growth factors. Among these, insulin growth factor 1 (IGF-1) and Transforming Growth Factor Beta 1 (TGF-ß1) are involved in cartilage homeostasis and chondrocyte responses to mechanical loading. Likewise, in healthy conditions, the articular cartilage expresses high levels of ECM components to guarantee the chondrocyte’s viability and proliferation. Additionally, a wide and remarkably tidemark is observed, as well as a regular surface that provides the ideal biomechanical properties to the joint. (B) The articular cartilage changes induced by caffeine intake. Caffeine consumption has been associated with several alterations in articular cartilage, similar to those that appear in osteoarthritis (OA). This alkaloid reduces the synthesis of major cartilage ECM components. It also diminishes chondrocyte proliferation, decreases the tidemark, and is associated with an irregular surface of the superficial zone of the cartilage. Additionally, caffeine is linked to lower chondrocyte quality due to cholesterol accumulation.
Although the aetiology of OA is not fully understood, it is known that OA chondrocyte phenotypic changes affect ECM composition and promote cartilage degradation[8][9] [8,9]. Among these changes, the acquisition of a hypertrophic-like phenotype stands out[8][9] [8,9]. Under physiological conditions, articular chondrocytes remain in a resting state and do not reach the terminal differentiation[8][9] [8,9]. However, OA chondrocytes express hypertrophic markers, such as collagen type X (COLX), matrix metalloproteinase 13 (MMP13), osteopontin (SPP1, also known as OPN), osteocalcin (OCN), osteonectin (SPARC), Runt-related transcription factor 2 (Runx2), Vascular Endothelial Growth Factor (VEGF), and alkaline phosphatase (ALP)[8] [8]. Moreover, OA chondrocytes also present lower expression levels of the SRY-Box Transcription factor 9 (Sox9)[8] [8]. As a result, all these changes contribute to ECM degradation.
There are several risk factors for OA development, such as certain genetic profiles, gender, aging, ethnicity, exercise, metabolic alterations, alcohol consumption, obesity, coffee intake, and diet[2][3][4][11] [2–4,11]. Notably, multiple modifiable risk factors are closely related to dietary habits, which suggests that diet could play a key role in OA pathogeny[2][3][4][11] [2–4,11]. Related to this, the consumption of caffeinated beverages like coffee has been associated with knee OA development, highlighting the negative role of caffeine in the pathophysiology of articular cartilage[11][12][13][14][15][16][17][18][19]. [11,12–15,16,17–19].
2. Prenatal Caffeine Exposure
Prenatal caffeine exposure
The potential effects of caffeine on the articular cartilage were well demonstrated in rat animal models[12][13][14][15][16][17] [12–15,16,17]. In these experiments, prenatal caffeine exposure (PCE) below the clinical dose of intoxication and in the range of some pregnant women[12][13][14][15][16][17][18][19] [12–15,16,17–19], significantly affected foetal articular cartilage integrity[12][13][14][15][16][17] [12–15,16,17]. Specifically, histological studies revealed that rat offspring with PCE possess irregular surface cartilage with uneven and altered chondrocytes in the tangential zone[15] [15]. The articular cartilage of these rats also exhibited an irregular and reduced ECM. Interestingly, the tidemark of this articular cartilage was absent, which suggests an altered mineralization process of the hypertrophic chondrocytes[15] [15] (Figure 1). According to all the alterations observed in the histological studies, the Mankin’s score (a scale used for the classification of OA cartilage lesions severity) of the cartilage from the PCE rats was found to be higher than that of their wild type littermates[15] [15]. Underpinning these studies, a molecular analysis of PCE articular cartilage revealed the reduced expression of key components of the ECM, such as COL2A1 and the levels of ACAN and COL1A1[12][14][15][17] [12,14,15,17]. Likewise, it was also reported that the cartilage of PCE rats exhibited a lower expression of several proteins of the IGF-1 signalling pathway, which suggests reduced anabolic metabolism in this tissue (Figure 1). Interestingly, the deleterious effects elicited by PCE on the rats’ articular cartilage were still present in adulthood[12][13] [12,13].
