Hoveyda–Grubbs-type complexes, ruthenium catalysts for olefin metathesis, have gained increased interest as a research target in the interdisciplinary research fields of chemistry and biology because of their high functional group selectivity in olefin metathesis reactions and stabilities in aqueous media.
- Hoveyda–Grubbs catalyst
- artificial metalloenzyme
- second-coordination sphere effect
1. Introduction
Transition metal-catalyzed olefin metathesis (OM), the rearrangement of C–C double bonds, has gained its importance in organic synthesis because a variety of OM reaction fashions (shown in Scheme 1) are applicable for the syntheses of natural products and bioactive compounds [1,2,3][1][2][3]. Among transition metal catalysts used for OM reactions, a series of Grubbs catalyst and its derivatives (1–5; shown in Figure 1), catalysts with a ruthenium center, are the most popular catalysts both in industrial and laboratory-scale reactions [4]. This is because these catalysts are properly stable under the air compared to other transition metal complexes for OM reactions, such as Schrock-type complexes with a molybdenum center. The usability of these catalysts has motivated researchers to develop more diverted ruthenium catalysts equipped with various substituent groups. Especially, Hoveyda–Grubbs second-generation catalyst (HG-II, 5) shows the stability to some extent even in protic media, resulting from the strong coordination of an N-heterocyclic carbene (NHC) ligand and the bidentate coordination fashion of a 2-alkoxybenzylidene ligand (so-called Hoveyda-type ligand) [5]. Furthermore, HG-II is applicable for all types of OM reaction shown in Scheme 1. Consequently, HG-II has been a target of structural modification to develop a variety of derivatives with controlled reactivities [6,7,8,9,10,11][6][7][8][9][10][11].
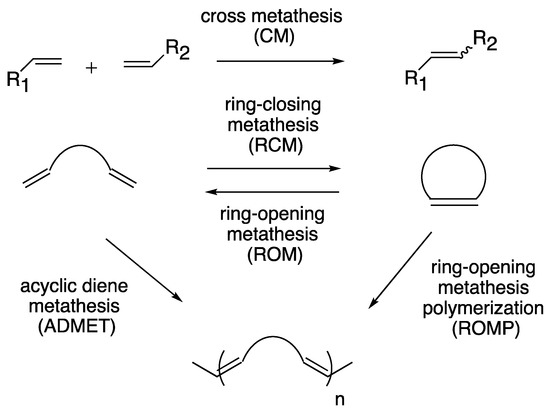
Scheme 1. Reaction fashions of olefin metathesis.
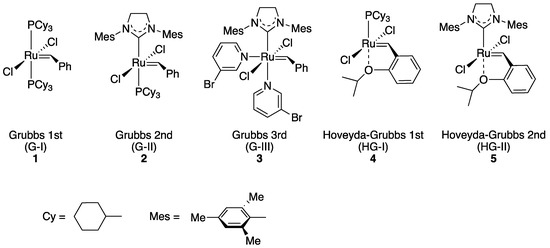
Figure 1. Grubbs catalyst and its derivatives 1–5.
Another advantage of ruthenium catalysts, other than the tolerance to moisture and air, is that the ruthenium centers in these catalysts have the higher affinity towards olefin than amide, ester, and carboxy groups (i.e., functional groups seen in biomolecules) [12]. Inspired by this fact, researchers have attempted to apply structurally modified HG-II-type complexes for biochemical demands (e.g., the construction of enzymatic olefin metathesis process, the regulation of bioreactions, and so on). This is an example for the marriage between organometallic chemistry and biological chemistry [13].
“No naturally occurring enzyme catalyzes olefin metathesis”—with this abiological aspect in OM reactions, a variety of structural modifications for HG-II-type complexes have been attempted to employ these complexes for biomolecule chemistry. Olefin metathesis in aqueous media has also been of importance as a diverted research topic. In this context, this review article introduces several types of structural modification of HG-II-type complexes for biochemical applications (Figure 2): At first, recent achievements on the development of hybrid materials composed of HG-II-type complexes and biomolecules are described. Next, the structural factors in HG-II-type complexes which influence OM reaction efficiencies in aqueous media will be discussed. Furthermore, in connection to the structural effects of biomacromolecules on OM reaction reactivities of HG-II-type complexes, the reactivity modulation of HG-II-type complexes on the basis of second-coordination sphere effect will be described. Finally, the application of ruthenium-olefin specific interaction, a driving force of OM reactions catalyzed by HG-II-type complexes, for a new type of protein modification will be presented.
