Preclinical evidence, accumulated over the past decade, indicates that the angiotensin II type 2 receptor (AT2R) stimulation exerts significant neuroprotective effects in various animal models of neuronal injury, notably in the central nervous system. Studies of brain AT2R distribution and function are outshining the recent findings about AT2R in peripheral sensory
neurons and pain modulation. While AT2R, as an atypical G protein-coupled receptor, and its related signaling are still under investigation, pharmacological studies have shown that stimulation of AT2R leads to neuritogenesis in vitro and in vivo. This review aims to report the evidence of potential neuroprotective and neuroregenerative roles of AT2R in the peripheral nervous system (PNS).
- angiotensin II type 2 receptor
- peripheral nervous system
- neuroregeneration
- neuroprotection
- pain
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
There is a large unmet clinical need for novel therapeutic approaches to reduce disabilities (sensory impairment, motor deficit) and to improve overall quality of life in patients with peripheral neuropathies. The development of effective therapeutic solutions is hampered by the vast etiological spectrum of underlying causes and the persistent gaps in our understanding of the pathophysiological processes involved in peripheral neuropathy. However, accumulating evidence suggests a significant contribution of the renin-angiotensin system (RAS) in both neuroprotection and neuroregeneration.
The RAS is well-described and known to regulate arterial blood pressure and ionic homeostasis [1]. The sequential enzymatic cascade of RAS is initiated with renin, a catalytic enzyme produced by kidney and secreted into the systemic circulation, which cleaves liver-derived angiotensinogen (AGT) to produce the decapeptide angiotensin I (Ang I). Pulmonary angiotensin converting enzyme (ACE) converts Ang I into angiotensin II (Ang II), the main active component of the RAS. Ang II binds with high and similar affinity to its two principal receptors in humans, the Ang II type 1 receptor (AT1R) and the Ang II type 2 receptor (AT2R) [2]. Other angiotensinogen-derived peptides have been described and are shown in Figure 1. The RAS was first described as an endocrine system. However, it is now considered to be a “ubiquitous” system that is expressed locally in many tissues exerting multiple autocrine/paracrine effects with implications in tissue physiology and homeostasis. The first demonstration of Ang II presence in tissues, in the arterial wall of sheep, dates back to 1980 [3]. Subsequent studies have quantified the synthesis of Ang II by the use of radiolabeled ligands in the heart, kidneys and adrenal glands [4–7][4][5][6][7]. Additionally, components of a local RAS have been detected in several tissues including skin, bone, adipose tissue and inflammatory cells [8–10][8][9][10]. The localization and effects of local RAS are described in further detail elsewhere [11].
AT1R and AT2R are both seven-transmembrane receptors displaying a similar affinity to Ang II although these two receptors differ in their amino acid sequence, tissue-specific expression and functional effects. The main role of AT2R is to inhibit actions mediated by AT1R by decreasing cell growth and proliferation while promoting cell differentiation, in addition to a vasodilatory action and a decrease in blood pressure [12]. As demonstrated in several studies, AT2R expression is temporally regulated. Ligand binding, in situ hybridization and autoradiography studies show that AT2R is widely expressed during fetal life, whereas its expression is maintained at low levels in all organs in adults, contrary to AT1R which is preferentially expressed in adults [13,14][13][14]. On the other hand, recent western blot and RT-PCR studies show that AT2R shows significantly higher expression in adult than in fetal and neonatal rodent tissues, with the exception of skin [15,16][15][16]. Such a discrepancy challenges the role of AT2R during development. Moreover, inverse expression profiles of AT1R and AT2R during development have been reported, with decreased expression of AT1R and an increased expression of AT2R in the adult versus fetal stage, suggesting a crucial interacting role between the two receptors [16]. Moreover, AT1R/AT2R heterodimerization has previously been shown in non-neuronal cell types, illustrating the strong link between these two receptors [17]. AT2R expression is also dramatically increased in tissue under pathological conditions, for example during nerve crush injury or inflammation, suggesting a possible role of AT2R in tissue repair and more particularly in neuroregeneration [18–20][18][19][20].
While numerous studies have focused on protective and regenerative properties of AT2R in the central nervous system [21–23][21][22][23], very few have considered a role for AT2R in the peripheral nervous system (PNS). Several reports have emphasized the role of peripheral AT2R in the modulation of pain [24–30][24][25][26][27][28][29][30]. However, the potential effects of Ang II/AT2R in neuroprotection/neuroregeneration in the PNS have been understudied and remains poorly understood. A few pharmacological studies, some from our team, highlight the beneficial effect of AT2R stimulation in a rodent model of traumatic- and drug-induced peripheral neuropathy [19,31–34][31][32][33][34]. Here, we aim to review the current knowledge on the role of AT2R and the therapeutic effect of respective agonists or antagonists in the treatment of various types of peripheral neuropathies. Basic information concerning the AT2R and its distribution within the PNS are introduced first.
Figure 1. Schematic representation of the renin-angiotensin system (RAS). Angiotensin (Ang) I is cleaved by angiotensin-converting enzyme (ACE) to Ang II which can be then cleaved to Ang III by aminopeptidase A, then further cleaved to Ang IV by aminopeptidase B. Ang I can also be cleaved by ACE2 to produce Ang (1–9) which can be cleaved into Ang (1–7) by ACE. Ang (1–7) can be directly generated from Ang II by ACE2. Ang (1–7) binds and activates the receptors Mas and Mas-related G protein-coupled receptor member D (MrgD), Ang IV binds to AT4R, Ang III activates AT1R and AT2R and Ang (1–9) directly activates AT2R. Functionally, it is possible to simplify the RAS into two distinctive pathways [12]. The first involves over-activation of AT1R by Ang II/Ang III, promoting cellular growth, vasoconstriction, fibrosis and inflammation. The second involves the interactions between AT2R and Ang II/Ang (1–9)/Ang III, but also Ang (1–7) and Mas/MrgD receptors, and Ang IV and AT4R, leading to vasodilatation and anti-proliferative, anti-fibrotic, anti-inflammatory effects.
