Zinc is an essential trace element, required for enzymatic, structural, and regulatory functions. As body reserves are scarce, an adequate zinc status relies on proper dietary supply and efficient homeostasis. Several biomarkers have been proposed that enable the detection of poor zinc status, but more sensitive and specific ones are needed to detect marginal deficiencies. The zinc content of commercial dry dog foods has great variability, with a more frequent non-compliance with the maximum authorized limit than with the nutritional requirement. The bioavailability of dietary zinc also plays a crucial role in ensuring an adequate zinc status.
- zinc
- dog
- nutrition
1. Introduction
Zinc has an acknowledged essential biological role for all living organisms. The importance of zinc for the metabolism of microorganisms was discovered in 1866 when Jules Raulin confirmed its essentiality for the growth of Aspergillus niger
[1]
. However, its essentiality for mammals was only recognized in 1934 for rats
[2]
, in 1961 for humans
[3]
, and in 1962 for dogs
[4]
. Under physiological conditions, the dogs’ access to zinc depends upon oral intake. To ensure an optimal zinc status and simultaneously meet dog requirements and legal limits for zinc content, zinc bioavailability additives in pet foods is of utmost importance. In this sense, recent studies have evaluated inorganic and organic sources of zinc at different levels of inclusion to enhance zinc bioavailability in dog foods
[5][6][7][8][9][10][11][12][13]
. The optimal zinc status of dogs promotes health, wellbeing, and increases lifespan.
Indeed, an adequate zinc status is required for the proper function of several systems. In dogs, the role of zinc in skin health has been well documented
[14][15][16]. Besides, clinical reports show that impairment of zinc status might be associated with disorders in other systems. However, to fully comprehend the mechanisms that link zinc to the clinical signs and laboratory findings reported in dogs, it is necessary to acknowledge zinc functions at cellular and molecular levels. As such studies are scarce in dogs, resorting to data extrapolation from other mammals is often required. Moreover, while heavily deficient zinc status often has clinical manifestations, more specific biomarkers are required to differentiate marginal deficiencies and optimal levels, which constitute an additional challenge.
. Besides, clinical reports show that impairment of zinc status might be associated with disorders in other systems. However, to fully comprehend the mechanisms that link zinc to the clinical signs and laboratory findings reported in dogs, it is necessary to acknowledge zinc functions at cellular and molecular levels. As such studies are scarce in dogs, resorting to data extrapolation from other mammals is often required. Moreover, while heavily deficient zinc status often has clinical manifestations, more specific biomarkers are required to differentiate marginal deficiencies and optimal levels, which constitute an additional challenge.
2. Zinc in Dog Foods
- Zinc in Dog Foods
2.1. Legal Limits and Authorized Sources of Zinc for Animal Feed Supplementation
Supplemental sources of zinc (additives) for use in animal feeds, including companion animals, require authorization in the European Union (EU). Moreover, when added, pet food producers have to comply with the legal limits concerning the total content of zinc in feeds described in Appendix 4(II) of Regulation 1831/2003/EU
[17]
. In addition to compliance with legal limits, pet food manufacturers are obliged to declare additives, including zinc, used and the concentration added in the product label as described in Regulation 2009/767/EU
[18]
.
The most recent regulation of the European Council (Regulation 2016/1095 of 6 July 2016) sets a maximum of 200 mg/kg as is of zinc for supplemented dog foods (≈227 mg/kg DM, assuming 12% of moisture), which represents a decrease of 20% compared to the previously authorized level, 250 mg/kg as is
[17]
. Although the previously authorized zinc level was considered safe for target species, the release of zinc into the environment was considered an unavoidable risk. Indeed, the accumulation of zinc in soils (mostly in acidic sandy soils) and the leaching from it to surface waters was pointed to as a risk for organisms that reside in soil and water
[19]
.
shows the authorized sources for animal feed, which include five inorganic and six organic sources.
Table 1.
Zinc sources authorized as additives for animal feed supplementation in the European Union.