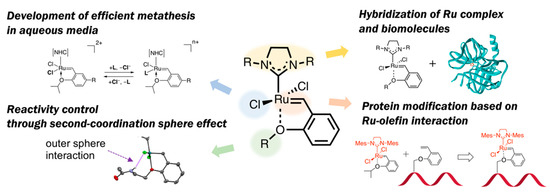
Figure 2. Outline of this review article: Structural modification of HG-II-type complexes for various demands.
2. Protein-Based Artificial Metalloenzymes with Olefin Metathesis Activity
The research field on organic syntheses using enzymes has a long history, which have been summarized in many review article and books including special issues in journals [14]. Most past works on enzymatic syntheses have mainly focused on the reaction types that are often seen in metabolism (e.g., oxidation, reduction, and hydrolysis). In contrast, artificial metalloenzymes (ArMs; ArM = artificial metalloenzyme), synthetically constructed enzymes with a metal complex embedded in protein scaffolds, have the potential to mediate abiological types of reaction, with the design of metal complexes inserted into the protein core. In this context, ArMs with OM activity (OM-ArMs) are attractive because no enzymes in nature mediate olefin metathesis: The scientific importance of ArMs with olefin metathesis activity can be seen in the fact that several review articles have taken up this topic [15,16,17,18,19,20,21,22,23,24][15][16][17][18][19][20][21][22][23][24]. Accordingly, this chapter, firstly, introduces the general concept for the construction of ArMs with olefin metathesis activity. Next, as one example, we introduce our work on the ArM with the scaffold of α-chymotrypsin (α-CT), a commercially available protease [25]. Furthermore, recent achievements in the application of OM-ArMs for in-cell systems will also be presented.
2.1. Choice of Host Proteins and Design of Hoveyda–Grubbs-Type Complexes
To construct ArMs with OM activity (i.e., OM-ArMs), the most frequently employed approach is to embed a HG-II-type complex, the active site for OM, into a host protein (so-called “hybrid metalloenzymes”). Figure 3 depicts typical strategies for the construction of OM-ArMs through the hybridization of a protein and an HG-II-type complex. For the fixation of a HG-II-type complex into a host protein, covalent conjugation and supramolecular anchoring are representative strategies [17].
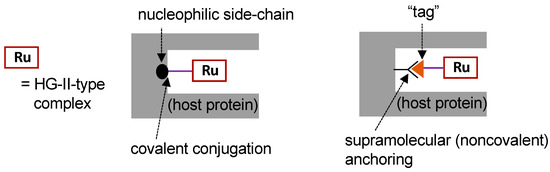
Figure 3. Strategies for the construction of artificial metalloenzymes with olefin metathesis activity (OM-ArMs) [17].
Protein scaffolds in ArMs provide a hydrophobic environment in the vicinity of the metal complex site. As the guideline for the choice of the protein scaffolds in the construction of ArMs, several structural demands should be considered:
-
Sufficient space to accommodate a HG-II-type complex inside the protein core;
-
Possession of the structural mechanism for regioselective binding of a HG-II-type complex to a protein part;
-
Proper thermostability.
Table 1 lists the research examples of hybrid OM-ArMs. For example, the heat shock protein from Methanocaldcoccus jannaschii (MjHSP) forms a 24-subunit spherical capsid with large pores. The structure provides sufficient space for a metal complex site [26]. (Strept)avidin also has a glove-like wide space near the protein surface [27]. Proteins are structurally robust and tolerant to acidic conditions. FhuAΔCVFTEV and nitrobindin construct a metal complex space in the inside of their β-barrel and cylinder-type structures [28,29][28][29]. α-CT, a protease recognizing a hydrophobic side chain of Phe, Tyr, and Trp, basically has a structural cleft for the accommodation of a peptide chain [30], which is used as a reaction space for OM reactions.
Table 1.
Hybrid artificial metalloenzymes with olefin metathesis activity (OM-ArMs).