2. Evidence for AT2R Expression in the Peripheral Nervous System
Over the past fifteen years, many studies have supported the concept of a local RAS and its potential role in the PNS, and particularly in the sensory nervous system [27,35–38][35][36][37][38]. Nevertheless, the distribution and expression level of AT2R in the PNS has been subject of controversy.
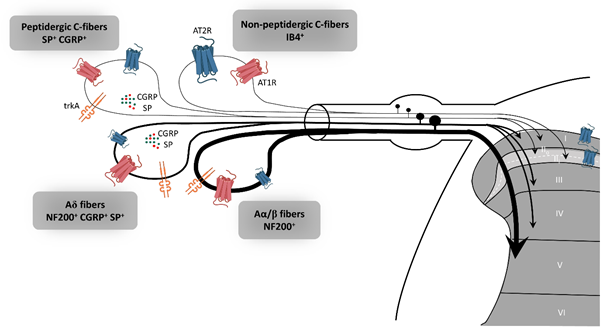
In rat dorsal root ganglion (DRG), AT2R expression, at the mRNA and protein levels, is tightly regulated throughout development to switch later to a restricted subpopulation of C-nociceptor neurons during adult life [39] [39]. Recently, preferential expression of AT2R in non-peptidergic isolectin B4 (IB4+) C-nociceptor neurons was confirmed in adult rat DRG neurons [18]. The authors showed that AT2R is also expressed by some peptidergic small C- and medium Aδ-neurons as well as by a few large Aα- and Aβ-neurons, and that almost all AT2R+ DRG neurons co-expressed AT1R [18] (Figure 2). In humans, positive AT2R-immunolabelling was also shown in small-diameter DRG neurons and in nerve endings in the sub-epidermis and dermis, in urinary bladder, in vestibule and in the myenteric plexus [27,38,40][40]. This expression profile of AT2R in the PNS suggests its involvement in the development of sensory and nociceptive functions. However, immunohistochemistry (IHC)-based results must be viewed with caution due to the poor specificity of commercially-available AT2R antibodies [24,40][40]. One study has demonstrated the specificity of one commercial AT2R antibody, ab19134 (Abcam), using AT2R-expressing HEK-cells vs. non-transfected HEK-cells [41]. Nevertheless, recently, a research group reported no difference in AT2R signal intensity in DRG sections from wild type- and agtr2 (the AT2R gene) KO-mice using these same AT2R antibodies [24].
Figure 2. Expression of AT2R in the sensory peripheral nervous system. According to the most recent immunohistochemistry study on the expression of AT2R in DRG and in the spinal cord of rat, AT2R would be expressed by almost all types of sensory neurons, though to different degrees [18]. Non-peptidergic C-nociceptors expressing IB4 are strongly stained for AT2R. Some small and medium DRG neurons co-expressed AT2R and trkA, a marker of nociceptive neurons (peptidergic C and Aδ neurons). The few large neurons which express AT2R are stained for NF200 and trkA, markers of Aα/β nociceptive neurons. Most ATR2+ neurons are also AT1R+. AT1R: angiotensin II type 1 receptor, AT2R: angiotensin II type 2 receptor, CGRP: calcitonin gene-related peptide, DRG: dorsal root ganglion, IB4: isolectin 4, NF200: neurofilament 200, SP: substance P, trkA: tropomyosin receptor kinase type A.
At the mRNA level, AT2R is upregulated in pathological conditions in the PNS, as has been shown in other tissues such as infarcted heart and regenerating skeletal muscle [42,43][42][43]. In response to nerve axotomy and crush injury, AT2R mRNA levels are strongly increased in adult DRG and sciatic nerve fibers in rats [44]. A role for AT2R in Schwann cell (SC)-mediated healing actions has been evoked since the kinetics of AT2R mRNA expression is closely linked to the kinetics of SCs differentiation during nerve recovery. It is important to note that quantification of total AT2R mRNA from DRG encompasses different cell types (i.e., sensory neurons, satellite cells, immune cells, and SCs). SCs themselves have been shown to express AT1R and AT2R in vitro and in fresh samples of rat sciatic nerve, with a higher proportion of AT2R than AT1R [45][45][46].
Recently, Shepherd et al. demonstrated that mouse and human DRG neurons do not express AT2R [24]. By combining different technical approaches to investigate the expression of AT2R, the authors pointed out the lack of AT2R expression in mouse DRG neurons, either at the protein- or mRNA-level. In line with this, Ang II did not induce either calcium influx and electrophysiological responses or intracellular signaling in cultured primary mouse DRG neuron. In addition, a lack of GFP signal was observed in DRG sections from AT2R-EGFP reporter mice, suggesting that agtr2 was not expressed either in neurons or in non-neuronal cells of mouse DRG. However, they revealed that peripheral macrophages express a functional AT2R and these could be implicated in Ang II-induced peripheral mechanical pain sensitization [24]. One could envision a paracrine system between DRG neurons that synthesize Ang II and non-neuronal AT2R+ cells such as SCs and/or immune cells that are recruited only during nerve injury [46]. In this respect, the CD3+ T-cells which are involved in mechanical pain in response to chronic constriction injury (CCI) [41] might express AT2R. This cell-cell dialogue, mediated by AT2R in the PNS, could play a key role in neuroprotective and neuroregenerative processes.
