Inflammatory diseases include a wide variety of highly prevalent conditions with high mortality rates in severe cases ranging from cardiovascular disease, to rheumatoid arthritis, to chronic obstructive pulmonary disease, to graft vs. host disease, to a number of gastrointestinal disorders. Many diseases that are not considered inflammatory per se are associated with varying levels of inflammation. Imaging of the immune system and inflammatory response is of interest as it can give insight into disease progression and severity. Clinical imaging technologies such as computed tomography (CT) and magnetic resonance imaging (MRI) are traditionally limited to the visualization of anatomical information; then, the presence or absence of an inflammatory state must be inferred from the structural abnormalities.
- molecular imaging
- inflammation
- cardiovascular disease
1. Cardiovascular Disease (CVD)
Cardiovascular disease (CVD) is the leading cause of death worldwide [1][2]. CVD is a broad term that encompasses many heart and circulatory system conditions, most of which develop gradually and are only diagnosed after the presentation of symptoms, which often result in fatality, mainly heart attack or stroke [3]. One person will die every 36 s from CVD in the United States alone, and with an increase in the number of smokers and growing obesity rates—two major risk factors for developing CVD—it is now more important than ever to focus on the development of early screening tools to identify the markers of CVD before it is too late [4][5][6].
Atherosclerosis occurs when plaque builds up inside the artery; over time, this plaque will harden and the artery will narrow, limiting blood flow, which often results in cardiovascular disease [7]. This plaque buildup is often only detected at the onset of symptoms, such as myocardial infarction or stroke, which are two of the most common causes of mortality in the United States and Europe [6]. At present, catheter-based X-ray angiography or intravascular ultrasound is used to identify coronary atherosclerosis, but this procedure is extremely invasive and only yields anatomical information about the degree of stenosis [1][8][9]. Non-invasive molecular imaging techniques must be utilized to characterize the plaque activity to determine which patients are extremely high-risk and require immediate intervention. Coronary CT angiography (CCTA) is a method for identifying the degree of stenosis and the plaque composition [10]. CCTA is able to score the degree of calcification of the coronary plaque, which is a strong predictor of a serious cardiovascular event [11][12]. While CCTA does provide functional information about CVD, it falls short of being a true molecular imaging technique, as it does not visualize changes on molecular level.
An increase in macrophage activity, reflective of inflammation, has been linked to a higher risk of plaque rupture; therefore, molecular imaging of macrophage activity in the arteries can help identify areas where plaque may be building [13][14][15].
1. Cardiovascular Disease (CVD)
Cardiovascular disease (CVD) is the leading cause of death worldwide [26,27]. CVD is a broad term that encompasses many heart and circulatory system conditions, most of which develop gradually and are only diagnosed after the presentation of symptoms, which often result in fatality, mainly heart attack or stroke [28]. One person will die every 36 s from CVD in the United States alone, and with an increase in the number of smokers and growing obesity rates—two major risk factors for developing CVD—it is now more important than ever to focus on the development of early screening tools to identify the markers of CVD before it is too late [29,30,31].
Atherosclerosis occurs when plaque builds up inside the artery; over time, this plaque will harden and the artery will narrow, limiting blood flow, which often results in cardiovascular disease [32]. This plaque buildup is often only detected at the onset of symptoms, such as myocardial infarction or stroke, which are two of the most common causes of mortality in the United States and Europe [31]. At present, catheter-based X-ray angiography or intravascular ultrasound is used to identify coronary atherosclerosis, but this procedure is extremely invasive and only yields anatomical information about the degree of stenosis [26,33,34]. Non-invasive molecular imaging techniques must be utilized to characterize the plaque activity to determine which patients are extremely high-risk and require immediate intervention. Coronary CT angiography (CCTA) is a method for identifying the degree of stenosis and the plaque composition [35]. CCTA is able to score the degree of calcification of the coronary plaque, which is a strong predictor of a serious cardiovascular event [36,37]. While CCTA does provide functional information about CVD, it falls short of being a true molecular imaging technique, as it does not visualize changes on molecular level.
An increase in macrophage activity, reflective of inflammation, has been linked to a higher risk of plaque rupture; therefore, molecular imaging of macrophage activity in the arteries can help identify areas where plaque may be building [38,39,40].
18F-Flourodeoxyglucose (FDG) PET imaging is commonly used to image the inflammatory component of atherosclerosis [16][17][18][19].
F-Flourodeoxyglucose (FDG) PET imaging is commonly used to image the inflammatory component of atherosclerosis [41,42,43,44].
18
F-FDG is a radiolabeled glucose molecule, which is internalized by cells through the same mechanism in which glucose is metabolized. Both
18
F-FDG and glucose are phosphorylated by hexokinase, where
18
F-FDG becomes
18
F-FDG-6-phosphate and glucose become glucose-6-phosphate.