According to the structural characteristics of the host protein, HG-II-type complexes incorporated into the protein should be decorated with some functional groups. In this case, the functional groups are attached to the NHC moiety in the complexes. Figure 4 presents several HG-II-type complexes developed for the construction of OM-ArMs. To introduce an HG-II-type complex through covalent interactions, the complex is equipped with a functional group to react with a nucleophilic amino acid side chain in host proteins (complexes 6–9) [25,26,28,29,31][25][26][28][29][31]. To apply the noncovalent anchoring strategy, the complexes should possess a “tag” moiety to attain a specific interaction with a host protein (complexes 10 and 11) [27,32][27][32].
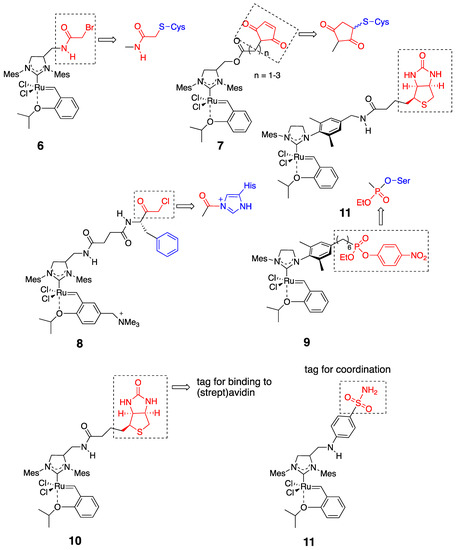
Figure 4. HG-II-type complexes used for the construction of hybrid artificial metalloenzymes with olefin metathesis activity (OM-ArMs) [24].
The combination of the two strategies (i.e., supramolecular anchoring followed by covalent conjugation) is also useful to situate a HG-II-type complex near a specific amino acid residue. For example, complex 8, a complex used for the α-CT-based OM-ArM has a phenylalanine moiety at the terminal of the complex, where α-CT accommodates the hydrophobic side-chain of the phenylalanine part at the S1-pocket at the protease active site (Figure 5) [30]. As a result, the chloromethyl ketone group is forced to position near His57 imidazole, followed by the regioselective conjugation at the place. His57 is located in the protease active site in α-CT. The blocking of His57 by complex 8 was confirmed by the abolishment of the intrinsic protease activity of α-CT.
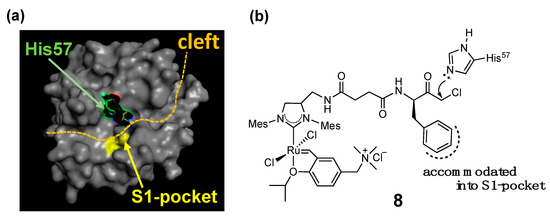
Figure 5. Mechanism explaining the regioselective conjugation of complex 8 onto His57 in α-chymotrypsin [18,25][18][25]; (a) whole structure of α-chymotrypsin from bovine pancreas (PDB (protein data bank) code: 4CHA); (b) structure of complex 8.
In the characterization of OM-ArMs, one of the most important issues to be clarified is the location of the metal center in the protein structure. In the studies of the α-CT-based OM-ArM with complex 8, the appearance of induced circular dichroism (CD) signals around 370 nm, the region of metal-to-ligand charge transfer (MLCT) in HG-II-type complexes [33[33][34],34], was used as an indicator to confirm the local environment around the metal center. The CD signals were observed for the complex in α-CT, whereas no CD signal appeared without α-CT. The finding proved that complex 8 attached to α-CT has structural torsion around the metal coordination site, resulted from the positioning of the complex in the cleft part of α-CT (see Figure 5).
2.2. Modulation of Catalytic Activities
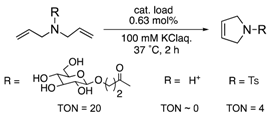
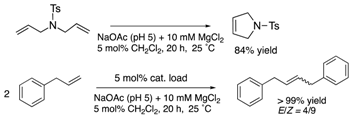
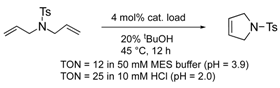
The protein scaffolds of OM-ArMs help to situate a HG-II-type complex under different environments from that in bulk, regulating the OM catalytic activities. For example, the OM-ArM in α-CT showed the higher ring-closing metathesis (RCM) activity toward a glucose-pendant substrate (a water-soluble and electrostatically neutral compound) compared to an ammonium compound or N-tosyldiallyamide although the substrate specificity was low without the protein (see Table 1). This is because the positively charged protein surface of α-CT (pI ~ 8.6) interfered the access of the positively charged or hydrophobic substrate to the metal center embedded in the protein core. As a result, the structural characteristic of α-CT (the surface charge) brought about the substrate specificity.