|
Source |
Authorized Zinc Source |
Regulation |
Reference |
|
Inorganic |
Zinc acetate dihydrate |
2016/1095/EU |
[20] |
|
Zinc chloride anhydrous |
|||
|
Zinc oxide |
|||
|
Zinc sulfate monohydrate/heptahydrate |
|||
|
Zinc chloride hydroxide monohydrate |
|||
|
Organic |
Zinc chelate of amino acids hydrate |
||
|
Zinc chelate of glycine hydrate |
|||
|
Zinc chelate of protein hydrolysates |
|||
|
Zinc bislysinate |
2016/973/EU |
[21] |
|
|
Zinc chelate of hydroxy analog of methionine |
2010/335/EU |
[22] |
|
|
Zinc chelate of methionine sulfate |
2019/1125/EU |
[23] |
2.2. Zinc Content in Commercial Dog Foods
A complete compound food is, by definition, a single diet capable of ensuring the animal requirements for energy, macro, and micronutrients
[24]
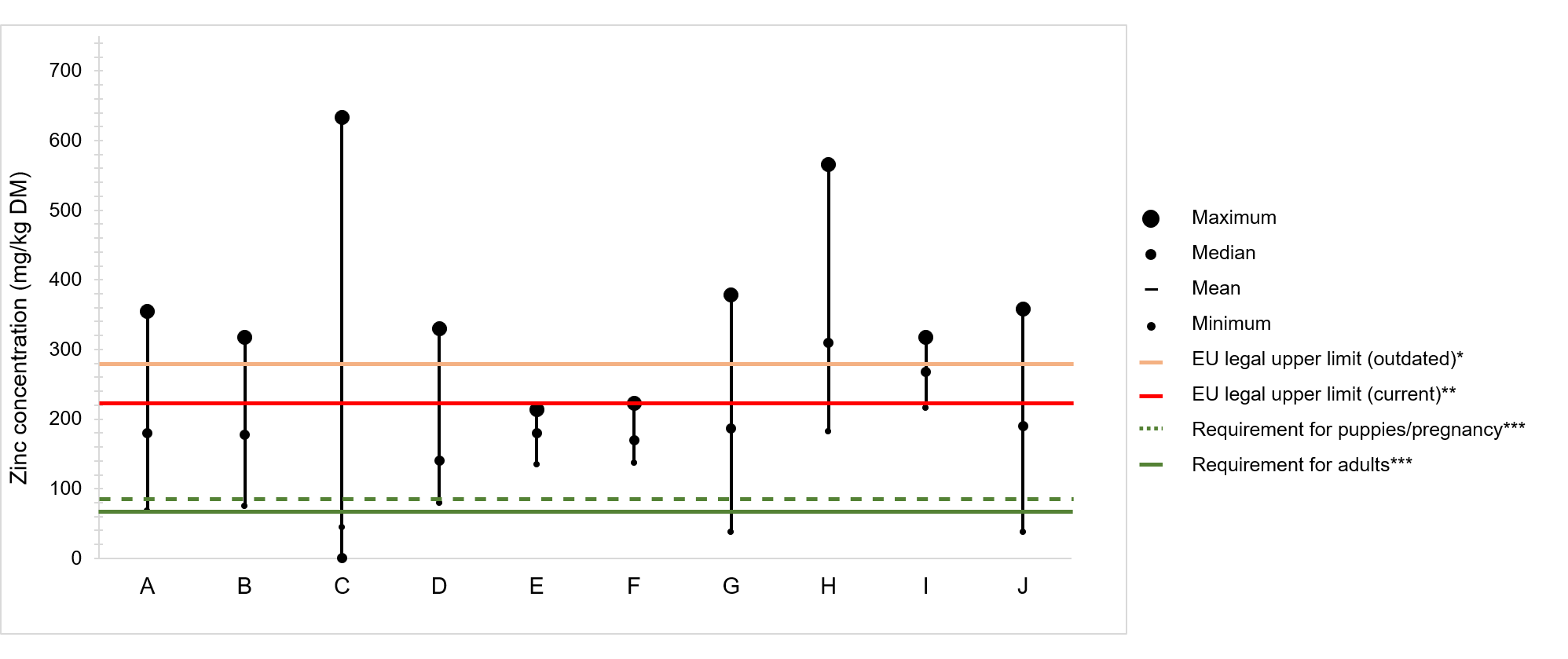
. Figure 1 displays a compilation of studies in which the zinc content of complete commercial foods for puppies and adult dogs has been determined. It should be noted the great variability between foods, and the more frequent non-compliance with the maximum authorized limit than with the nutritional requirement.
Figure 1.
Zinc concentration in commercial dog foods reported in several studies, classified according to the type of food, dry food (DF), wet food (WF); and the age category, puppy (P), adult (A), and senior (S), whenever the information was provided in the publications: A – n = 59, DF, P + A
[25]
; B – n = 26, DF, P
[26]
; C – n = 34; DF, P + A + S
[27]
; D – n = 18, DF
[28]
; E – n = 15, DF, P + A + S
[29]
; F – n = 49, WF, P + A + S
[29]
; G – n = 24, DF
[30]
; H – n = 20, DF, A
[31]
; I – n = 6, DF, P
[31]
; J – n = 119, DF, P + A
[32]
. * Maximum legal limit in the European Union (EU) Regulation No 636/2013; ** Maximum legal limit in the EU Regulation No 2016/1095; *** Zinc requirements published by FEDIAF
[24]. DM—dry matter.
. DM—dry matter.
The eight studies presented in
compile zinc determinations of 18 to 119 food samples both for puppies, adult, and senior dogs, mainly commercialized in the United States of America, Europe, and Latin America. Adult formulas were the most share of the samples analyzed, and the results were rarely presented according to age category. Five out of eight, report similar ranges of zinc content, from <100 to ≤378 mg/kg DM. In two studies, the lowest content of 38 mg/kg DM was found
, which corresponds to 50% of the minimum requirement for zinc in dog foods. In addition to dry foods, Davies et al. analyzed 49 wet samples from different brands and found that the content of zinc in wet and dry foods ranged from 137 to 223 and 145 to 214 mg/kg DM, respectively, meaning that both nutritional requirements and legal impositions were respected in all samples
[29]
. In contrast, Elias et al. reported the greatest variation of all studies, with values ranging from 44 to 633 mg/kg DM
[27]
. Similarly, zinc excess was reported by Pereira et al., with contents ranging from 248 to 317 mg/kg in puppy and 182 to 566 mg/kg in adult foods
[31]
. Moreover, the study reported that the zinc content was not affected by the market segment and that the percent of zinc labeled was on median 51.1% of the total content, although in some cases, it was as low as ≈ 20%.