With regard to the expression of AT2R by DRG neurons, it appears that species differences likely exist. Most studies conducted in rats have found AT2R to be expressed in DRG neurons while studies performed in mouse DRG neurons have given opposite results [24]. One explanation might be that two isoforms of AT2R exist in mice, as is the case for AT1R (AT1a and AT1b) in mice and rats, and as has already been suggested in the rat brain [47]. In this respect, AT2R-antibodies could detect another isoform of AT2R, which could be expressed in DRG neurons of AT2R KO-mice. It is also important to note that AT2R-deficient mice often only have part of the receptor affected, thus potentially allowing the expression of a truncated form of the protein, which is possibly detected by some antibodies [48,49][48][49]. Another hypothesis is that AT2R mRNA is synthesized in DRG neurons under pathological conditions (lesion, crush, section) and transported along microtubules to nerve terminals where it is then translated by a local system [50]. This would provide a rationale for the discrepancies in results obtained from IHC on cultured DRG and DRG sections. Further studies are required to elucidate the questions concerning AT2R expression in the PNS and to understand their function.
3. AT2R Signaling
The AT2R gene was cloned in the early 90′s and the receptor has been attributed numerous functions. However, its signaling pathway remains difficult to elucidate [51,52] [51][52]. AT2R belongs to the G protein-coupled receptor superfamily (GPCR). However, aside from signaling through G-protein-dependent mechanisms, activation of this receptor by G protein-independent intracellular signaling in neurons makes it an “atypical” or “non-canonical” GPCR. The recently described crystal structure of human AT2R bound to either Ang II or an AT2R-selective ligand, allowed the receptor to be captured in an active-like conformation and provided structural insights into a moderate coupling to G proteins [53,54][53][54]. Continuing efforts investigating the conformational arrangement of this receptor are necessary to complete our understanding of AT2R activation and signaling and will aid in better design of targeted compounds [55]. In the following section, we will specifically review the role of AT2R signaling implicate in neuronal survival and neurite outgrowth and summarize the current findings in a schematic (Figure 3).
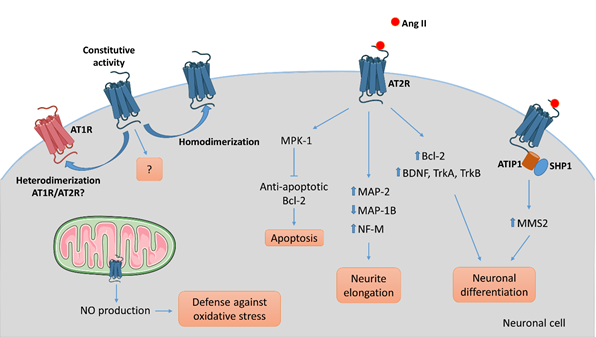
To understand AT2R signal transduction, several cellular tools have been used. Among them, the rat pheochromocytoma PC12W cell line, of neuronal origin, has been particularly useful since these cells mostly express AT2R rather than AT1R. Several studies, focused on the effect of Ang II on PC12W cells, have demonstrated that AT2R mediates programmed cell death through inactivation of mitogen-activated protein kinase (MAPK) inhibition of the anti-apoptotic Bcl-2 protein resulting eventually in induction of apoptosis [56,57][56][57]. This apoptotic function of AT2R was once hypothesized to be involved in developmental biology and pathophysiology. However, it was later demonstrated that AT2R stimulation by C21, a specific agonist [58], induced RNA expression of Bcl-2 and increased the level of neurotrophins (BDNF, TrkA and TrkB) in vitro in primary neurons and in vivo in a model of spinal cord injury [59]. In quiescent PC12W cells, AT2R stimulation by Ang II treatment leads to neurite formation [60]. In this case, the signaling involves an increase in polymerized β-tubulin, upregulation of microtubule-associated protein (MAP)-2 and down-regulation of MAP-1B levels. Similar observations were made in PC12W cells differentiated by nerve growth factor (NGF) and in undifferentiated NG108-15 cells (mouse neuroblastoma x rat glioma hybrid cell line) [60,61][60][61]. MAP-2 proteins are known to interact with microtubules, neurofilaments and actin, and contribute to the maintenance of the neuronal cytoskeleton. Similarly, MAP-1B regulates branching and neurite direction during DRG neuron regeneration [62]. A significant decrease in MAP-2 expression was observed in DRG following CCI to the sciatic nerve in rats, suggesting the involvement of MAP-2 in the early response to nerve injury [63]. Ang II treatment also diminishes the expression of neurofilament-M at the protein and mRNA levels in PC12W cells, and this effect is suppressed by preventive treatment with PD123177 (an AT2R antagonist) [64]. Thus, AT2R stimulation in vitro decreases proliferation and leads to neuronal differentiation and to anarchic neurite elongation via reorganization of cytoskeletal components. In undifferentiated NG108-15 and PC12W cells, both cell types expressing AT2R but not AT1R, Ang II-induced neuronal differentiation, and thus neurite elongation, is counteracted by AT1R stimulation in differentiated neuronal cells expressing AT1R [61]. Therefore, these data highlight the precise regulation existing between AT2R and AT1R during neuronal differentiation and neurite elongation. In NG108-15 cells, Ang II/AT2R interaction leads to neurite outgrowth through a sustained activation of p42/p44 MAPK and phosphorylation of trkA [65,66][65][66]. The link between AT2R and activation of p42/p44 MAPK leading to neurite outgrowth was further confirmed in cultured adult rat DRG neurons, another optimal in vitro model in which to investigate the effects of AT2R modulation on PNS neurons [67]. In this later study, neurons treated with Ang II presented denser and much longer neurites than controls, confirming results obtained in neuronal cell lines.