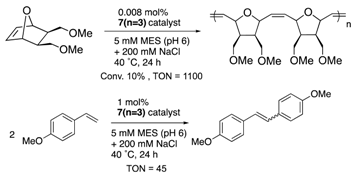
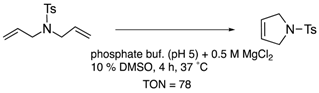
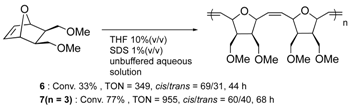
Furthermore, the structural optimization of reaction space in OM-ArMs have also been performed using genetic mutation. The mutation of (an) amino acid residue(s) near the metal center can change the shape of the reaction space to regulate the access of a substrate to the metal center. As an example, the OM-ArM constructed by the mutant nitrobindin (denoted as NB4; see Table 1) showed the activity of ring-opening metathesis polymerization (ROMP) for 7-oxonorbornene; however, the conversion was very low (10% conversion, turnover numeber (TON) = 1100). This is because the inserted ruthenium complex in the protein almost occupied a small cavity in the protein core (the estimated volume: 855 Å3 vs. 795 Å3 for 7 (n = 3)). Consequently, the access of a substrate molecule was limited. After inserting an additional β-strand part into the original protein structure to expand the narrow cavity (up to 1389 Å3), the conversion was successfully increased up to 75% (TON = 9300) [35]. As demonstrated in this work, genetic mutation around the reaction center is a useful strategy for modulating the substrate specificity and the stereoselectivity in substrate/product as well as the increase in turnover numbers of catalytic reactions. In this context, the concept of “directed molecular evolution” (i.e., repetition of mutation and screening to find the best class of ArMs) has been recognized as a general idea in the development of ArMs [36,37][36][37]. For the optimization of the catalytic activities of OM-ArMs, one example based on in-cell systems will be introduced in the next chapter.
To utilize the structural effects of a host protein on the catalytic activities of OM-ArMs, the metal center in an OM-ArM should be placed inside of the protein core but be situated around the entry of the core to achieve a smooth catalytic cycle (i.e., approach of a substrate molecule, chemical processes, and a fast release of a product). In this situation, the reactivities of OM-ArMs are partially influenced by components in reaction media (e.g., pH, buffer, additives, and so on). For example, the OM-ArM with α-CT exhibited the RCM activities in 100 mM KCl aq, whereas the addition of buffer reagent significantly decreased the catalytic activity. Several OM-ArMs showed the higher OM activities under weakly acidic conditions rather than neutral conditions in spite of no significant change in the whole structures of the host proteins. This fact is a generally considerable issue in the OM reactions in aqueous media. This topic will be discussed later.
References
- Hughes, D.; Wheeler, P.; Ene, D. Olefin Metathesis in Drug Discovery and Development-Examples from Recent Patent Literature. Org. Process Res. Dev. 2017, 21, 1938–1962.
- Ravindar, L.; Lekkala, R.; Rakesh, K.P.; Asiri, A.M.; Marwani, H.M.; Qin, H.L. Carbonyl-Olefin Metathesis: A Key Review. Org. Chem. Front. 2018, 5, 1381–1391.
- Lecourt, C.; Dhambri, S.; Allievi, L.; Sanogo, Y.; Zeghbib, N.; Ben Othman, R.; Lannou, M.I.; Sorin, G.; Ardisson, J. Natural Products And Ring-Closing Metathesis: Synthesis of Sterically Congested Olefins. Nat. Prod. Rep. 2018, 35, 105–124.
- Grubbs, R.H. Olefin Metathesis. Tetrahedron 2004, 60, 7117–7140.
- Garber, S.B.; Kingsbury, J.S.; Gray, B.L.; Hoveyda, A.H. Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts. J. Am. Chem. Soc. 2001, 123, 3186.
- Grela, K.; Harutyunyan, S.; Michrowska, A. A Highly Efficient Ruthenium Catalyst for Metathesis Reaction. Angew. Chem. Int. Ed. 2002, 41, 4038–4040.
