Aflatoxins family includes a great number of lipophilic molecules produced by aerobic micro fungi belonging to the genus Aspergillus. Aflatoxins are secondary metabolites produced by the microfungi.
Aflatoxins family includes a great number of lipophilic molecules produced by aerobic micro fungi belonging to the genus Aspergillus. Aflatoxins are secondary metabolites produced by the microfungi, and their presence in food is considered a real and often severe risk to consumers for their potential toxicity. Aflatoxins levels and presence in different foods matrices is a natural contamination as reported in the scientific literature. Different foodstuffs may be at risk of contamination by Aspergillus, and the accumulation of aflatoxins in the food chain can be a safety issue still open due to the difficulties connected to de contamination of the foodstuff, mainly crops, involved. Bioavailability and bioaccessibility of aflatoxins are also an issue, since these unwanted molecules can be assumed by the humans with the diet. Bioaccessibility, that deals with the fraction of micro-nutrients released from the food matrix during digestion and gastro-intestinal available for absorption, is related to aflatoxins bioaccessibility during the digestion process. Bioavailability of the aflatoxins assumed from the diet depends on their stability during digestion, since they are released from the food matrix (bioaccessibility) and on the efficiency of their passage through the gastro-intestinal mucosa. Despite of the practical difficulties in measuring the distribution and bioactivity of aflatoxins on a specific human body organ, the bioavailability is the fraction of an oral dose of a compound or precursor of an active metabolite that reaches the bloodstream. Bioaccessibility includes the entire sequence of events that take place during the digestion of food material that can be assimilated by the body through the epithelial cells of the gastro-intestinal mucosa. Aflatoxins are often present in very small amounts or in traces and, for this reason, advanced new chromatographic and spectrometric analytical methods are currently explored and used to detect even trace amounts, due to possible accumulation in the body due the carry over and to their liposolubility.
- Aflatoxins
- Secondary metabolites
- Contaminants
- Safety
- Crops
- Decontamination
- Health
1. Introduction
Aflatoxins in food is considered a real and often severe risk to consumers for their potential toxicity. Aflatoxins levels and presence in different foods matrices is a natural contamination as reported in the scientific literature. Different foodstuffs may be at risk of contamination by Aspergillus, and the accumulation of aflatoxins in the food chain can be a safety issue still open due to the difficulties connected to de contamination of the foodstuff, mainly crops, involved. Bioavailability and bioaccessibility of aflatoxins are also an issue, since these unwanted molecules can be assumed by the humans with the diet. Bioaccessibility, that deals with the fraction of micro-nutrients released from the food matrix during digestion and gastro-intestinal available for absorption, is related to aflatoxins bioaccessibility during the digestion process. Bioavailability of the aflatoxins assumed from the diet depends on their stability during digestion, since they are released from the food matrix (bioaccessibility) and on the efficiency of their passage through the gastro-intestinal mucosa. Despite of the practical difficulties in measuring the distribution and bioactivity of aflatoxins on a specific human body organ, the bioavailability is the fraction of an oral dose of a compound or precursor of an active metabolite that reaches the bloodstream. Bioaccessibility includes the entire sequence of events that take place during the digestion of food material that can be assimilated by the body through the epithelial cells of the gastro-intestinal mucosa. Aflatoxins are often present in very small amounts or in traces and, for this reason, advanced new chromatographic and spectrometric analytical methods are currently explored and used to detect even trace amounts, due to possible accumulation in the body due the carry over and to their liposolubility.