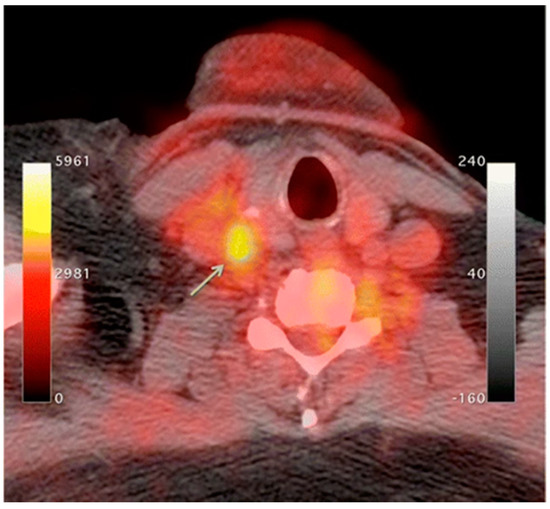
18F-FDG-6-phosphate cannot be further metabolized by glucose-6-phosphate isomerase; therefore, it remains inside the cell for PET imaging [20]. In atherosclerosis, the accumulation of macrophages at locations of active plaque buildup requires a large amount of glucose, thus causing the upregulation of glucose transporters on the surface of these macrophages. Therefore, increased
F-FDG-6-phosphate cannot be further metabolized by glucose-6-phosphate isomerase; therefore, it remains inside the cell for PET imaging [45]. In atherosclerosis, the accumulation of macrophages at locations of active plaque buildup requires a large amount of glucose, thus causing the upregulation of glucose transporters on the surface of these macrophages. Therefore, increased
18
F-FDG uptake will be seen at locations of increased macrophage density, which is reflective of inflammation and active plaque buildup (
Figure 1) [13][21]. It is unknown what the influence of
18F-FDG uptake from other cells, such as, neutrophils, endothelial cells, and lymphocytes, has on the observed signal [8][15]. Once the plaque cells have calcified,
F-FDG uptake from other cells, such as, neutrophils, endothelial cells, and lymphocytes, has on the observed signal [33,40]. Once the plaque cells have calcified,
18
F-FDG uptake will subside substantially, making this type of PET imaging ineffective. PET imaging of atherosclerosis using
18
F-FDG requires a circulation time of 2–3 h to allow for accumulation in the arterial wall and the decay or excretion of background levels of
18F-FDG [15].
F-FDG [40].
18
F-FDG PET imaging in oncology typically needs 1 h of circulation time before imaging can begin.
18F-FDG PET imaging is non-specific; therefore, it is complicated by highly metabolic neighboring tissues such as myocardial cells and neurons [13][22][23]. The suppression of myocardial
F-FDG PET imaging is non-specific; therefore, it is complicated by highly metabolic neighboring tissues such as myocardial cells and neurons [38,47,48]. The suppression of myocardial
18F-FDG uptake can be achieved through dietary manipulation (high-fat, low-carb) to shift the body into beta-oxidation of fatty acids instead of metabolizing glucose as a primary energy source to try and limit this background activity [24][25]. Other radiotracers can be utilized that are specific to macrophages, limiting the effects from other highly metabolic cells. Translocator protein (TSPO)/peripheral benzodiazepine (PBR) receptors are overexpressed in activated macrophages, which is a great option for active targeting [26].
F-FDG uptake can be achieved through dietary manipulation (high-fat, low-carb) to shift the body into beta-oxidation of fatty acids instead of metabolizing glucose as a primary energy source to try and limit this background activity [49,50]. Other radiotracers can be utilized that are specific to macrophages, limiting the effects from other highly metabolic cells. Translocator protein (TSPO)/peripheral benzodiazepine (PBR) receptors are overexpressed in activated macrophages, which is a great option for active targeting [51].
11C-PK11195, (1-(2-Chlorophenyl)-N-(11C)methyl-N- (1-methylpropyl) -3-isoquinoline carboxamide) is a radiolabeled TSPO ligand that has been used as a PET tracer to visualize inflammatory plaque in atherosclerosis [26][27][28][29].
C-PK11195, (1-(2-Chlorophenyl)-N-(11C)methyl-N- (1-methylpropyl) -3-isoquinoline carboxamide) is a radiolabeled TSPO ligand that has been used as a PET tracer to visualize inflammatory plaque in atherosclerosis [51,52,53,54].
11C-PK11195 uptake in patients with atherosclerosis was higher in patients who had a myocardial infarction or stroke compared to patients who were asymptomatic [28]. Other radiolabeled TSPO targeted ligands include
C-PK11195 uptake in patients with atherosclerosis was higher in patients who had a myocardial infarction or stroke compared to patients who were asymptomatic [53]. Other radiolabeled TSPO targeted ligands include
18F-GE-180, which showed a better signal-to-noise ratio and lower non-specific binding; more work must be done to validate this radiotracer [30].
F-GE-180, which showed a better signal-to-noise ratio and lower non-specific binding; more work must be done to validate this radiotracer [55].
68Ga-DOTATATE is another radiolabeled tracer that can be used to target inflammatory plaque in atherosclerosis by targeting the somatostatin receptor subtype 2 (SSR-2), which is also overexpressed on activated macrophages [31][32]. A copper radiolabel (
Ga-DOTATATE is another radiolabeled tracer that can be used to target inflammatory plaque in atherosclerosis by targeting the somatostatin receptor subtype 2 (SSR-2), which is also overexpressed on activated macrophages [56,57]. A copper radiolabel (
64Cu) is frequently substituted for gallium because of the longer half-life and shorter positron range, which allow for better spatial resolution [22][33]. CXC-motif chemokine receptor 4 (CXCR-4) is also overexpressed on many immune cells, particularly monocytes and macrophages, making this receptor a good target for imaging inflammatory plaques of atherosclerosis [34]. Radiolabeled pentixafor,
Cu) is frequently substituted for gallium because of the longer half-life and shorter positron range, which allow for better spatial resolution [47,58]. CXC-motif chemokine receptor 4 (CXCR-4) is also overexpressed on many immune cells, particularly monocytes and macrophages, making this receptor a good target for imaging inflammatory plaques of atherosclerosis [59]. Radiolabeled pentixafor,
68Ga-pentixafor, targets this CXCR-4 receptor for the quantification of arterial inflammation in atherosclerotic plaques [34][35][36].