It has also been demonstrated that the morphological differentiation induced by Ang II/AT2R involves an increase in nitric oxide (NO) production in the NG108-05 cell line [68]. An increase of neuronal NO synthase (nNOS) was observed in cultures of rat DRG neurons under stress conditions [69]. The neuroprotective role of NO has already been described in vitro and in vivo in rat DRG neurons[70][71] [69–71]. In vivo, inhibition of nNOS aggravates DRG neuron injuries, such as in a rat model of sciatic nerve transection, suggesting a neuroprotective role of NO [71]. A more detailed review of the role of NO in neuronal proliferation, survival and differentiation can be found elsewhere [72]. A functional mitochondrial angiotensin system has been reported in several cellular types, including neuronal cells [73]. AT2R was localized on the inner mitochondrial membrane by immunogold electron microscopy, and its stimulation increased NO production, thus decreasing mitochondrial respiration [74]. This phenomenon could represent a defense against oxidative stress, and a neuroprotective function of AT2R. However, the presence of AT2R in mitochondria from PNS neurons has not thus far been investigated.
In 2007, Li et al., showed, in neuronal cells, that AT2R interacts with ATIP1, the first member of the AT2-interacting protein (ATIP) family, to induce neuronal differentiation via upregulation of methane methylsulfonate-sensitive 2 (MMS2) [75]. ATIPs are a family of proteins encoded by alternative splicing of a single gene called mtus1 (Microtubule associated tumor suppressor 1); ATIP1, ATIP3 and ATIP4 being the major isoforms. ATIP1 and ATIP3 are widely distributed whereas ATIP4 is restricted to the central nervous system [76]. The ATIPs are cytosolic proteins that constitutively interact with the C-terminal domain of AT2R and are involved in intracellular transport and signaling pathways of AT2R, depending on the cell-type and on the isoform [77,78][77][78]. ATBP50, the murine ATIP1, was identified as a Golgi-associated protein involved in the transport of the AT2R to the cell membrane [78][78]. In neuronal cells, AT2R activation induces the ATIP1-Src homology phosphatase 1 (SHP1) complex, leading to transcriptional activation of the DNA repair enzyme MMS2, and then induction of neuronal differentiation [75].
Figure 3. AT2R-mediated intracellular signaling pathways involved in neuronal cells. Several intracellular signaling pathways are associated with AT2R activation in neuronal cells leading to apoptosis via activation of the phosphatase MPK-1, neurite elongation via reorganization of the cytoskeleton, or neuronal differentiation in part via upregulation of growth-factors, depending on the cell type, the context, and the environment. To date, ATIP1 is identified as a partner of AT2R involved in intracellular signaling pathway and trafficking in neuronal cells. While the evidence of an ATIP/AT2R complex comes from experiments on central nervous system neurons, we could hypothesize that a similar system exists in PNS neurons. AT2R signaling may antagonize AT1R-mediated signaling by a direct heterodimerization between the two. As well, homodimerization of AT2R might result in ligand-independent signaling.
While progress has been made in understanding AT2R signaling, this receptor remains an enigma. Other review articles have emphasized in great detail the limitations of AT2R signaling studies [12,79–81][79][80][81].
References
- Rettig, R.; Healy, D.P.; Printz, M.P. Cardiovascular Effects of Microinjections of Angiotensin II into the Nucleus Tractus Solitarii. Brain research 1986, 364, 233–240.
- Bosnyak, S.; Jones, E.S.; Christopoulos, A.; Aguilar, M.I.; Thomas, W.G.; Widdop, R.E. Relative Affinity of Angiotensin Peptides and Novel Ligands at AT1 and AT2 Receptors. Clinical science (London, England : 1979) 2011, 121, 297–303, doi:10.1042/CS20110036.
- Fei, D.T.; Coghlan, J.P.; Fernley, R.T.; Scoggins, B.A.; Tregear, G.W. Peripheral Production of Angiotensin II and III in Sheep. Circ Res 1980, 46, I135-137.
- Karlsson, C.; Lindell, K.; Ottosson, M.; Carlsson, B.R.; Carlsson, L.M.S. Human Adipose Tissue Expresses Angiotensinogen and Enzymes Required for Its Conversion to Angiotensin II. 1998, 83, 5.
- van Kats, J.P.; Danser, A.H.; van Meegen, J.R.; Sassen, L.M.; Verdouw, P.D.; Schalekamp, M.A. Angiotensin Production by the Heart: A Quantitative Study in Pigs with the Use of Radiolabeled Angiotensin Infusions. Circulation 1998, 98, 73–81, doi:10.1161/01.cir.98.1.73.
- van Kats, J.P.; van Meegen, J.R.; Verdouw, P.D.; Duncker, D.J.; Schalekamp, M.A.; Danser, A.H. Subcellular Localization of Angiotensin II in Kidney and Adrenal. J Hypertens 2001, 19, 583–589, doi:10.1097/00004872-200103001-00010.
- van Thiel Bibi S.; Góes Martini Alexandre; te Riet Luuk; Severs David; Uijl Estrellita; Garrelds Ingrid M.; Leijten Frank P.J.; van der Pluijm Ingrid; Essers Jeroen; Qadri Fatimunnisa; et al. Brain Renin–Angiotensin System. Hypertension 2017, 69, 1136–1144, doi:10.1161/HYPERTENSIONAHA.116.08922.
- Jurewicz, M.; McDermott, D.H.; Sechler, J.M.; Tinckam, K.; Takakura, A.; Carpenter, C.B.; Milford, E.; Abdi, R. Human T and Natural Killer Cells Possess a Functional Renin-Angiotensin System: Further Mechanisms of Angiotensin II-Induced Inflammation. J Am Soc Nephrol 2007, 18, 1093–1102, doi:10.1681/ASN.2006070707.
- Steckelings, U.M.; Wollschläger, T.; Peters, J.; Henz, B.M.; Hermes, B.; Artuc, M. Human Skin: Source of and Target Organ for Angiotensin II. Experimental Dermatology 2004, 13, 148–154, doi:10.1111/j.0906-6705.2004.0139.x.
- Nehme, A.; Zouein, F.A.; Zayeri, Z.D.; Zibara, K. An Update on the Tissue Renin Angiotensin System and Its Role in Physiology and Pathology. JCDD 2019, 6, 14, doi:10.3390/jcdd6020014.