2. Characteristics, Health issues, and Decontamination
Aflatoxiare chemically derived from difuranocoumarin with a coumarins are chemically derived from difuranocoumarin with a coumarin nucnucleus-based bifuran group and a lactone ring (AFGs) or a pentanone ring (AFBs and AFMs). Aflatoxin contamination is highly influenced by environmental factors. Battilani et al., in 2016, reported that the risk of AF contamination can be increased in cereals following an elevation in the rate of temperature for every 2°C in European Countries, including Italy, Spain, Portugal, Turkey, Cyprus, Albania, Bulgaria, and Greece. Moreover, Moretti et al., in 2019 estimated that the risk of AF contamination in maize may be enhanced in Europe because of desired climatic conditions in the next thirty years. Aflatoxin-forming species require temperatures of 25–37 °C and moisture of 80%-85% for growth. Therefore, climate changes can alter the temperature and water activity (aw) of foods and feeds that affect the expression level of structural (aflD) and regulatory (aflS and aflR) genes and thus induce AF secretion by Aspergillus fungi. Reverse transcription polymerase chain reaction (RT-PCR) findings showed that the minimum and maximum expression levels of regulatory genes were at the temperatures of 20–37 °C and 28 °C, respectively, highlighting the importance of temperature in the synthesis of AF. Bernáldez et al. in 2017 found that the temperature of 30 °C and the water activity of 0.99 in maize were the optimal conditions for the growth of A. flavus according to the analysis of temperature and aw interaction affecting the expression level of aflR. In a study by Lv et al., the maximum production of AFB1 was at the temperature of 33°C and the water activity of 0.96 aw. Gizachew et al. in 2019 reported that the maximum level of AF production was at the temperature of 27 °C and the water activity of 0.90 aw in A. flavus and A. parasiticus in ground Nyjer seeds. pH is another factor affecting AF production, where maximum and minimum AF production occurs in acidic and basic conditions, respectively.
During the process of AF biosynthesis in crops by A. flavus and A. parasiticus, the primary substrate of hexanoyl is converted to polyketide using a polyketide synthase and two fatty acid synthases[1][2][3][4][5] [67–71], followed by the production of norsolorinic acid anthrone from the polyketide using polyketide synthase and then the conversion of norsolorinic acid anthrone to norsolorinic acid (NOR) as the first stable precursor of AF as shown in Figure 1. Then, NOR converted to averantin via reductase enzyme, see Figure 1 (1) and then 5′-hydroxyaverantin (HAVN) produced from averantin by monooxygenase enzyme see Figure 1 (2). Next, the HAVN forms 5′-oxoaverantin (OAVN) using dehydrogenase, see Figure 1 (3), and subsequently OAVN is converted to averufin (AVF) using cyclase, see Figure 1 (4), followed by the conversion of AVF to hydroxyversicolorone (HVN) via the Baeyer–Villiger reaction, see Figure 1 (5). After that, versiconal hemiacetal acetate (VHA) is formed via the oxidation of HVN, see Figure 1 (6) that is converted to versiconol acetate (VOAc) and then versiconol (VOH), see Figure 1 (7); the VOH then uses esterase to produce versiconal, see Figure 1 (8) that is subsequently converted to versicolorin B via cyclase, see Figure 1 (9), followed by the conversion of versicolorin B to versicolorin A and dimethyl-dihydro-sterigmatocystin (DMDHST) as shown Figure 1 (10); then the conversion of versicolorin A and DMDHST to dimethyl-sterigmatocystin (DMST) and dihydro-sterigmatocystin (DHST), respectively, see Figure 1 (11).
Next, O-methyltransferases plays central role in the biosynthesis of AFs to convert the intermediates of DMST and DHST to sterigmatocystin (ST) and dihydro-O-methylsterigmatocystin (DHOMST), respectively, as shown in Figure 1. Afterwards, O-methylsterigmatocystin (OMST) is produced from ST, see Figure 1 (13); finally, OMST and DHOMST lead to the production of AFs, as shown in Figure 1 (13b and 14). Over 20 AF have been identified so far, of which, aflatoxins B1 (AFB1), B2 (AFB2), G1 (AFG1) and G2 (AFG2) have been characterized under UV radiation where AFB1 and AFB2 exhibit a strong blue fluorescence while AFG1 and AFG2 show greenish yellow fluorescence (Figure 2). According to the evidence, only AFB1/B2 are produced by A. flavus and AFB1/ B2/G1/ G2 are produced by A. parasiticus, indicating a difference in the origin of AF. Aflatoxin M1 (AFM1) and AFM2 are not normally present in crops, but the metabolites of these compounds can be separated from the meat and milk products because of consuming AF-B1/AF-B2-contaminated feed. The toxicity levels of AF are different according to the following order of toxicity: AFG2< AFB2< AFG1< AFB1 [100]. Aflatoxins are soluble in organic solvents (e.g., chloroform and methanol) and slightly soluble in water, but insoluble in non-polar solutions (e.g., phenyl, cyclohexyl, ethyl, octyl, and octadecyl). Furthermore, the acid pKa of AF as a heat-stable compound is 17.787, with a molecular weight range of 312-346 Daltons.