As plaque builds up inside the artery, macrophages become active, and the region often becomes hypoxic due to the reduced oxygen diffusion efficiency from the thickening of the vessel wall. As active macrophages reflect sites of inflammation, it is suspected that macrophage activity is partially mediated by hypoxia as atherosclerotic plaques overexpress hypoxia-inducible factor 1-alpha (HIF-1α) [8][22][37]. There is ongoing research that focuses on the imaging of hypoxia as a surrogate biomarker of plaque inflammation and atherosclerosis. Radiolabeled ligands such as
Ga-pentixafor, targets this CXCR-4 receptor for the quantification of arterial inflammation in atherosclerotic plaques [59,60,61].
As plaque builds up inside the artery, macrophages become active, and the region often becomes hypoxic due to the reduced oxygen diffusion efficiency from the thickening of the vessel wall. As active macrophages reflect sites of inflammation, it is suspected that macrophage activity is partially mediated by hypoxia as atherosclerotic plaques overexpress hypoxia-inducible factor 1-alpha (HIF-1α) [33,47,62]. There is ongoing research that focuses on the imaging of hypoxia as a surrogate biomarker of plaque inflammation and atherosclerosis. Radiolabeled ligands such as
18
F-fluoromisonidazole (FMISO) or
18F-EF5 have been used to detect atherosclerotic plaques through PET imaging of hypoxia preclinically; more work must be done to advance these findings to the clinic [38][39][40].
F-EF5 have been used to detect atherosclerotic plaques through PET imaging of hypoxia preclinically; more work must be done to advance these findings to the clinic [63,64,65].
2. Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune disorder that is characterized by chronic inflammation of the joints often causing degradation of the cartilage and bone, leading to a diminished quality of life due to musculoskeletal deficits and chronic pain [41]. For every 1000 adults, five will have RA, making it one of the most prevalent chronic inflammatory conditions worldwide [42]. RA etiology is not exactly known due to the synergistic effects of epigenetics [43] and environmental factors (smoking [44][45], obesity [46][47][48], and alcohol consumption [49][50][51]). Autoantibodies such as antibodies to citrullinated protein antigens (ACPAs) or rheumatoid factor (RF) have well-established roles in RA as accurate predictors of disease severity [52][53][54]. The current standard of care for the diagnosis of RA is through blood work to monitor the erythrocyte sedimentation rate (ESR), C-reactive protein levels (CRP), RF, and ACPAs [52][55] or anatomical imaging through MRI and ultrasound [56]. Power Doppler ultrasound (PDUS) is an US technique that is commonly used in the evaluation of RA, as it can visualize blood flow as well as anatomical information. The locations of active inflammation will have increased blood flow, making PDUS a good choice for not only diagnosing RA but also for assessing the severity and response to treatment [57][58].
Rheumatoid arthritis (RA) is an autoimmune disorder that is characterized by chronic inflammation of the joints often causing degradation of the cartilage and bone, leading to a diminished quality of life due to musculoskeletal deficits and chronic pain [66]. For every 1000 adults, five will have RA, making it one of the most prevalent chronic inflammatory conditions worldwide [67]. RA etiology is not exactly known due to the synergistic effects of epigenetics [68] and environmental factors (smoking [69,70], obesity [71,72,73], and alcohol consumption [74,75,76]). Autoantibodies such as antibodies to citrullinated protein antigens (ACPAs) or rheumatoid factor (RF) have well-established roles in RA as accurate predictors of disease severity [77,78,79]. The current standard of care for the diagnosis of RA is through blood work to monitor the erythrocyte sedimentation rate (ESR), C-reactive protein levels (CRP), RF, and ACPAs [77,80] or anatomical imaging through MRI and ultrasound [81]. Power Doppler ultrasound (PDUS) is an US technique that is commonly used in the evaluation of RA, as it can visualize blood flow as well as anatomical information. The locations of active inflammation will have increased blood flow, making PDUS a good choice for not only diagnosing RA but also for assessing the severity and response to treatment [82,83].
Synovial membrane inflammation (synovitis) is a key characteristic of RA that involves the upregulation of both innate and adaptive immune cells and fibroblast-like synoviocytes (FLS) [59]. This immune response coupled with FLS results in inflammation and the activation of osteoclasts that leads to the degradation of cartilage [60][61]. The synovial fluid contains a variety of activated macrophages, B cells, and T cells, all of which are good targets for the molecular imaging of RA. The overexpression of inflammatory biomarkers can damage the existing vasculature, resulting in the enhanced permeability and retention (EPR) effect [62]. The newly permeable environment allows for the passive targeting of the immune cells of an inflammatory response. SPION-based contrast agents are small enough to penetrate the synovial fluid where they are phagocytized by active macrophages and can be visualized by T2-weighted MRI [63][64][65].
Synovial membrane inflammation (synovitis) is a key characteristic of RA that involves the upregulation of both innate and adaptive immune cells and fibroblast-like synoviocytes (FLS) [84]. This immune response coupled with FLS results in inflammation and the activation of osteoclasts that leads to the degradation of cartilage [85,86]. The synovial fluid contains a variety of activated macrophages, B cells, and T cells, all of which are good targets for the molecular imaging of RA. The overexpression of inflammatory biomarkers can damage the existing vasculature, resulting in the enhanced permeability and retention (EPR) effect [87]. The newly permeable environment allows for the passive targeting of the immune cells of an inflammatory response. SPION-based contrast agents are small enough to penetrate the synovial fluid where they are phagocytized by active macrophages and can be visualized by T2-weighted MRI [18,88,89].