New attitudes and practices of companion animal feeding have emerged in the past years, namely, vegan
[33]
and raw and homemade diets
[34]
; thus, zinc content in trendy dog foods has already been reported. Zafalon et al. observed that one of three commercial vegan dog foods analyzed had a content of zinc above the EU legal limits; however, none of the three were below the nutritional requirements, which is somewhat expected since all diets were supplemented with trace element additives
[35]
. In contrast, the zinc content of home-prepared diets was found below the nutritional requirements in 79% of 75 recipes of dog foods available on the internet
[36]
. Similarly, Dillitzer et al., who evaluated zinc content in bone and raw food rations, reported that more than half of the samples analyzed failed to supply zinc daily recommended allowance (range 27—400 mg/kg DM; median 76 mg/kg DM)
[37]
. In their study, the low zinc rations usually consisted of meat with only small amounts of bone and without either offal, zinc-containing supplements, or nuts bone
[37]
.
2.2.1 Variation of Zinc Content in Raw Ingredients
The total content of zinc in complete dry dog foods reflects the amount sourced by the raw ingredients (background level), and the amount that is added through supplementation (labeled). Although zinc content of ingredients is available in feed tables, several factors can influence the accuracy of those estimations, being advisable to routinely analyze the content of zinc in raw ingredients. However, that is still not current practice, probably because the analytical techniques require expertise and sophisticated equipment, which inevitably translates into high costs
[38]
.
Plants can be zinc accumulators or not, with tolerance or non-tolerance to high concentrations of zinc in soils
[39]
. The zinc content of plants depends on the soil characteristics and zinc concentration, and the presence of fertilizers
[40]
. The zinc uptake by plant roots is facilitated by acidic pH and high content of organic matter of soil, whereas higher phosphorus, iron, aluminum oxides, bicarbonate, clay, and alkaline pH decrease zinc solubility and mobility
[41]
. In general, plant roots secrete reductants, organic acids (acting as chelators), and H+ ions to enhance zinc solubility, and this element is mainly taken up from soil as Zn2+
[39]
. From the roots, zinc is transported through the epidermis, cortex, endodermis, and pericycle until it reaches the xylem to be mobilized to the shoots
. The distribution of zinc in plant tissues depends upon the plant species, maturity, and zinc status. If the level of zinc in plants is low to adequate, zinc is mainly found in growing tissues (roots, vegetative shoots, and reproductive tissues), whereas in plants that tolerate zinc at toxic levels, zinc is accumulated in cell walls or vacuoles in the root cortex or leaves
[42]
.
In animal by-products, a great variation in zinc content is often observed as zinc retention varies among tissues. For instance, Henry et al. reported that the content of zinc was 87, 94, 119, 126, and 146 mg/kg DM, respectively, in the heart, spleen, liver, kidney, and muscle of sheep fed diets non supplemented with zinc
[43]
. Also, the concentration of zinc in bone, heart, and muscle did not respond to an increase of zinc supplementation (700, 1400, and 2100 mg/kg) for 30 days, whereas the concentration in the spleen, kidney, and liver increased, having in kidney and liver been proportional to increment of zinc supplemented
[43]
. In goats, the zinc content of bone and muscle was 87 and 122 mg/kg DM, respectively
[44]
, and in broilers, the content of zinc in tibia ash, fresh breast muscle, and fresh liver was c.a. 360, 8.7, and 31 mg/kg, respectively
[45]
, when fed the requirement of zinc for each species. Thus, animal species, presence/proportion of offal/bone, and feeding regime of animals comprising the meat meals affect the zinc content of the raw ingredients from animal origin.
2.3. Bioavailability of Zinc Sources
The bioaccessibility of a nutrient comprises the fraction released from the food matrix into the intestinal lumen, ready to be absorbed. Bioavailability refers to the bioaccessible fraction that is absorbed through the intestinal mucosa and effectively reaches the bloodstream
[46]
. In addition to the inherent animal variables (e.g., preexisting tissue reserves, physiological state, disease, and genetics), the bioavailability is affected by the zinc source
[47]
, as it determines how the molecules interact with the conditions it encounters, e.g., the pH and presence of antagonistic compounds in the gastrointestinal compartment
[48]
.