OA development dramatically affects the chondrocyte phenotype, function, and metabolism, in part reducing the expression of the transcription factor Sox9[8] [8], which is essential for the preservation of the chondrocyte phenotype and its function[9][20][21] [9,20,21]. In this regard, it is noteworthy that caffeine intake in pregnant rats reduced Sox9 expression in the articular chondrocytes of their offspring[17] [17], which is consistent with the reduced vitality and altered differentiation of these cells[17] [17]. This, in turn, involves low-quality articular cartilage [8], which might increase OA susceptibility in adulthood.
Several studies have suggested a relationship between cholesterol accumulation, metabolic syndrome, and the risk of OA[22][23] [22,23]. Consistent with this, PCE induced low-quality articular cartilage in foetal rats, which was associated with local and systemically altered cholesterol metabolism[15] [15].
Together with these effects, PCE also reduced the IGF-1 signalling pathway in rat foetal cartilage, maintaining its detrimental effects into adulthood[15] [15]. Accordingly, it has been suggested that PCE could support the existence of a subset of OA with a foetal origin[15] [15].
3. Direct and Indirect Effect of Caffeine on Articular Chondrocytes
Direct and Indirect Effect of Caffeine on Articular Chondrocytes
In vitro experiments have revealed that caffeine also exerts direct actions on chondrocyte’s primary cultures[12][24][25] [12,24,25]. Caffeine stimulation in a dose-dependent manner was shown to reduce rat chondrocyte proliferation and viability[24] [24]. Similarly, caffeine also reduced the mRNA expression of key ECM components (COL2A1 and ACAN) in these cells[12][24] [12,24]. It also reduced the mRNA expression of several IGF-1 signalling pathway members that participate in chondrocytes’ anabolic responses[12][26] [12,26] (Figure 1).
References
- T.W. O’Neill; Paul McCabe; John McBeth; Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Practice & Research Clinical Rheumatology 2018, 32, 312-326, 10.1016/j.berh.2018.10.007.
- Rodolfo Gómez; Amanda Villalvilla; Raquel Largo; Oreste Gualillo; Gabriel Herrero-Beaumont; TLR4 signalling in osteoarthritis—finding targets for candidate DMOADs. Nature Reviews Rheumatology 2014, 11, 159-170, 10.1038/nrrheum.2014.209.
- Anna Litwic; Mark H. Edwards; Elaine M. Dennison; C. Cooper; Epidemiology and burden of osteoarthritis.. British Medical Bulletin 2013, 105, 185-99, 10.1093/bmb/lds038.
- Amanda Villalvilla; Jame's A. Da Silva; Raquel Largo; Oreste Gualillo; Paulo Cezar Vieira; Gabriel Herrero-Beaumont; Rodolfo Gómez; 6-Shogaol inhibits chondrocytes’ innate immune responses and cathepsin-K activity. Molecular Nutrition & Food Research 2013, 58, 256-266, 10.1002/mnfr.201200833.
- X. Edward Guo; Helen H. Lu; Morakot Likhitpanichkul; Van C. Mow; The Role of Biomechanics in Functional Tissue Engineering for Articular Cartilage. Frontiers in Biomedical Engineering 2003, null, 37-60, 10.1007/978-1-4419-8967-3_3.
- Maryam Rahmati; Giovanna Nalesso; Ali Mobasheri; Masoud Mozafari; Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Research Reviews 2017, 40, 20-30, 10.1016/j.arr.2017.07.004.
- T Pracke; K Mikes; [Multiple calcifications of the articular cartilages].. Acta chirurgiae orthopaedicae et traumatologiae Cechoslovaca 1959, 26, , null.
- Van der Kraan, P.M.; van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232.
- Rita Dreier; Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders.. Arthritis Research & Therapy 2010, 12, 216-216, 10.1186/ar3117.
- J A Buckwalter; H J Mankin; Articular cartilage: tissue design and chondrocyte-matrix interactions.. Instructional course lectures 1998, 47, , null.
- Bang, C.H.; Kim, C.; Kim, J.H.; Choi, S.J.; Song, G.G.; Jung, J.H. Is knee osteoarthritis related to coffee drinking? A nationwide cross-sectional observational study. Clin. Rheumatol. 2019, 38, 817–825.
- Yang Tan; Kaihang Lu; Jing Li; Qubo Ni; Zhe Zhao; Jacques Magdalou; Liaobin Chen; Liao-Bin Chen; Prenatal caffeine exprosure increases adult female offspring rat’s susceptibility to osteoarthritis via low-functional programming of cartilage IGF-1 with histone acetylation. Toxicology Letters 2018, 295, 229-236, 10.1016/j.toxlet.2018.06.1221.