Activated macrophages can also be imaged using
18F-FDG PET imaging in the same manner described above [66][67][68]. While
F-FDG PET imaging in the same manner described above [90,91,92]. While
18F-FDG PET imaging targets activated macrophages through elevated levels of glucose metabolism, there are more specific methods used to image active macrophages in RA. Folate receptor β (FRβ), a glycosylphosphatidyl plasma membrane anchored protein used to internalize folate needed for DNA synthesis and cell division, is overexpressed on activated macrophages in the synovial fluid, making it an attractive target for the molecular imaging of RA [69][70]. Radiolabeled folic acid can be imaged through scintigraphy or PET imaging for the detection of inflammation in the joints (
F-FDG PET imaging targets activated macrophages through elevated levels of glucose metabolism, there are more specific methods used to image active macrophages in RA. Folate receptor β (FRβ), a glycosylphosphatidyl plasma membrane anchored protein used to internalize folate needed for DNA synthesis and cell division, is overexpressed on activated macrophages in the synovial fluid, making it an attractive target for the molecular imaging of RA [93,94]. Radiolabeled folic acid can be imaged through scintigraphy or PET imaging for the detection of inflammation in the joints (
Table 1) [69][71][72][73]. Spatial resolution of PET images is poor; a fluorescently labeled folate probe (NIR2-folate) can be visualized with NIR fluorescence imaging with greater spatial resolution, but this technique is limited by penetration depth due to light scattering in tissue [74]. Many other methods exist for targeting activated macrophages in RA [75].
) [93,95,96,97]. Spatial resolution of PET images is poor; a fluorescently labeled folate probe (NIR2-folate) can be visualized with NIR fluorescence imaging with greater spatial resolution, but this technique is limited by penetration depth due to light scattering in tissue [98]. Many other methods exist for targeting activated macrophages in RA [99].
Table 1. Summary of the molecular targets and tracers used to identify inflammatory disease that are discussed in this review.
| Disease | Target | Tracer | Inflammatory Component | Source |
|---|---|---|---|---|
| Cardiovascular Disease | Glucose Metabolism | 18F-Flourodeoxyglucose (FDG) | Activated macrophage accumulation | [16][17][18][19][41,42,43,44] |
| Translocator protein (TSPO) receptors | 11C- PK11195 18F-GE-180 |
Overexpressed on activated macrophages | [26]][51[27],52[28][29,53,54] | |
| Somatostatin receptor subtype-2 (SSR-2) | 68Ga-DOTATATE/ 64Cu-DOTATATE |
Overexpressed on activated macrophages | [31][32][56,57] | |
| Chemokine receptor 4 | 68Ga-pentixafor | Overexpressed on activated macrophages | [34][35][36][59,60,61] | |
| Hypoxia | 18F-fluoromisonidazole (FMISO | Activated macrophage accumulation → inflammation and thickening of the vessel wall → decreased oxygen diffusion efficiency → Hypoxia | [39][64] | |
| 18F-EF5 | [40][65] | |||
| Rheumatoid Arthritis | Glucose metabolism | 18F-Flourodeoxyglucose (FDG) | Activated macrophage accumulation | [66][67][68][90,91,92] |
| Folate receptor β (FRβ) | 18F-Fluoro-PEG-folate 111In-folate conjugate |
Overexpressed on activated macrophages within the synovial fluid | [69]][93[71],95[72][73,96,97] | |
| NIR2-Folate | [74][98] | |||
| E-selectin | 111In-labeled anti-E-selectin MAb | Overexpressed on endothelial cells due to TNFα | [76][100] | |
| DyLight 750/anti-E-selectin Mab probe | [62][87] | |||
| 99mTc-labelled anti-E-selectin FAb | [77][102] | |||
| MMPs | 18F-pyriminde-2,4,6,-triones | Elevated levels in synovial fluid correlate with inflammatory response | [78][104] | |
| NIR fluorescent MMP-3 specific chitosan nanoparticle | [79][103] | |||
| CD20 | 124I-Rituximab 89Zr-Rituximab |
Overexpressed on B lymphocytes as they accumulate in synovial fluid | [80][81][105,106] | |
| TNFα | 99mTc-Infliximab | Overexpressed in synovial fluid | [82][83][107,109] | |
| L-selectin/P-selectin | NIR Fluorescent Polyanionic dendritic polyglycerol sulfate (dPGS) | Movement of immune cells to the inflammatory location | [84][85][111,113] | |
| COPD | Pulmonary perfusion | 99mTc-labeled macroaggregated albumin | Ventilation/Perfusion (V/Q) scintigraphy to regional inflammatory/airflow differences | [86][87][123,125] |
| Pulmonary ventilation | 81mKr or 133Xe 99mTc-labeled DTPA 99mTc-labeled carbon particles (Technegas) |
[87][125] | ||
| Glucose metabolism | 18F-Flourodeoxyglucose (FDG) | Activated macrophage accumulation | [88][89][90][91][130,131,132,133] | |
| Translocator protein (TSPO) receptors | 11C-PK11195 | Overexpressed on activated macrophages | [92][134] | |
| MMPs | 18F-IPFP | Produced by active macrophages at the inflammatory location | [93][135] | |
| 99mTc-labeled RP805 | [94][136] | |||
| Gastrointestinal | Glucose metabolism | 18F-Flourodeoxyglucose (FDG) | Activated macrophage accumulation | [95][96][143,144] |
| CXCL8 receptor | 99mTc-CXCL8 | Overexpression on activated neutrophils | [97][150] | |
| Interleukin 1 β | 89Zr-lα-IL-1β | Secreted by immune cells indicating an inflammatory response | [98][151] | |
| CD11b | 89Zr-α-CD11b | Pan-myeloid innate immune marker | [98][151] | |
| CD4 | 89Zr-GK1.5 cys diabody (cDb) | CD4 positive T-Cells characterize IBD inflammatory response | [99][152] | |
| EGFR | 64Cu-Cetuximab fragment-DOTA | Overexpression in inflammatory cells | [100][158] |