- Bieniek, M.; Samojłowicz, C.; Sashuk, V.; Bujok, R.; Śledź, P.; Lugan, N.; Lavigne, G.; Arlt, D.; Grela, K. Rational Design and Evaluation of Upgraded Grubbs/Hoveyda Olefin Metathesis Catalysts: Polyfunctional Benzylidene Ethers on the Test Bench. Organometallics 2011, 30, 4144–4158.
- Keitz, B.K.; Endo, K.; Patel, P.R.; Herbert, M.B.; Grubbs, R.H. Improved Ruthenium Catalysts for Z-Selective Olefin Metathesis. J. Am. Chem. Soc. 2012, 134, 693–699.
- Szczepaniak, G.; Kosiński, K.; Grela, K. Towards “Cleaner” Olefin Metathesis: Tailoring the NHC Ligand of Second Generation Ruthenium Catalysts to Afford Auxiliary Traits. Green Chem. 2014, 16, 4474–4492.
- Wang, Z.J.; Jackson, W.R.; Robinson, A.J. A Simple and Practical Preparation of an Efficient Water Soluble Olefin Metathesis Catalyst. Green Chem. 2015, 17, 3407–3414.
- Paradiso, V.; Bertolasi, V.; Costabile, C.; Caruso, T.; Dąbrowski, M.; Grela, K.; Grisi, F. Expanding the Family of Hoveyda-Grubbs Catalysts Containing Unsymmetrical NHC Ligands. Organometallics 2017, 36, 3692–3708.
- Trnka, T.M.; Grubbs, R.H. The Development Of L2X2Ru = CHR Olefin Metathesis Catalysts: An Organometallic Success Story. Acc. Chem. Res. 2001, 34, 18–29.
- Fish, R.H.; Jaouen, G. Bioorganometallic Chemistry: Structural Diversity of Organometallic Complexes with Bioligands and Molecular Recognition Studies of Several Supramolecular Hosts with Biomolecules, Alkali-Metal Ions, and Organometallic Pharmaceuticals. Organometallics 2003, 22, 2166–2177.
- Hartwig, J.F.; Ward, T.R. New “Cats” in the House: Chemistry Meets Biology in Artificial Metalloenzymes and Repurposed Metalloenzymes. Acc. Chem. Res. 2019, 52, 1145.
- Binder, J.B.; Raines, R.T. Olefin Metathesis for Chemical Biology. Curr. Opin. Chem. Biol. 2008, 12, 767–773.
- Rosati, F.; Roelfes, G. Artificial Metalloenzymes. ChemCatChem 2010, 2, 916–927.
- Lewis, J.C. Artificial Metalloenzymes and Metallopeptide Catalysts for Organic Synthesis. ACS Catal. 2013, 3, 2954–2975.
- Matsuo, T.; Hirota, S. Artificial Enzymes with Protein Scaffolds: Structural Design and Modification. Bioorg. Med. Chem. 2014, 22, 5638–5656.
- Sauer, D.F.; Gotzen, S.; Okuda, J. Metatheases: Artificial Metalloproteins for Olefin Metathesis. Org. Biomol. Chem. 2016, 14, 9174–9183.
- Schwizer, F.; Okamoto, Y.; Heinisch, T.; Gu, Y.; Pellizzoni, M.M.; Lebrun, V.; Reuter, R.; Köhler, V.; Lewis, J.C.; Ward, T.R. Artificial Metalloenzymes: Reaction Scope and Optimization Strategies. Chem. Rev. 2018, 118, 142–231.
- Sauer, D.F.; Matsuo, T.; Onoda, A.; Okuda, J.; Hayashi, T. Artificially Created Metathease Consisting of an Organometallic Complex Immobilized to a Protein Matrix. In Advances in Bioorganometallic Chemistry; Hirao, T., Moriuchi, T., Eds.; Elsevier Science Publishing Co Inc.: Chennai, India, 2018; pp. 302–326.
- Sauer, D.F.; Schiffels, J.; Hayashi, T.; Schwaneberg, U.; Okuda, J. Olefin Metathesis Catalysts Embedded In Beta-Barrel Proteins: Creating Artificial Metalloproteins for Olefin Metathesis. Beilstein J. Org. Chem. 2018, 14, 2861–2871.