The intestinal absorption of zinc molecules first requires a dissociation that, in the case of salts (inorganic sources), easily occurs in the acidic pH of the stomach. Then, the dissociated cation (Zn2+) may bond to amino acids from the chyme or to carrier proteins, embedded in the luminal membranes of the mucosal cells to be transported across the intestinal membrane
[49]
. However, the dissociated cation may also interact with food components, such as phytates, and eventually form insoluble complexes that lead to the excretion of zinc in feces
[13]
, or with other mineral elements, e.g., calcium, copper, and iron that can interfere with Zn2+ absorption and vice-versa
[50]
. Conversely, if elemental zinc is bond to an organic molecule (zinc chelate), it will not, in theory, dissociate so easily before intestinal absorption, being less prone to precipitate and form insoluble complexes with food components
[49]
. The absorption of intact zinc chelates might be carried out by zinc transporters, but also by peptide and amino acid transport mechanisms. According to an in vitro study performed with Caco-2 cells, zinc methionine regulates the mRNA expression of ZIP4, ZnT1, and PepT1, suggesting that this chelate is transported either by the traditional zinc ion channel or by the small peptide transport pathway (PepT1)
[51]
. The permeation of peptides is affected by the chain-length and sequence of the peptides, whereas PepT1 and the proton gradient (the driven force for PepT1 mediated transport) are affected by dietary and pharmacologic compounds (e.g., flavonoids, fatty acids)
[52]
. In that sense, the absorption of zinc chelates is likely influenced by organic molecules, as it might share their mechanism of uptake.
2.4. Supplementation Strategies to Enhance Zinc Bioavailability
The study of zinc bioavailability sources draws strategies of zinc supplementation in dog foods.
summarizes studies that evaluated sources of dietary zinc supplementation in dog foods. Overall, the results show differences between inorganic forms and have pointed benefits of organics over inorganics.
Table 2.
Studies of zinc source supplementation in dog foods.
|
Design/Duration/Zn Restriction 1 |
Subjects |
Zn Forms |
Level Zn 3 |
Biomarker of Zinc Status |
Reference |
|
2 wks length for 3 Zn sources; 3 wks washout between each Zn source/2 wks 2 |
6 male Beagles/ |
Zinc sulfate |
0 |
Fecal, plasma, and urinary [Zn], apparent fecal absorption |
[5] |
|
Zinc acetate |
2 mg/kg BW |
||||
|
Zinc oxide |
4 mg/kg BW |
||||
|
Randomized block design/35 days/ |
42 puppies/11 wks |
Zinc oxide |
40 4 |
Weight gain, plasma [Zn], declaws, teeth, and testes [Zn] |
[8] |
|
1 meal test for each Zn form with 1 wk between them/2 wks/ |
4 adult Beagles |
Zinc oxide |
50 |
Fecal, plasma and urinary [Zn], AUC |
[10] |
|
6 × 4 randomized block design/25 days per treatment/no restriction |
4 adult Beagles |
Zinc oxide 5 |
50 |
Fecal [Zn], hair growth rate, hair [Zn] |
[9] |
|
Randomized block design/20 days/30 days/ |
27 adult Beagles |
Zinc oxide |
50 |
Hair growth, hair [Zn], serum AP, AUC5 |
[7] |
|
Zinc amino acid |
75 |
||||
|
Zinc polysaccharide |
100 |
||||
|
Cross-over design/3 wks 2 wks/58.5 mg/kg DM |
4 female Beagles |
Zinc sulfate |
≈61.5 |
Hair growth, hair [Zn], plasma and fecal [Zn], serum ALT, zinc absorption |
[53] |
|
3 Latin Squares |
12 Beagles/ |
Zinc sulfate 7 |
75 |
Plasma, hair, and urinary [Zn], serum, ALT, SOD activity and CRP, CD4/CD8 ratio in peripheral blood, coat quality (brightness, softness, greasiness, and scale) and growth (trichogram), CTTAD, flatulence |
[13] |
|
Zinc proteinate 7 |
|||||
|
Randomized block design/28 d/50 mg/kg DM (7 days) |
30 Hound-cross Mongrel/ |
Zinc oxide |
50 8 |
Weight gain, hair weight and length, plasma and hair [Zn], liver, cortisol, bone, and total AP, concentrations, blood MT gene expression |
[12] |
|
Zinc methionine |
100 8 |
||||
|
Randomized block design/30 days/no restriction |
18 dogs many breeds/ |
Zinc oxide |
40 |
Coat quality (brightness, looseness, texture, greasiness), whole-blood and hair [Zn], antibody against sheep red blood cells |
[11] |
|
Randomized block design/reproduction (≈12 wks) 10/ |
34 female Beagles/> 1 year + newborn puppies |
Zinc oxide |
53 |
BW (dams) and weight gain (lactation from birth to 6 wks), litter size, hair [Zn], hair and root morphology by scanning electron microscopy |
[6] |
A comparison between three inorganic forms of zinc (zinc oxide, zinc acetate, and zinc sulfate) performed by Ozpinar et al., showed higher apparent absorption of zinc sulfate, although zinc concentration in blood, urine, and feces was not different among sources
[5]
.
Wedekind and Lowry evaluated the bioavailability of zinc oxide and zinc propionate under three conditions: (1) Recommended dietary level of calcium, (2) high calcium level, and (3) high calcium and fiber level, under the assumption that those conditions would, by that order, compromise zinc bioavailability
[8]
. Multiple regression slope-ratio analysis between zinc intake and plasma concentration of zinc revealed that zinc propionate was more bioavailable. Moreover, calcium reduced the bioavailability of both forms with less impact on zinc propionate. Retention of zinc in declaws, teeth, and testis was unaffected by the source of supplemental dietary zinc. However, the intake of zinc was different due to variation in zinc content of diets that averaged ≈ 46 mg/kg for zinc oxide and 76 mg/kg for zinc propionate, precluding a direct comparison of zinc bioavailability. Indeed, tissue retention may not be linear, and in that case, the low ratio of tissue concentration:intake of zinc propionate would indicate the worst bioavailability for this source.