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of Local Renin-Angiotensin Systems. Physiol Rev 2006, 86, 747–803, doi:10.1152/physrev.00036.2005.
- Nouet, S.; Nahmias, C. Signal Transduction from the Angiotensin II AT2 Receptor. Trends in Endocrinology & Metabolism 2000, 11, 1–6, doi:10.1016/S1043-2760(99)00205-2.
- Grady, E.F.; Sechi, L.A.; Griffin, C.A.; Schambelan, M.; Kalinyak, J.E. Expression of AT2 Receptors in the Developing Rat Fetus. J Clin Invest 1991, 88, 921–933, doi:10.1172/JCI115395.
- Lenkei, Z.; Palkovits, M.; Corvol, P.; Llorens-Cortès, C. Expression of Angiotensin Type-1 (AT1) and Type-2 (AT2) Receptor MRNAs in the Adult Rat Brain: A Functional Neuroanatomical Review. Frontiers in Neuroendocrinology 1997, 18, 383–439, doi:10.1006/frne.1997.0155.
- Gao, J.; Chao, J.; Parbhu, K.-J.K.; Yu, L.; Xiao, L.; Gao, F.; Gao, L. Ontogeny of Angiotensin Type 2 and Type 1 Receptor Expression in Mice. J Renin Angiotensin Aldosterone Syst 2012, 13, 341–352, doi:10.1177/1470320312443720.
- Yu, L.; Shao, C.; Gao, L. Developmental Expression Patterns for Angiotensin Receptors in Mouse Skin and Brain. Journal of the renin-angiotensin-aldosterone system : JRAAS 2014, 15, 139–49, doi:10.1177/1470320312467557.
- AbdAlla, S.; Lother, H.; Abdel-tawab, A.M.; Quitterer, U. The Angiotensin II AT2 Receptor Is an AT1 Receptor Antagonist. J Biol Chem 2001, 276, 39721–39726, doi:10.1074/jbc.M105253200.
- Benitez, S.G.; Seltzer, A.M.; Messina, D.N.; Foscolo, M.R.; Patterson, S.I.; Acosta, C.G. Cutaneous Inflammation Differentially Regulates the Expression and Function of Angiotensin-II Types 1 and 2 Receptors in Rat Primary Sensory Neurons. J Neurochem 2020, 152, 675–696, doi:10.1111/jnc.14848.
- Bessaguet, F.; Danigo, A.; Bouchenaki, H.; Duchesne, M.; Magy, L.; Richard, L.; Sturtz, F.; Desmoulière, A.; Demiot, C. Neuroprotective Effect of Angiotensin II Type 2 Receptor Stimulation in Vincristine-Induced Mechanical Allodynia. PAIN 2018, 159, 2538–2546, doi:10.1097/j.pain.0000000000001361.
- Lucius, R.; Gallinat, S.; Rosenstiel, P.; Herdegen, T.; Sievers, J.; Unger, T. The Angiotensin II Type 2 (AT2) Receptor Promotes Axonal Regeneration in the Optic Nerve of Adult Rats. The Journal of experimental medicine 1998, 188, 661–70.
- Guimond, M.-O.; Gallo-Payet, N. How Does Angiotensin AT2 Receptor Activation Help Neuronal Differentiation and Improve Neuronal Pathological Situations? Frontiers in Endocrinology 2012, 3, 164, doi:10.3389/fendo.2012.00164.
- Namsolleck, P.; Recarti, C.; Foulquier, S.; Steckelings, U.M.; Unger, T. AT(2) Receptor and Tissue Injury: Therapeutic Implications. Current hypertension reports 2014, 16, 416, doi:10.1007/s11906-013-0416-6.
- Wilms, H.; Rosenstiel, P.; Unger, T.; Deuschl, G.; Lucius, R. Neuroprotection with Angiotensin Receptor Antagonists: A Review of the Evidence and Potential Mechanisms. American Journal of Cardiovascular Drugs 2005, 5, 245–253.
- Shepherd, A.J.; Copits, B.A.; Mickle, A.D.; Karlsson, P.; Kadunganattil, S.; Haroutounian, S.; Tadinada, S.M.; de Kloet, A.D.; Valtcheva, M. V; McIlvried, L.A.; et al. Angiotensin II Triggers Peripheral Macrophage-to-Sensory Neuron Redox Crosstalk to Elicit Pain. The Journal of Neuroscience 2018, 38, 7032–7057, doi:10.1523/jneurosci.3542-17.2018.
- Shepherd, A.J.; Mickle, A.D.; Golden, J.P.; Mack, M.R.; Halabi, C.M.; de Kloet, A.D.; Samineni, V.K.; Kim, B.S.; Krause, E.G.; Gereau, R.W.; et al. Macrophage Angiotensin II Type 2 Receptor Triggers Neuropathic Pain. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, E8057–E8066, doi:10.1073/pnas.1721815115.
- Smith, A.K.; O’Hara, C.L.; Stucky, C.L. Mechanical Sensitization of Cutaneous Sensory Fibers in the Spared Nerve Injury Mouse Model. Molecular Pain 2013, 9, 1744-8069-9–61, doi:10.1186/1744-8069-9-61.
- Anand, U.; Yiangou, Y.; Sinisi, M.; Fox, M.; MacQuillan, A.; Quick, T.; Korchev, Y.E.; Bountra, C.; McCarthy, T.; Anand, P. Mechanisms Underlying Clinical Efficacy of Angiotensin II Type 2 Receptor (AT2R) Antagonist EMA401 in Neuropathic Pain: Clinical Tissue and in Vitro Studies. Molecular Pain 2015, 11, 38, doi:10.1186/s12990-015-0038-x.