Due to the abundance of immune cells in the synovial fluid, there is an overexpression of inflammatory cytokines that elicit certain cellular responses that can then be targeted for imaging. The presence of interleukin-1 and tumor necrosis factor alpha (TNF-α) stimulate the transient expression of surface protein E-selectin on vascular endothelial cells and the overexpression of matrix metalloproteases (MMPs) in the synovial fluid. Anti E-selectin antibodies and MMP-targeted probes can be either radiolabeled or conjugated to an NIR dye and visualized through scintillation/PET or NIR fluorescence imaging [76][101][77][79][78]. Biologicals used as therapeutics for RA can also be radiolabeled and used to image RA. Rituximab, a monoclonal antibody that targets CD20, a cell surface marker that is expressed on most B cells, can be radiolabeled and used as a probe for the in vivo molecular imaging of RA based on B lymphocyte accumulation in the synovial fluid (
Due to the abundance of immune cells in the synovial fluid, there is an overexpression of inflammatory cytokines that elicit certain cellular responses that can then be targeted for imaging. The presence of interleukin-1 and tumor necrosis factor alpha (TNF-α) stimulate the transient expression of surface protein E-selectin on vascular endothelial cells and the overexpression of matrix metalloproteases (MMPs) in the synovial fluid. Anti E-selectin antibodies and MMP-targeted probes can be either radiolabeled or conjugated to an NIR dye and visualized through scintillation/PET or NIR fluorescence imaging [100,101,102,103,104]. Biologicals used as therapeutics for RA can also be radiolabeled and used to image RA. Rituximab, a monoclonal antibody that targets CD20, a cell surface marker that is expressed on most B cells, can be radiolabeled and used as a probe for the in vivo molecular imaging of RA based on B lymphocyte accumulation in the synovial fluid (
Figure 2) [102][80][81]. Infliximab, a monoclonal antibody that targets tumor necrosis factor alpha (TNFα), has also been radiolabeled with
) [4,105,106]. Infliximab, a monoclonal antibody that targets tumor necrosis factor alpha (TNFα), has also been radiolabeled with
99mTc, which demonstrated a superior sensitivity to inflammation than MRI and clinical examinations in patients with RA [82][103][83].
Tc, which demonstrated a superior sensitivity to inflammation than MRI and clinical examinations in patients with RA [107,108,109].
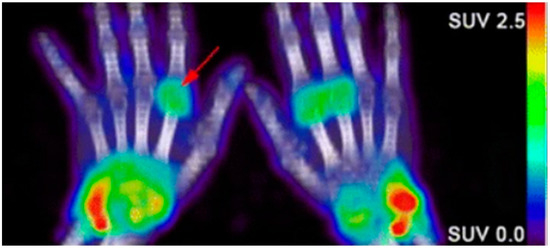
Figure 2. Confirmation of rheumatoid arthritis (RA) in the wrists/hands of patients using 89Zr-rituximab PET imaging to target B-cell accumulation [80][105].
Carbohydrate-binding proteins, L-selection and P-selection, are involved in the movement of immune cells before and during the inflammatory response [104]. Polyanionic dendritic polyglycerol sulfate (dPGS) targets inflammation through binding with these selectins. Conjugation with indocyanine green (ICG), an NIR fluorescent dye, allowed for the in vivo differentiation of RA-positive joints from RA negative joints in a preclinical rat arthritis model as seen by a 3.5-fold greater fluorescence imaging signal [84]. As the clinical translation of NIR fluorescence is limited by low penetration depth, multispectral optoacoustic tomography (MSOT) can overcome those limitations. MSOT imaging is based on a light-in, sound-out approach, having all the benefits of optical imaging but allowing for increased depth penetration, since photon scattering is irrelevant to acoustic waves [105]. Then, NIR-labeled dPGS can be imaged at much greater depths using MSOT [85].
Carbohydrate-binding proteins, L-selection and P-selection, are involved in the movement of immune cells before and during the inflammatory response [110]. Polyanionic dendritic polyglycerol sulfate (dPGS) targets inflammation through binding with these selectins. Conjugation with indocyanine green (ICG), an NIR fluorescent dye, allowed for the in vivo differentiation of RA-positive joints from RA negative joints in a preclinical rat arthritis model as seen by a 3.5-fold greater fluorescence imaging signal [111]. As the clinical translation of NIR fluorescence is limited by low penetration depth, multispectral optoacoustic tomography (MSOT) can overcome those limitations. MSOT imaging is based on a light-in, sound-out approach, having all the benefits of optical imaging but allowing for increased depth penetration, since photon scattering is irrelevant to acoustic waves [112]. Then, NIR-labeled dPGS can be imaged at much greater depths using MSOT [113].
2.3. Chronic Obstructive Pulmonary Disease (COPD)
Chronic obstructive pulmonary disease (COPD) is a preventable, but underdiagnosed inflammatory disease with an extremely high morbidity and mortality rate [106]. Approximately 90% of all COPD cases are related to smoking, yet only 20% of smokers will develop COPD, suggesting that other environmental and genetic factors must also play a role [107][108]. COPD is characterized by airway obstruction due to chronic inflammation and tissue damage caused by a decrease in alveolar elasticity and gas exchange, which ultimately leads to an irreversible decrease in lung function [109]. Pulmonary function testing (PFT) to measure airflow coupled with conventional imaging modalities, CT or MRI, to visualize morphological changes in the airway, is the current standard for diagnosing COPD [110]. Since COPD is an inflammatory disease, these imaging modalities must infer about the inflammatory state through surrogate biomarkers such as airway thickness and airway wall area [109]. Emphysema and chronic bronchitis are two subtypes of COPD that have very distinct molecular characteristics. Emphysema is an irreversible condition induced by smoking or inhaling irritants that destroys the alveoli; this leads to a decrease in the surface area of the lungs, making it difficult to obtain oxygen, causing inflammation of the lung parenchyma [111][112]. Chronic bronchitis is the persistent inflammation of the bronchial tubes due to a chronic cough, which leads to sputum build up in the airways, restricting airflow [113][114]. Early identification of COPD and proper differentiation of different phenotypes is imperative for the development of a proper treatment plan.