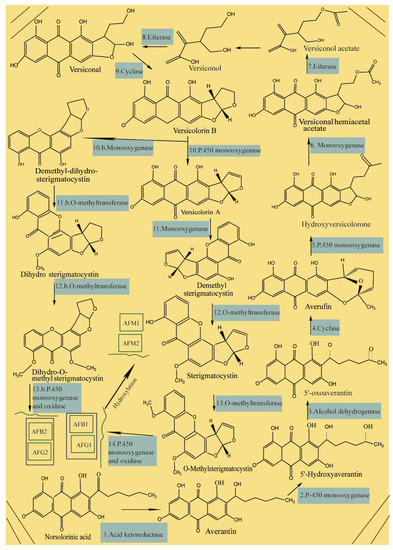
Figure 1. Overview of aflatoxins’ biosynthesis.
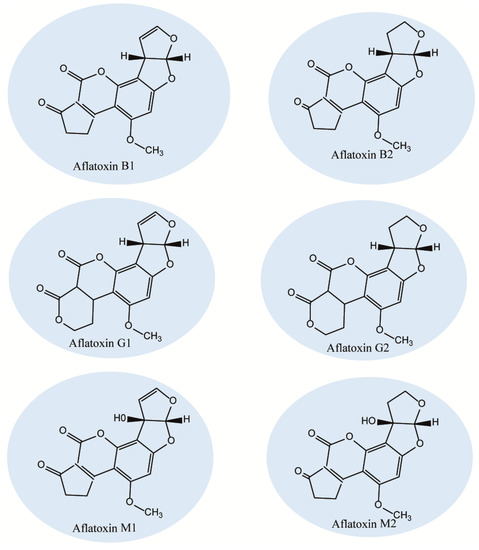
Figure 2. Chemical structures of group 1 carcinogenic aflatoxins.
Mycotoxin contamination is still a food safety global concern, the most toxic being the aflatoxins. Aflatoxins, during food transit or storage, directly or indirectly, may result in the contamination of foods, which may affects the liver, immune system, and reproduction after infiltration into human beings and animals. Detoxification methods are nowadays a big challenge since aflatoxin contamination of foods and feeds results in economic losses and may affects human and animal health, either directly or indirectly. Future research is recommended to focus on field-applicable new technologies for the control of aflatoxins with the aim of protecting human and nimal food/feed safety and health to promote food safety, increase awareness about public health and prevention, raise economic benefits, and decrease costs.
References
Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.;
Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change.
Sci. Rep. 2016, 6, 24328.
Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends
Food Sci. Technol. 2019, 84, 38-40.
Bernáldez, V.; Córdoba, J.J.; Magan, N.; Peromingo, B.; Rodríguez, A. The influence of ecophysiological
factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus.
LWT Food Sci. Technol. 2017, 83, 283-291.
Lv, C.; Jin, J.; Wang, P.; Dai, X.; Liu, Y.; Zheng, M.; Xing, F. Interaction of water activity and temperature on the growth, gene expression and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chem. 2019, 293, 472-478.
Gizachew, D.; Chang, C.H.; Szonyi, B.; De La Torre, S.; Ting, W.T.E. Aflatoxin B1 (AFB1) production by
Aspergillus flavus and Aspergillus parasiticus on ground Nyjer seeds: The effect of water activity and
temperature. Int. J. Food Microbiol. 2019, 296, 8-13.