- Rice, A.S.C.; Dworkin, R.H.; McCarthy, T.D.; Anand, P.; Bountra, C.; McCloud, P.I.; Hill, J.; Cutter, G.; Kitson, G.; Desem, N.; et al. EMA401, an Orally Administered Highly Selective Angiotensin II Type 2 Receptor Antagonist, as a Novel Treatment for Postherpetic Neuralgia: A Randomised, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. The Lancet 2014, 383, 1637–1647, doi:10.1016/S0140-6736(13)62337-5.
- Marion, E.; Song, O.R.; Christophe, T.; Babonneau, J.; Fenistein, D.; Eyer, J.; Letournel, F.; Henrion, D.; Clere, N.; Paille, V.; et al. Mycobacterial Toxin Induces Analgesia in Buruli Ulcer by Targeting the Angiotensin Pathways. Cell 2014, 157, 1565–1576, doi:10.1016/j.cell.2014.04.040.
- Song, O.-R.; Kim, H.-B.; Jouny, S.; Ricard, I.; Vandeputte, A.; Deboosere, N.; Marion, E.; Queval, C.J.; Lesport, P.; Bourinet, E.; et al. A Bacterial Toxin with Analgesic Properties: Hyperpolarization of DRG Neurons by Mycolactone. Toxins (Basel) 2017, 9, doi:10.3390/toxins9070227.
- Bessaguet, F.; Danigo, A.; Magy, L.; Sturtz, F.; Desmoulière, A.; Demiot, C. Candesartan Prevents Resiniferatoxin-Induced Sensory Small-Fiber Neuropathy in Mice by Promoting Angiotensin II-Mediated AT2 Receptor Stimulation. Neuropharmacology 2017, 126, 142–150, doi:10.1016/j.neuropharm.2017.08.039.
- Hashikawa-Hobara, N.; Hashikawa, N.; Inoue, Y.; Sanda, H.; Zamami, Y.; Takatori, S.; Kawasaki, H. Candesartan Cilexetil Improves Angiotensin II Type 2 Receptor-Mediated Neurite Outgrowth via the PI3K-Akt Pathway in Fructose-Induced Insulin-Resistant Rats. Diabetes 2012, 61, 925–932, doi:10.2337/db11-1468.
- Hobara, N.; Goda, M.; Yoshida, N.; Takatori, S.; Kitamura, Y.; Mio, M.; Kawasaki, H. Angiotensin II Type 2 Receptors Facilitate Reinnervation of Phenol-Lesioned Vascular Calcitonin Gene-Related Peptide-Containing Nerves in Rat Mesenteric Arteries. Neuroscience 2007, 150, 730–741, doi:10.1016/j.neuroscience.2007.09.026.
- Reinecke, K.; Lucius, R.; Reinecke, A.; Rickert, U.; Herdegen, T.; Unger, T. Angiotensin II Accelerates Functional Recovery in the Rat Sciatic Nerve in Vivo: Role of the AT2 Receptor and the Transcription Factor NF-KappaB. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2003, 17, 2094–2096, doi:10.1096/fj.02-1193fje.
- Pavel, J.; Tang, H.; Brimijoin, S.; Moughamian, A.; Nishioku, T.; Benicky, J.; Saavedra, J.M. Expression and Transport of Angiotensin II AT1 Receptors in Spinal Cord, Dorsal Root Ganglia and Sciatic Nerve of the Rat. Brain Research 2008, 1246, 111–122, doi:10.1016/j.brainres.2008.09.099.
- Patil, J.; Schwab, A.; Nussberger, J.; Schaffner, T.; Saavedra, J.M.; Imboden, H. Intraneuronal Angiotensinergic System in Rat and Human Dorsal Root Ganglia. Regulatory Peptides 2010, 162, 90–98, doi:10.1016/j.regpep.2010.03.004.
- Chakrabarty, A.; Blacklock, A.; Svojanovsky, S.; Smith, P.G. Estrogen Elicits Dorsal Root Ganglion Axon Sprouting via a Renin-Angiotensin System. Endocrinology 2008, 149, 3452–3460, doi:10.1210/en.2008-0061.
- Liao, Z.; Chakrabarty, A.; Mu, Y.; Bhattacherjee, A.; Goestch, M.; Leclair, C.M.; Smith, P.G. A Local Inflammatory Renin-Angiotensin System Drives Sensory Axon Sprouting in Provoked Vestibulodynia. Journal of Pain 2017, 18, 511–525, doi:10.1016/j.jpain.2016.12.008.
- Benitez, S.; Seltzer, A.; Acosta, C. Nociceptor-like Rat Dorsal Root Ganglion Neurons Express the Angiotensin-II AT2 Receptor throughout Development. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2017, 56, 10–17, doi:10.1016/j.ijdevneu.2016.11.001.
- Hafko, R.; Villapol, S.; Nostramo, R.; Symes, A.; Sabban, E.L. Commercially Available Angiotensin II At 2 Receptor Antibodies Are Nonspecific. PLoS ONE 2013, 8, 69234, doi:10.1371/journal.pone.0069234.
- Khan, N.; Muralidharan, A.; Smith, M.T. Attenuation of the Infiltration of Angiotensin II Expressing CD3+ T-Cells and the Modulation of Nerve Growth Factor in Lumbar Dorsal Root Ganglia – A Possible Mechanism Underpinning Analgesia Produced by EMA300, An Angiotensin II Type 2 (AT2) Receptor Ant. Frontiers in Molecular Neuroscience 2017, 10, doi:10.3389/fnmol.2017.00389.
- Nio, Y.; Matsubara, H.; Murasawa, S.; Kanasaki, M.; Inada, M. Regulation of Gene Transcription of Angiotensin II Receptor Subtypes in Myocardial Infarction. J Clin Invest 1995, 95, 46–54, doi:10.1172/JCI117675.
- Yoshida, T.; Huq, T.S.; Delafontaine, P. Angiotensin Type 2 Receptor Signaling in Satellite Cells Potentiates Skeletal Muscle Regeneration. J Biol Chem 2014, 289, 26239–26248, doi:10.1074/jbc.M114.585521.