Chronic obstructive pulmonary disease (COPD) is a preventable, but underdiagnosed inflammatory disease with an extremely high morbidity and mortality rate [114]. Approximately 90% of all COPD cases are related to smoking, yet only 20% of smokers will develop COPD, suggesting that other environmental and genetic factors must also play a role [115,116]. COPD is characterized by airway obstruction due to chronic inflammation and tissue damage caused by a decrease in alveolar elasticity and gas exchange, which ultimately leads to an irreversible decrease in lung function [117]. Pulmonary function testing (PFT) to measure airflow coupled with conventional imaging modalities, CT or MRI, to visualize morphological changes in the airway, is the current standard for diagnosing COPD [118]. Since COPD is an inflammatory disease, these imaging modalities must infer about the inflammatory state through surrogate biomarkers such as airway thickness and airway wall area [117]. Emphysema and chronic bronchitis are two subtypes of COPD that have very distinct molecular characteristics. Emphysema is an irreversible condition induced by smoking or inhaling irritants that destroys the alveoli; this leads to a decrease in the surface area of the lungs, making it difficult to obtain oxygen, causing inflammation of the lung parenchyma [119,120]. Chronic bronchitis is the persistent inflammation of the bronchial tubes due to a chronic cough, which leads to sputum build up in the airways, restricting airflow [121,122]. Early identification of COPD and proper differentiation of different phenotypes is imperative for the development of a proper treatment plan.
Molecular imaging techniques have been developed to target the inflammatory response of COPD. As the airways become inflamed, there will be changes in the pulmonary blood flow as well as airflow. These changes often precede morphological changes that can be detected by CT. Perfusion scintigraphy through the injection of
99m
Tc-labeled macroaggregated albumin coupled with ventilation scintigraphy through the inhalation of either an inert radioactive gas (
81m
Kr or
133
Xe), an aerosol-based
99m
Tc-labeled DTPA, or Technegas (
99mTc-labeled carbon particles) will uncover aspects of the heterogeneity of the disease that cannot be seen using PFT or CT [86]. A great comparative study of these radiolabeled tracers in ventilation scintigraphy is found here [115]. The Ventilation to Perfusion (V/Q) ratio obtained will yield important information about regional differences in airflow and inflammation, where larger V/Q values indicate emphysema and lower values reflect chronic bronchitis [87][116]. Similarly, MRI using hyperpolarized noble gas (
Tc-labeled carbon particles) will uncover aspects of the heterogeneity of the disease that cannot be seen using PFT or CT [123]. A great comparative study of these radiolabeled tracers in ventilation scintigraphy is found here [124]. The Ventilation to Perfusion (V/Q) ratio obtained will yield important information about regional differences in airflow and inflammation, where larger V/Q values indicate emphysema and lower values reflect chronic bronchitis [125,126]. Similarly, MRI using hyperpolarized noble gas (
3
He or
129Xe) can also be used to assess the ventilation status through imaging of the airspaces of the lungs rather than the tissue [117]. Apparent diffusion coefficient (ADC) maps of the hyperpolarized gas can be obtained on a voxel-wise basis using diffusion-weighted MRI (DWI-MRI), where high ADC values reflect areas of severe disease [118][119]. While hyperpolarized MRI is able to visualize the ventilation deficiencies associated with COPD, it is limited by spatial resolution and the ability of the patient to hold their breath.
Xe) can also be used to assess the ventilation status through imaging of the airspaces of the lungs rather than the tissue [127]. Apparent diffusion coefficient (ADC) maps of the hyperpolarized gas can be obtained on a voxel-wise basis using diffusion-weighted MRI (DWI-MRI), where high ADC values reflect areas of severe disease [128,129]. While hyperpolarized MRI is able to visualize the ventilation deficiencies associated with COPD, it is limited by spatial resolution and the ability of the patient to hold their breath.
As with other inflammatory diseases, COPD can also be visualized through immune cells.
18F-FDG PET/CT imaging is commonly used to monitor the metabolic activity of immune cells to diagnose and identify disease severity [88][89][90][91]. Since
F-FDG PET/CT imaging is commonly used to monitor the metabolic activity of immune cells to diagnose and identify disease severity [130,131,132,133]. Since
18
F-FDG is a non-specific biomarker of immune activity, the addition of
11
C-PK11195, a macrophage-targeted radiotracer, allows for the non-specific visualization of neutrophil activity as well as the more specific visualization of macrophage accumulation. A study involving six patients with COPD and five control subjects saw a greater accumulation of
18
F-FDG in all COPD patients compared to control, and greater
11C-PK11195 accumulation in four of six COPD patients compared to control [92]. Macrophages will secrete matrix metalloproteases (MMPs) and many other cytokines, which are all attractive options for the molecular imaging of COPD. Using a mouse model of COPD, a radiofluorinated probe,
C-PK11195 accumulation in four of six COPD patients compared to control [134]. Macrophages will secrete matrix metalloproteases (MMPs) and many other cytokines, which are all attractive options for the molecular imaging of COPD. Using a mouse model of COPD, a radiofluorinated probe,
18
F-IPFP, was developed and tested to target MMP-9 and MMP-12; the accumulation of
18F-IPFP was 4× higher in the lungs of COPD mice than in normal mice [93].