- Sabatino, V.; Ward, T.R. Aqueous Olefin Metathesis: Recent Developments and Applications. Beilstein J. Org. Chem. 2019, 15, 445–468.
- Matsuo, T.; Miyake, T.; Hirota, S. Recent Developments on Creation of Artificial Metalloenzymes. Tetrahedron Lett. 2019, 60, 151226.
- Matsuo, T.; Imai, C.; Yoshida, T.; Saito, T.; Hayashi, T.; Hirota, S. Creation of an Artificial Metalloprotein With a Hoveyda-Grubbs Catalyst Moiety through the Intrinsic Inhibition Mechanism of α-Chymotrypsin. Chem. Commun. 2012, 48, 1662–1664.
- Mayer, C.; Gillingham, D.G.; Ward, T.R.; Hilvert, D. An Artificial Metalloenzyme for Olefin Metathesis. Chem. Commun. 2011, 47, 12068–12070.
- Lo, C.; Ringenberg, M.R.; Gnandt, D.; Wilson, Y.; Ward, T.R. Artificial Metalloenzymes for Olefin Metathesis Based on the Biotin-(strept)Avidin Technology. Chem. Commun. 2011, 47, 12065–12067.
- Philippart, F.; Arlt, M.; Gotzen, S.; Tenne, S.J.; Bocola, M.; Chen, H.H.; Zhu, L.L.; Schwaneberg, U.; Okuda, J. A Hybrid Ring-Opening Metathesis Polymerization Catalyst Based on an Engineered Variant of the β-Barrel Protein FhuA. Chem. Eur. J. 2013, 19, 13865–13871.
- Sauer, D.F.; Himiyama, T.; Tachikawa, K.; Fukumoto, K.; Onoda, A.; Mizohata, E.; Inoue, T.; Bocola, M.; Schwaneberg, U.; Hayashi, T. A Highly Active Biohybrid Catalyst for Olefin Metathesis in Water: Impact of a Hydrophobic Cavity in a β-Barrel Protein. ACS Catal. 2015, 5, 7519–7522.
- Kraut, J. Chymotrypsin-Chemical Properties and Catalysis. In The Enzymes; Paul, D.B., Krebs, E.G., Eds.; Academic Press: Cambdige, MA, USA, 1971; Volume 3, pp. 213–248.
- Basauri-Molina, M.; Verhoeven, D.G.A.; van Schaik, A.J.; Kleijn, H.; Klein Gebbink, R.J.M. Ring-Closing and Cross-Metathesis with Artificial Metalloenzymes Created by Covalent Active Site-Directed Hybridization of a Lipase. Chem. Eur. J. 2015, 21, 15676–15685.
- Zhao, J.; Kajetanowicz, A.; Ward, T.R. Carbonic Anhydrase II as Host Protein for the Creation of a Biocompatible Artificial Metathesase. Org. Biomol. Chem. 2015, 13, 5652–5655.
- Mingotaud, A.F.; Mingotaud, C.; Moussa, W. Characterization of the Micellar Ring Opening Metathesis Polymerization in Water of a Norbornene Derivative Initiated by Hoveyda-Grubbs’ Catalyst. J. Polym. Sci. Pol. Chem. 2008, 46, 2833–2844.
- Thiel, V.; Hendann, M.; Wannowius, K.J.; Plenio, H. On the Mechanism of the Initiation Reaction in Grubbs-Hoveyda Complexes. J. Am. Chem. Soc. 2012, 134, 1104–1114.
- Grimm, A.R.; Sauer, D.F.; Davari, M.D.; Zhu, L.L.; Bocola, M.; Kato, S.; Onoda, A.; Hayashi, T.; Okuda, J.; Schwaneberg, U. Cavity Size Engineering of a β-Barrel Protein Generates Efficient Biohybrid Catalysts for Olefin Metathesis. ACS Catal. 2018, 8, 3358–3364.
- Lutz, S. Beyond Directed Evolution—Semi-Rational Protein Engineering and Design. Curr. Opin. Biotechnol. 2010, 21, 734–743.
- Reetz, M.T. Directed Evolution of Selective Enzymes: Catalysts for Organic Chemistry and Biotechnology; Wiley-VCH: Weinheim, Germany, 2016.