Lowe et al. studied the absorption of zinc oxide and zinc amino acid chelate, concluding that the organic form was twice as bioavailable as the inorganic
[10]
. Zinc amino acid chelate (methionine and glycine) promoted higher hair retention and growth rate when compared to zinc oxide and zinc polysaccharide
[9]
. Moreover, the negative effect of dietary calcium was only noticed for zinc oxide, increasing its fecal excretion, and decreasing the quality of hair parameters.
According to Lowe and Wiseman , zinc sulfate is more bioavailable than zinc oxide but less available than zinc amino acid chelate (methionylglycinate)
[7]
, which also agrees with data of Jamikorn and Preedapattarapong
[53]
. Indeed, serum alkaline phosphatase activity, hair growth, zinc deposition, and absorption were higher for dogs fed diets supplemented with zinc amino acid chelate than with zinc sulfate
[7]
. It must be emphasized that the absorption of zinc reported in this study (37% and 29.8% for zinc amino acid chelate and zinc sulfate, respectively) was calculated from total fecal content without considering the endogenous losses.
Pereira et al. tested the supplementation of zinc sulfate and zinc proteinate at an adequate level in dog foods with high phytate content, with and without the concomitant addition of an enzymatic complex to degrade phytates, which are known to affect zinc bioavailability
[13]
. Results showed that zinc proteinate was associated with higher bioavailability of phosphorus and a higher percentage of circulating CD4+ T-cells suggesting an improved T-cell differentiation. However, no other biomarkers (e.g., plasma and hair zinc concentration, coat quality, serum SOD activity) responded to the zinc source
[13]
. These results are in line with another study in which weaning puppies were fed a control diet with a background level of zinc of 50 mg/kg and compared to four other dietary treatments consisting of a control diet supplemented with 50 or 100 mg/kg of zinc, either as zinc oxide or zinc methionine
[12]
. Although weight gain and plasma zinc concentration were lower in dogs fed the control diet (zinc 50 mg/kg) in comparison to the other four dietary treatments, only total alkaline phosphatase was affected by zinc source, being higher in dogs fed zinc methionine
[12]
. Both studies highlight the need to find more sensitive zinc biomarkers, that can differentiate the bioavailability of zinc sources within adequate zinc status.
Another aspect to be considered when studying mineral supplementation is the source of all supplemental mineral elements. Trevizan et al. compared the total replacement of essential inorganic trace elements (zinc, manganese, copper, and selenium) by an organic source consisting of a 2-hydroxy-4-(methylthio)butanoate (HMTBa) complexed with zinc, manganese, copper, and selenium and observed that despite blood zinc and zinc retention in hair were unaffected, the total replacement of inorganic elements improved immunity by preventing the decrease in antibodies against sheep red blood cells, and improved coat quality
[11]
. The benefits of replacement of inorganic trace elements by chelated also extend to reproductive performance. Female dogs fed chelated zinc, manganese, and copper during estrus, pregnancy, and lactation (up to the 6th week) had more pups than the ones fed inorganic zinc, manganese, and copper
[6]
. However, no differences were reported in bodyweight of both damns and puppies nor in the concentration of zinc in hair, although damns fed chelated trace elements had smoother and less fragmented hair follicles, only visible through scanning electron microscopy
[6]
.
The above-mentioned studies seem to point towards a positive effect of organic zinc regardless of the type of chelate (e.g., proteinate, amino acid) in comparison to inorganic, even though some reports are contradictory. Indeed, chelates behave differently in the gastrointestinal tract, as their stability depends upon several factors, e.g., pH, temperature, ionic strength, and characteristics of the ligand
[49]
. For instance, the stability of the bond between the amino acids and proteins is variable, and if only moderately strong, they might dissociate at pH values lower than 3 or higher than 9, being in those conditions as susceptible as inorganic minerals
[48]
. Additionally, the ligands are susceptible to different digestive processes, which conditioned the bioavailability of the chelate. For instance, peptide-chelates are susceptible to pepsin action, whereas amino acid chelates do not have peptide bonds to be targeted. This is relevant since some peptides are absorbed and are bioactive as is, but others require dissociation of peptide-zinc complexes to release the metal for absorption. In that case, it is not advantageous when a high molecular weight ligand with high-chelating zinc capacity, resists peptide digestion, and thus, prevents the absorption of zinc
[54]
.
It seems consensual that the effect of zinc source becomes more evident after a restriction period of dietary zinc, likely due to the alteration in zinc pools and status. If so, the differences in zinc status at the beginning of each of the supplementation studies might contribute to the contradictory results observed. Nevertheless, and as previously stressed, more sensitive biomarkers are required to precisely evaluate the zinc sources in dogs at different physiological stages, without the need to deplete zinc status.
The use of zinc nanoparticles (zinc oxide) for supplementation of animal feed has been documented in other species, with improved absorption and quality of animal products
. However, to the authors’ knowledge, the use of zinc nanoparticles has not yet been reported to improve zinc bioavailability in dog foods, being, therefore, a topic suggested for future research.