F-IPFP was 4× higher in the lungs of COPD mice than in normal mice [135].
99m
Tc-labeled RP805 is another MMP targeted radiotracer that saw significantly greater accumulation in IL-13 transgenic mice than control mice using SPECT/CT (
) [136].
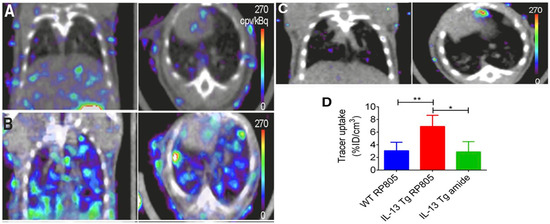
Figure 3.
Coronal (left) and transversal (right) SPECT/CT imaging of matrix metalloproteases (MMPs). (
A
) Wild-type mice injected with
99m
Tc-labeled RP805 (
B
) IL-13 transgenic mice injected with
99m
Tc-labeled RP805 (
C
) IL-13 transgenic mice injected with an amide analog tracer as a control. (
D
) Quantification of uptake in SPECT images. *
p
< 0.01 **
p < 0.001 [94].
< 0.001 [136].
34. Gastrointestinal
Different gastrointestinal (GI) diseases can present with common, non-specific symptoms such as diarrhea and abdominal pain, making accurate diagnosis challenging without molecular information in addition to history and physical exam. [120][121]. Globally, the prevalence of inflammatory GI conditions such as inflammatory bowel disease (IBD) has increased significantly over time [122], particularly in developing countries [123]. Several causes, including genetic factors, diet, and infection, can result in inflammation of the GI tract. Identification of GI inflammation can aid in monitoring response to interventions. Subsequently, appropriate treatment can be administered to relieve symptoms or prevent disease progression. This can be especially critical in lowering patient risk for colorectal cancers [124].
Different gastrointestinal (GI) diseases can present with common, non-specific symptoms such as diarrhea and abdominal pain, making accurate diagnosis challenging without molecular information in addition to history and physical exam. [137,138]. Globally, the prevalence of inflammatory GI conditions such as inflammatory bowel disease (IBD) has increased significantly over time [139], particularly in developing countries [140]. Several causes, including genetic factors, diet, and infection, can result in inflammation of the GI tract. Identification of GI inflammation can aid in monitoring response to interventions. Subsequently, appropriate treatment can be administered to relieve symptoms or prevent disease progression. This can be especially critical in lowering patient risk for colorectal cancers [141].
Historically, tests using blood, stool, or biopsied tissue samples have been paired with invasive imaging techniques, such as endoscopy, to diagnose and assess patient GI disease [124]. Currently available invasive and non-invasive imaging techniques such as endoscopy, CT, MRI, and US, can show the macroscopic structural abnormalities associated with inflammatory bowel disease such as bowel wall thickening, abscesses, or fistulas to identify the scope of disease [125]. When combining multiple standard imaging modalities, the presence of inter-clinician reader variability and the lack of molecular information contained in the images (
Historically, tests using blood, stool, or biopsied tissue samples have been paired with invasive imaging techniques, such as endoscopy, to diagnose and assess patient GI disease [141]. Currently available invasive and non-invasive imaging techniques such as endoscopy, CT, MRI, and US, can show the macroscopic structural abnormalities associated with inflammatory bowel disease such as bowel wall thickening, abscesses, or fistulas to identify the scope of disease [142]. When combining multiple standard imaging modalities, the presence of inter-clinician reader variability and the lack of molecular information contained in the images (
) often requires a biopsy for an accurate diagnosis. In the context of
, the numerous lesions within the colon result in a higher potential of a biopsy sampling error and the possibility to miss areas of early-stage colon cancer.
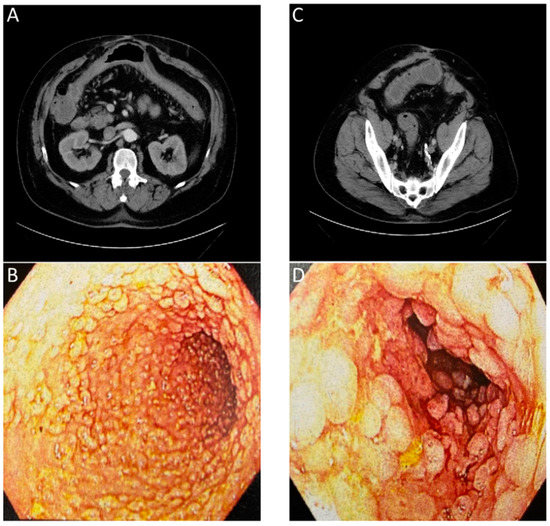
Figure 4.
Images of a patient with history of chronic diarrhea that is occasionally bloody. CT ordered for unrelated reasons incidentally showed non-specific inflammation. Correlation with endoscopy showed substantial chronic inflammation. (
A
) CT with arrows showing inflammation of transverse colon. (
B
) Endoscopic images of transverse colon with diffuse pseudopolyps. (
C
) CT with arrows showing inflammation of sigmoid colon. (
D
) Endoscopic images of sigmoid colon with diffuse pseudopolyps. The lack of an inflammation or cancer specific contrast agent for the CT or endoscopic evaluation required a biopsy to confirm a lack of neoplasia.