- Yangfan Shangguan; Hongqiang Jiang; Zhengqi Pan; Hao Xiao; Yang Tan; Kai Tie; Jun Qin; Yu Deng; Liaobin Chen; Liao-Bin Chen; Glucocorticoid mediates prenatal caffeine exposure-induced endochondral ossification retardation and its molecular mechanism in female fetal rats. Cell Death & Disease 2017, 8, e3157-e3157, 10.1038/cddis.2017.546.
- Yang Tan; Jin Liu; Yu Deng; Hong Cao; Dan Xu; Fenglong Cu; Youying Lei; Jacques Magdalou; Min Wu; Liao-Bin Chen; Liao-Bin Chen; Caffeine-induced fetal rat over-exposure to maternal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation. Toxicology Letters 2012, 214, 279-287, 10.1016/j.toxlet.2012.09.007.
- Hanwen Luo; Jing Li; Hong Cao; Yang Tan; Jacques Magdalou; Liaobin Chen; Liao-Bin Chen; Prenatal caffeine exposure induces a poor quality of articular cartilage in male adult offspring rats via cholesterol accumulation in cartilage. Scientific Reports 2015, 5, 17746, 10.1038/srep17746.
- L. M. Barone; M. S. Tassinari; R. Bortell; T. A. Owen; J. Zerogian; K. Gagne; G. S. Stein; Jane B. Lian; Inhibition of induced endochondral bone development in caffeine-treated rats. Journal of Cellular Biochemistry 1993, 52, 171-182, 10.1002/jcb.240520209.
- Amanda Maria Sena Reis; Karina Pessoa Oliveira; Isabela Helena Fagundes De Paula; Alisson Paulo Da Silva; Júlia Fahrion Tarragô; Natália De Melo Ocarino; R. Serakides; Nonlinear effects of caffeine on the viability, synthesis and gene expression of chondrocytes from the offspring of rats treated during pregnancy. Acta Histochemica 2018, 120, 505-512, 10.1016/j.acthis.2018.06.001.
- Ana Maria Baptista Oliveira Dias Malva Vaz; Caffeine consumption during pregnancy and the underweight newborn. Revista INFAD de Psicología. International Journal of Developmental and Educational Psychology. 2016, 1, 53, 10.17060/ijodaep.2015.n1.v1.22.
- CARE Study Group; Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ 2008, 337, a2332-a2332, 10.1136/bmj.a2332.
- Eleanor Mackie; Yasser Ahmed; Liliana Tatarczuch; K.-S. Chen; M. Mirams; Endochondral ossification: How cartilage is converted into bone in the developing skeleton. The International Journal of Biochemistry & Cell Biology 2008, 40, 46-62, 10.1016/j.biocel.2007.06.009.
- Karl H. Plate; Georg Breier; Werner Risau; Molecular Mechanisms of Developmental and Tumor Angiogenesis. Brain Pathology 1994, 4, 207-218, 10.1111/j.1750-3639.1994.tb00835.x.
- Abdurhman S Al-Arfaj; Radiographic osteoarthritis and serum cholesterol.. Saudi Medical Journal 2003, 24, , null.
- Qi Zhuo; Wei Yang; Jiying Chen; Yan Wang; Metabolic syndrome meets osteoarthritis. Nature Reviews Rheumatology 2012, 8, 729-737, 10.1038/nrrheum.2012.135.
- Hyeonhae Choi; Yuri Choi; Jisook Kim; Jaeman Bae; Jaesook Roh; Longitudinal bone growth is impaired by direct involvement of caffeine with chondrocyte differentiation in the growth plate. Journal of Anatomy 2016, 230, 117-127, 10.1111/joa.12530.
- A.M. Tesch; M.H. Macdonald; C. Kollias-Baker; H.P. Benton; Endogenously produced adenosine regulates articular cartilage matrix homeostasis: enzymatic depletion of adenosine stimulates matrix degradation. Osteoarthritis and Cartilage 2004, 12, 349-359, 10.1016/j.joca.2004.01.002.
- Patil, A.S.; Sable, R.B.; Kothari, R.M. Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J. Cell. Physiol. 2012, 227, 1796–1804.