A fine supplementation strategy ensures the efficacy of products with benefits for dogs and improves the product at a technical level, optimizing the cost and complying with both nutritional requirements and legal impositions.
References
- Raulin, Jules. Études chimiques sur la végétation; Masson & cie: the University of California, 1905; pp. -.
- W. R. Todd; C. A. Elvehjem; E. B. Hart; ZINC IN THE NUTRITION OF THE RAT. American Journal of Physiology-Legacy Content 1933, 107, 146-156, 10.1152/ajplegacy.1933.107.1.146.
- Ananda S. Prasad; James A. Halsted; Manucher Nadimi; Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. The American Journal of Medicine 1961, 31, 532-546, 10.1016/0002-9343(61)90137-1.
- NRC. Nutrient Requirements of Dogs; The National Academies Press: Washington DC, WA, USA, 1962; pp. -.
- H. Ozpinar; I. Abas; T. Bilal; G. Demirel; Investigation of excretion and absorption of different zinc salts in puppies. Laboratory Animals 2001, 35, 282-287, 10.1258/0023677011911615.
- Gail Kuhlman; Ronald E. Rompala; The Influence of Dietary Sources of Zinc, Copper and Manganese on Canine Reproductive Performance and Hair Mineral Content. The Journal of Nutrition 1998, 128, 2603S-2605S, 10.1093/jn/128.12.2603s.
- John A Lowe; Julian Wiseman; A comparison of the bioavailability of three dietary zinc sources using four different physiologic parameters in dogs.. The Journal of Nutrition 1998, 128, 2809S-2811S, 10.1093/jn/128.12.2809s.
- Karen J. Wedekind; Stephen R. Lowry; Are organic zinc sources efficacious in puppies?. The Journal of Nutrition 1998, 128, 2593S-2595S, 10.1093/jn/128.12.2593s.
- John A. Lowe; Julian Wiseman; D. J. A. Cole; Zinc Source Influences Zinc Retention in Hair and Hair Growth in the Dog. The Journal of Nutrition 1994, 124, 2575S-2576S, 10.1093/jn/124.suppl_12.2575s.
- John A. Lowe; Julian Wiseman; D. J. A. Cole; Absorption and Retention of Zinc when Administered as an Amino-Acid Chelate in the Dog. The Journal of Nutrition 1994, 124, 2572S-2574S, 10.1093/jn/124.suppl_12.2572s.
- Trevizan, L.; Fischer, M.M.; Rodenbusch, C.R.; Labres, R.V.; Kessler, A. de Mello; Effects of diets containing organic and inorganic zinc sources on hair characteristics, zinc concentration in blood and hair, and the immune response of dogs. Acta Scientiae Veterinariae 2013, 41, 1-7.
- B.M. Vester; L.K. Karr-Lilienthal; D.J. Tomlinson; K.S. Swanson; G.C. Fahey; Indicators of Zinc Status of Weanling Puppies Are Affected by Zinc Dietary Concentration. The Professional Animal Scientist 2007, 23, 448-453, 10.15232/s1080-7446(15)31000-7.
- Ana Margarida Pereira; Margarida Guedes; Elisabete Matos; Edgar Pinto; Agostinho A. Almeida; Marcela A. Segundo; Alexandra Correia; Manuel Vilanova; António J. M. Fonseca; Ana Rita J. Cabrita; et al. Effect of Zinc Source and Exogenous Enzymes Supplementation on Zinc Status in Dogs Fed High Phytate Diets. Animals 2020, 10, 400, 10.3390/ani10030400.
- S.D. White; P. Bourdeau; R.A.W. Rosychuk; B. Cohen; T. Bonenberger; K.V. Fieseler; P. Ihrke; P.L. Chapman; P. Schultheiss; G. Zur; et al.A. CannonC. Outerbridge Zinc-responsive dermatosis in dogs: 41 cases and literature review. Veterinary Dermatology 2001, 12, 101-109, 10.1046/j.1365-3164.2001.00233.x.
- K.A. Marsh; F.L. Ruedisueli; S.L. Coe; T.G.D. Watson; Effects of zinc and linoleic acid supplementation on the skin and coat quality of dogs receiving a complete and balanced diet. Veterinary Dermatology 2000, 11, 277-284, 10.1046/j.1365-3164.2000.00202.x.
- Sarah Colombini; Canine zinc-responsive dermatosis.. Veterinary Clinics of North America: Small Animal Practice 1999, 29, 1373-1383, 10.1016/s0195-5616(99)50133-2.
- European Council. European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003 - Appendix 4(II). - released 05.08.2020.
- European Council. Regulation (EC) No 767/2009 of 13 July 2009 on the placing on the market and use of feed. 2009.
- EFSA. Scientific Opinion on the safety and efficacy of zinc compounds (E6) as feed additives for all animal species (zinc acetate, dihydrate; zinc chloride, anhydrous; zinc oxide; zinc sulphate, heptahydrate; zinc sulphate, monohydrate; zinc chelate of amino acids, hydrate; zinc chelate of glycine, hydrate), based on a dossier submitted by FEFANA asbl. EFSA Journal 2015, 13, 46.