PET imaging is currently the only clinically approved molecular imaging approach for GI inflammation [95][96]. Specifically,
PET imaging is currently the only clinically approved molecular imaging approach for GI inflammation [143,144]. Specifically,
18F-FDG PET is used to measure the extent and magnitude of GI inflammation, indicating areas of low or high inflammation based on metabolic differences throughout the GI tract. The high metabolic need of inflamed tissue alongside the increased presence and activity of immune cells, such as leukocytes, results in increased glucose metabolism at sites of inflammation [126]. Differences in
F-FDG PET is used to measure the extent and magnitude of GI inflammation, indicating areas of low or high inflammation based on metabolic differences throughout the GI tract. The high metabolic need of inflamed tissue alongside the increased presence and activity of immune cells, such as leukocytes, results in increased glucose metabolism at sites of inflammation [145]. Differences in
18
F-FDG consumption highlight areas of increased inflammation while contrasting against normal healthy tissue. PET alone offers limited spatial resolution despite its potential for high contrast imaging. Additionally, the uptake of
18
F-FDG occurs in off-target sites, resulting in high background signal. As such, PET is frequently paired with either CT or MRI imaging to better monitor disease status and accurately assess disease location, as shown in (
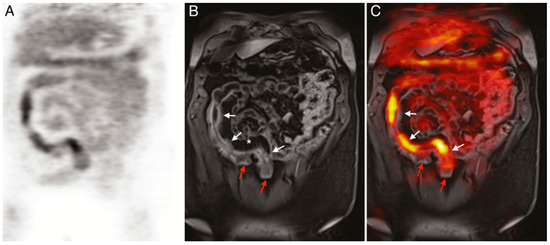
Figure 5.
(
A
)
18
F-FDG PET image of human patient with Crohn’s disease. (
B
) T1-weighted magnetic resonance imaging (MRI) image of the same patient. (
C
) Merged PET/MRI. White arrows indicate locations of acute inflammation while red arrows highlight damage resulting from earlier disease action. The asterisk (*) shows a site of proliferation of fibrofatty compounds in the mesentery. SUVmax of
18F-FDG 5.6–9.2 vs. SUVmax of background bowel 1.5–2.8 [128].
F-FDG 5.6–9.2 vs. SUVmax of background bowel 1.5–2.8 [147].
Current molecular imaging techniques prove mostly effective for verifying the extent and magnitude of GI inflammation. Preclinically, there has been investigation into the manipulation of contrast agents for the molecular imaging of GI inflammation. Wang et al. quantified inflammation in acute colitis mouse models using ultrasound with a P- and E-selectin targeted contrast agent and
18F-FDG-PET/CT. Similar results were obtained with both modalities [130]. P- and E-selectin are overexpressed on endothelial cells at sites of active inflammation, suggesting the future utility of this work in inflammatory GI disorders. While not practiced in the clinic at this time, immuno-PET techniques use radiolabeled proteins to target the upregulated immune cell presence or biochemical activity around inflamed tissues [97][98]. For example, antibody fragments targeting mouse CD4 cells, which are increasingly present at sites of GI inflammation, indicated the location and intensity of colorectal inflammation in mouse models [99]. Another modality undergoing preclinical assessment for the imaging of inflammation is multispectral optoacoustic tomography (MSOT). MSOT permits accurate, non-invasive imaging of the molecular characteristics of the disease through the visualization of exogenous or endogenous contrast agents [131][132]. Preclinical MSOT analysis has been shown to accurately detect in vivo colitis through measuring hypervascularity, which is common in inflamed tissue, and oxyhemoglobin levels in inoculated mouse models [133]. Alongside imaging modalities, new molecular targets are being investigated for improved diagnostic capabilities. α
F-FDG-PET/CT. Similar results were obtained with both modalities [149]. P- and E-selectin are overexpressed on endothelial cells at sites of active inflammation, suggesting the future utility of this work in inflammatory GI disorders. While not practiced in the clinic at this time, immuno-PET techniques use radiolabeled proteins to target the upregulated immune cell presence or biochemical activity around inflamed tissues [150,151]. For example, antibody fragments targeting mouse CD4 cells, which are increasingly present at sites of GI inflammation, indicated the location and intensity of colorectal inflammation in mouse models [152]. Another modality undergoing preclinical assessment for the imaging of inflammation is multispectral optoacoustic tomography (MSOT). MSOT permits accurate, non-invasive imaging of the molecular characteristics of the disease through the visualization of exogenous or endogenous contrast agents [153,154]. Preclinical MSOT analysis has been shown to accurately detect in vivo colitis through measuring hypervascularity, which is common in inflamed tissue, and oxyhemoglobin levels in inoculated mouse models [155]. Alongside imaging modalities, new molecular targets are being investigated for improved diagnostic capabilities. α
4
β
7
integrin is currently under investigation to determine whether it has the potential to increase the accuracy of IBD imaging. This is based on the increased presence of α
4
β
7 integrin on the activated lymphocytes found in inflamed tissue [134][135]. Endothelial growth factor receptor (EGFR) may be another target for imaging given its overexpression in inflamed and malignant cells. One study demonstrated the ability of radiolabeled anti-EGFR antibody fragments to successfully detect sites of IBD in mouse models, presenting greater target specificity and signal intensity relative to
integrin on the activated lymphocytes found in inflamed tissue [156,157]. Endothelial growth factor receptor (EGFR) may be another target for imaging given its overexpression in inflamed and malignant cells. One study demonstrated the ability of radiolabeled anti-EGFR antibody fragments to successfully detect sites of IBD in mouse models, presenting greater target specificity and signal intensity relative to
18F-FDG [100]. As new markers, probes, and imaging modalities are developed or found, accuracy in imaging diagnoses and tracking of GI inflammation is sure to improve.
F-FDG [158]. As new markers, probes, and imaging modalities are developed or found, accuracy in imaging diagnoses and tracking of GI inflammation is sure to improve.