- Gallinat, S.; Yu, M.; Dorst, A.; Unger, T.; Herdegen, T. Sciatic Nerve Transection Evokes Lasting Up-Regulation of Angiotensin AT2 and AT1 Receptor MRNA in Adult Rat Dorsal Root Ganglia and Sciatic Nerves. Molecular Brain Research 1998, 57, 111–122, doi:10.1016/S0169-328X(98)00079-5.
- Bleuel, A.; de Gasparo, M.; Whitebread, S.; Püttner, I.; Monard, D. Regulation of Protease Nexin-1 Expression in Cultured Schwann Cells Is Mediated by Angiotensin II Receptors. The Journal of neuroscience 1995, 15, 750–761.
- Hu, P.; Bembrick, A.L.; Keay, K.A.; McLachlan, E.M. Immune Cell Involvement in Dorsal Root Ganglia and Spinal Cord after Chronic Constriction or Transection of the Rat Sciatic Nerve. Brain, Behavior, and Immunity 2007, 21, 599–616, doi:10.1016/j.bbi.2006.10.013.
- Tsutsumi, K.; Saavedra, J.M. Heterogeneity of Angiotensin II AT2 Receptors in the Rat Brain. Molecular pharmacology 1992, 41, 290–7.
- Hein, L.; Barsh, G.S.; Pratt, R.E.; Dzau, V.J.; Kobilka, B.K. Behavioural and Cardiovascular Effects of Disrupting the Angiotensin II Type-2 Receptor in Mice. Nature 1995, 377, 744–747, doi:10.1038/377744a0.
- Ichiki, T.; Labosky, P.A.; Shiota, C.; Okuyama, S.; Imagawa, Y.; Fogo, A.; Niimura, F.; Ichikawa, I.; Hogan, B.L.; Inagami, T. Effects on Blood Pressure and Exploratory Behaviour of Mice Lacking Angiotensin II Type-2 Receptor. Nature 1995, 377, 748–750, doi:10.1038/377748a0.
- Wong, H.H.W.; Lin, J.Q.; Ströhl, F.; Roque, C.G.; Cioni, J.M.; Cagnetta, R.; Turner-Bridger, B.; Laine, R.F.; Harris, W.A.; Kaminski, C.F.; et al. RNA Docking and Local Translation Regulate Site-Specific Axon Remodeling In Vivo. Neuron 2017, 95, 852-868.e8, doi:10.1016/j.neuron.2017.07.016.
- Nakajima, M.; Mukoyama, M.; Pratt, R.E.; Horiuchi, M.; Dzau, V.J. Cloning of CDNA and Analysis of the Gene for Mouse Angiotensin II Type 2 Receptor. Biochemical and Biophysical Research Communications 1993, 197, 393–399, doi:10.1006/bbrc.1993.2492.
- Mukoyama, M.; Nakajima, M.; Horiuchi, M.; Sasamura, H.; Pratt, R.E.; Dzau, V.J. Expression Cloning of Type 2 Angiotensin II Receptor Reveals a Unique Class of Seven-Transmembrane Receptors. The Journal of biological chemistry 1993, 268, 24539–42.
- Asada, H.; Horita, S.; Hirata, K.; Shiroishi, M.; Shiimura, Y.; Iwanari, H.; Hamakubo, T.; Shimamura, T.; Nomura, N.; Kusano-Arai, O.; et al. Crystal Structure of the Human Angiotensin II Type 2 Receptor Bound to an Angiotensin II Analog. Nature Structural & Molecular Biology 2018, 25, 570–576, doi:10.1038/s41594-018-0079-8.
- Asada, H.; Inoue, A.; Ngako Kadji, F.M.; Hirata, K.; Shiimura, Y.; Im, D.; Shimamura, T.; Nomura, N.; Iwanari, H.; Hamakubo, T.; et al. The Crystal Structure of Angiotensin II Type 2 Receptor with Endogenous Peptide Hormone. Structure 2020, 28, 418-425.e4, doi:10.1016/j.str.2019.12.003.
- Sadybekov, A.; Katritch, V. Breaking the Enigma Code of Angiotensin II Type 2 Receptor Signaling. Structure 2020, 28, 390–392, doi:10.1016/j.str.2020.03.004.
- Yamada, T.; Horiuchi, M.; Dzau, V.J. Angiotensin II Type 2 Receptor Mediates Programmed Cell Death. Proceedings of the National Academy of Sciences of the United States of America 1996, 93, 156–160, doi:10.1073/pnas.93.1.156.
- Horiuchi, M.; Hayashida, W.; Kambe, T.; Yamada, T.; Dzau, V.J. Angiotensin Type 2 Receptor Dephosphorylates Bcl-2 by Activating Mitogen-Activated Protein Kinase Phosphatase-1 and Induces Apoptosis. Journal of Biological Chemistry 1997, 272, 19022–19026, doi:10.1074/jbc.272.30.19022.
- Wan, Y.; Wallinder, C.; Plouffe, B.; Beaudry, H.; Mahalingam, a. K.; Wu, X.; Johansson, B.; Holm, M.; Botoros, M.; Karlén, A.; et al. Design, Synthesis, and Biological Evaluation, of the First Selective Nonpeptide AT2 Receptor Agonist. Journal of Medicinal Chemistry 2004, 47, 5995–6008, doi:10.1021/jm049715t.
- Namsolleck, P.; Boato, F.; Schwengel, K.; Paulis, L.; Matho, K.S.; Geurts, N.; Thöne-Reineke, C.; Lucht, K.; Seidel, K.; Hallberg, A.; et al. AT2-Receptor Stimulation Enhances Axonal Plasticity after Spinal Cord Injury by Upregulating BDNF Expression. Neurobiology of Disease 2013, 51, 177–191, doi:10.1016/j.nbd.2012.11.008.