- European Council. Regulation (EC) 2016/1095 of 6 July 2016 concerning the authorisation of Zinc acetate dihydrate, Zinc chloride anhydrous, Zinc oxide, Zinc sulphate heptahydrate, Zinc sulphate monohydrate, Zinc chelate of amino acids hydrate, Zinc chelate of protein hydrolysates, Zinc chelate of glycine hydrate (solid) and Zinc chelate of glycine hydrate (liquid) as feed additives for all animal species. 2016.
- European Council. Regulation (EC) 2016/973 of 17 June 2016 concerning the authorisation of zinc bislysinate as a feed additive for all animal species. 2016.
- European Council. Regulation (EC) No 335/2010 of 22 April 2010 concerning the authorisation of zinc chelate of hydroxy analogue of methionine as a feed additive for all animal species. 2010.
- European Council. Regulation (EC) 2019/1125 of 5 June 2019 concerning the authorisation of zinc chelate of methionine sulfate as a feed additive for all animal species 2019.
- FEDIAF. Nutritional Guidelines For Complete and Complementary Pet Food for Cats and Dogs; Bruxelles, Belgium, September 2020.
- D. AlOmar; S. Hodgkinson; D. Abarzua; R. Fuchslocher; C. Alvarado; E. Rosales; Nutritional evaluation of commercial dry dog foods by near infrared reflectance spectroscopy. Journal of Animal Physiology and Animal Nutrition 2006, 90, 223-229, 10.1111/j.1439-0396.2005.00585.x.
- C.A. Alvarado; S.M. Hodgkinson; D. AlOmar; D. Boroschek; Evaluation of the chemical composition of dry dogfoods commercialized in Chile used for growing dogs. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2008, 60, 218-226, 10.1590/s0102-09352008000100030.
- Camila Elias; Elisabete A. De Nadai Fernandes; Márcio Arruda Bacchi; Neutron activation analysis for assessing chemical composition of dry dog foods. Journal of Radioanalytical and Nuclear Chemistry 2011, 291, 245-250, 10.1007/s10967-011-1285-6.
- David G. Kelly; Steven D. White; Ron D. Weir; Elemental composition of dog foods using nitric acid and simulated gastric digestions. Food and Chemical Toxicology 2013, 55, 568-577, 10.1016/j.fct.2013.01.057.
- M. Davies; R. Alborough; L. Jones; C. Davis; C. Williams; D. S. Gardner; Mineral analysis of complete dog and cat foods in the UK and compliance with European guidelines. Scientific Reports 2017, 7, 1-9, 10.1038/s41598-017-17159-7.
- Silvânio Costa; Ana Pereira; Elisangela Passos; José Alves; Carlos Garcia; Rennan Araujo; Evaluation of the Chemical Composition of Dry Feeds for Dogs and Cats. Journal of the Brazilian Chemical Society 2018, 29, 2616-2625, 10.21577/0103-5053.20180142.
- Ana Margarida Pereira; Edgar Pinto; Elisabete Matos; Francisco Castanheira; Agostinho Almiro Almeida; Cláudia S. Baptista; Marcela A. Segundo; António Mira Da Fonseca; Ana R. J. Cabrita; Mineral Composition of Dry Dog Foods: Impact on Nutrition and Potential Toxicity. Journal of Agricultural and Food Chemistry 2018, 66, 7822-7830, 10.1021/acs.jafc.8b02552.
- Arianna Goi; Carmen L. Manuelian; Sarah Currò; Massimo De Marchi; Prediction of Mineral Composition in Commercial Extruded Dry Dog Food by Near-Infrared Reflectance Spectroscopy. Animals 2019, 9, 640, 10.3390/ani9090640.
- Sarah A. S. Dodd; Nick J. Cave; Jennifer L. Adolphe; Anna K. Shoveller; Adronie Verbrugghe; Plant-based (vegan) diets for pets: A survey of pet owner attitudes and feeding practices. PLOS ONE 2019, 14, e0210806, 10.1371/journal.pone.0210806.
- Giada Morelli; Sofia Bastianello; Paolo Catellani; Rebecca Ricci; Raw meat-based diets for dogs: survey of owners’ motivations, attitudes and practices. BMC Veterinary Research 2019, 15, 74, 10.1186/s12917-019-1824-x.
- Rafael Vessecchi Amorim Zafalon; Larissa Wünsche Risolia; Thiago Henrique Annibale Vendramini; Roberta Bueno Ayres Rodrigues; Vivian Pedrinelli; Fabio Alves Teixeira; Mariana Fragoso Rentas; Mariana Pamplona Perini; Isabella Corsato Alvarenga; Marcio Antonio Brunetto; et al. Nutritional inadequacies in commercial vegan foods for dogs and cats.. PLOS ONE 2020, 15, e0227046, 10.1371/journal.pone.0227046.
- Vivian Pedrinelli; Rafael Vessecchi Amorim Zafalon; Roberta Bueno Ayres Rodrigues; Mariana Pamplona Perini; Renata Maria Consentino Conti; Thiago Henrique Annibale Vendramini; Júlio César De Carvalho Balieiro; Márcio Antonio Brunetto; Concentrations of macronutrients, minerals and heavy metals in home-prepared diets for adult dogs and cats. Scientific Reports 2019, 9, 1-12, 10.1038/s41598-019-49087-z.
- Natalie Dillitzer; Nicola Becker; Ellen Kienzle; Intake of minerals, trace elements and vitamins in bone and raw food rations in adult dogs. British Journal of Nutrition 2011, 106, S53-S56, 10.1017/s0007114511002765.
- Dayara Virgínia L Ávila; Sidnei O Souza; Silvânio Silvério L Costa; Rennan Geovanny O Araujo; Carlos Alexandre B Garcia; José Do Patrocínio H Alves; Elisangela A Passos; Determination of Zn in Dry Feeds for Cats and Dogs by Energy-Dispersive X-Ray Fluorescence Spectrometry. Journal of AOAC INTERNATIONAL 2016, 99, 1572-1575, 10.5740/jaoacint.16-0105.
- Neha Gupta; Hari Ram; Balwinder Kumar; Mechanism of Zinc absorption in plants: uptake, transport, translocation and accumulation. Reviews in Environmental Science and Bio/Technology 2016, 15, 89-109, 10.1007/s11157-016-9390-1.
- Michela Schiavon; Elizabeth A. H. Pilon‐Smits; The fascinating facets of plant selenium accumulation – biochemistry, physiology, evolution and ecology. New Phytologist 2016, 213, 1582-1596, 10.1111/nph.14378.
- Marschner, H.. Zinc Uptake from Soils; Robson, A. D., Eds.; Springer: Dordrecht, Netherlands, 1993; pp. 59-77.
- Longnecker, N.E.; Robson, A.D.. Distribution and transport of zinc in plants.; Robson, A.D., Eds.; Springer: Dordrecht, Netherlands, 1993; pp. 79-91.
- P.R. Henry; R.C. Littell; C.B. Ammerman; Effect of high dietary zinc concentration and length of zinc feeding on feed intake and tissue zinc concentration in sheep. Animal Feed Science and Technology 1997, 66, 237-245, 10.1016/s0377-8401(96)01104-2.
- G. Bellof; E. Most; J. Pallauf; Concentration of copper, iron, manganese and zinc in muscle, fat and bone tissue of lambs of the breed German Merino Landsheep in the course of the growing period and different feeding intensities. Journal of Animal Physiology and Animal Nutrition 2007, 91, 100-108, 10.1111/j.1439-0396.2006.00648.x.
- Yi Zhang; Terry Lynn Ward; Fei Ji; Chucai Peng; Lin Zhu; Limin Gong; Bing Dong; Effects of zinc sources and levels of zinc amino acid complex on growth performance, hematological and biochemical parameters in weanling pigs. Asian-Australasian Journal of Animal Sciences 2018, 31, 1267-1274, 10.5713/ajas.17.0739.
- Susan J. Fairweather-Tait; Bioavailability of trace elements. Food Chemistry 1992, 43, 213-217, 10.1016/0308-8146(92)90176-3.
- NRC. Nutrient Requirements of Dogs and Cats; National Academies Press: Washington DC, WA, USA,, 2006; pp. -.
- J. Cao; P. R. Henry; R. Guo; R. A. Holwerda; J. P. Toth; R. C. Littell; R. D. Miles; C. B. Ammerman; Chemical characteristics and relative bioavailability of supplemental organic zinc sources for poultry and ruminants.. Journal of Animal Science 2000, 78, 2039-2054, 10.2527/2000.7882039x.
- Ashmead, H.. Amino Acid Chelation in Human and Animal Nutrition; CRC Press: Boca Raton, FL, USA, 2012; pp. -.
- Jesse P. Goff; Invited review: Mineral absorption mechanisms, mineral interactions that affect acid–base and antioxidant status, and diet considerations to improve mineral status. Journal of Dairy Science 2018, 101, 2763-2813, 10.3168/jds.2017-13112.
- Jianpeng Li; Chen Gong; Zaiyang Wang; Ruichang Gao; Jiaoyan Ren; Xiaodong Zhou; He Xu; Feng Xiao; Yuhui Cao; Yuanhui Zhao; et al. Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Marine Drugs 2019, 17, 341, 10.3390/md17060341.
- Weilin Shen; Toshiro Matsui; Intestinal absorption of small peptides: a review. International Journal of Food Science & Technology 2018, 54, 1942-1948, 10.1111/ijfs.14048.
- Uttra Jamikorn; Thanisara Preedapattarapong; Comparative effects of zinc methionylglycinate and zinc sulfate on hair coat characteristics and zinc concentration in plasma, hair, and stool of dogs. Thai Journal of Veterinary Medicine 2008, 38, 9-16.
- M. Chinonye Udechukwu; Stephanie A. Collins; Chibuike C. Udenigwe; Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food & Function 2016, 7, 4137-4144, 10.1039/c6fo00706f.
- Partha S. Swain; Somu B.N. Rao; Duraisamy Rajendran; George Dominic; Sellappan Selvaraju; Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Animal Nutrition 2016, 2, 134-141, 10.1016/j.aninu.2016.06.003.
- Damian Konkol; Konrad Wojnarowski; The Use of Nanominerals in Animal Nutrition as a Way to Improve the Composition and Quality of Animal Products. Journal of Chemistry 2018, 2018, 1-7, 10.1155/2018/5927058.