- Stroth, U.; Meffert, S.; Gallinat, S.; Unger, T. Angiotensin II and NGF Differentially Influence Microtubule Proteins in PC12W Cells: Role of the AT2 Receptor. Molecular Brain Research 1998, 53, 187–195, doi:10.1016/S0169-328X(97)00298-2.
- Laflamme, L.; de Gasparo, M.; Gallo, J.-M.; Payet, M.D.; Gallo-Payet, N. Angiotensin II Induction of Neurite Outgrowth by AT 2 Receptors in NG108-15 Cells. Journal of Biological Chemistry 1996, 271, 22729–22735, doi:10.1074/jbc.271.37.22729.
- Bouquet, C.; Soares, S.; Boxberg, Y. von; Ravaille-Veron, M.; Propst, F.; Nothias, F. Microtubule-Associated Protein 1B Controls Directionality of Growth Cone Migration and Axonal Branching in Regeneration of Adult Dorsal Root Ganglia Neurons. J. Neurosci. 2004, 24, 7204–7213, doi:10.1523/JNEUROSCI.2254-04.2004.
- Cao, M.; Ji, F.; Liu, L.; Li, F. Expression Changes of Parvalbumin and Microtubule- Associated Protein 2 Induced by Chronic Constriction Injury in Rat Dorsal Root Ganglia. Chinese Medical Journal 2011, 124, 2184–2190, doi:10.3760/cma.j.issn.0366-6999.2011.14.019.
- Gallinat, S.; Csikos, T.; Meffert, S.; Herdegen, T.; Stoll, M.; Unger, T. The Angiotensin AT2 Receptor Down-Regulates Neurofilament M in PC12W Cells. Neuroscience Letters 1997, 227, 29–32, doi:10.1016/S0304-3940(97)00291-7.
- Gendron, L.; Laflamme, L.; Rivard, N.; Asselin, C.; Payet, M.D.; Gallo-Payet, N. Signals from the AT2 (Angiotensin Type 2) Receptor of Angiotensin II Inhibit P21ras and Activate MAPK (Mitogen-Activated Protein Kinase) to Induce Morphological Neuronal Differentiation in NG108-15 Cells. Mol Endocrinol 1999, 13, 1615–1626, doi:10.1210/mend.13.9.0344.
- Plouffe, B.; Guimond, M.O.; Beaudry, H.; Gallo-Payet, N. Role of Tyrosine Kinase Receptors in Angiotensin II AT2 Receptor Signaling: Involvement in Neurite Outgrowth and in P42/P44mapk Activation in NG108-15 Cells. Endocrinology 2006, 147, 4646–4654, doi:10.1210/en.2005-1315.
- Anand, U.; Facer, P.; Yiangou, Y.; Sinisi, M.; Fox, M.; McCarthy, T.; Bountra, C.; Korchev, Y.E.; Anand, P. Angiotensin II Type 2 Receptor (AT2R) Localization and Antagonist-Mediated Inhibition of Capsaicin Responses and Neurite Outgrowth in Human and Rat Sensory Neurons. European Journal of Pain (United Kingdom) 2013, 17, 1012–1026, doi:10.1002/j.1532-2149.2012.00269.x.
- Côté, F.; Laflamme, L.; Payet, M.D.; Gallo-Payet, N. Nitric Oxide, a New Second Messenger Involved in the Action of Angiotensin II on Neuronal Differentiation of NG108-15 Cells. Endocr Res 1998, 24, 403–407, doi:10.3109/07435809809032622.
- Thippeswamy, T.; Morris, R. Nerve Growth Factor Inhibits the Expression of Nitric Oxide Synthase in Neurones in Dissociated Cultures of Rat Dorsal Root Ganglia. Neurosci Lett 1997, 230, 9–12, doi:10.1016/s0304-3940(97)00459-x.
- Gendron, L.; Côté, F.; Payet, M.D.; Gallo-Payet, N. Nitric Oxide and Cyclic GMP Are Involved in Angiotensin II AT(2) Receptor Effects on Neurite Outgrowth in NG108-15 Cells. Neuroendocrinology 2002, 75, 70–81, doi:10.1159/000048222.
- Thippeswamy, T.; Jain, R.K.; Mumtaz, N.; Morris, R. Inhibition of Neuronal Nitric Oxide Synthase Results in Neurodegenerative Changes in the Axotomised Dorsal Root Ganglion Neurons: Evidence for a Neuroprotective Role of Nitric Oxide in Vivo. Neurosci Res 2001, 40, 37–44, doi:10.1016/s0168-0102(01)00205-x.
- Contestabile, A.; Ciani, E. Role of Nitric Oxide in the Regulation of Neuronal Proliferation, Survival and Differentiation. Neurochemistry International 2004, 45, 903–914, doi:10.1016/j.neuint.2004.03.021.
- Abadir, P.M.; Foster, D.B.; Crow, M.; Cooke, C.A.; Rucker, J.J.; Jain, A.; Smith, B.J.; Burks, T.N.; Cohn, R.D.; Fedarko, N.S.; et al. Identification and Characterization of a Functional Mitochondrial Angiotensin System. CELL BIOLOGY 2011, 108, 6.
- Valenzuela, R.; Costa-Besada, M.A.; Iglesias-Gonzalez, J.; Perez-Costas, E.; Villar-Cheda, B.; Garrido-Gil, P.; Melendez-Ferro, M.; Soto-Otero, R.; Lanciego, J.L.; Henrion, D.; et al. Mitochondrial Angiotensin Receptors in Dopaminergic Neurons. Role in Cell Protection and Aging-Related Vulnerability to Neurodegeneration. Cell Death Dis 2016, 7, e2427, doi:10.1038/cddis.2016.327.
- Li, J.-M.; Mogi, M.; Tsukuda, K.; Tomochika, H.; Iwanami, J.; Min, L.-J.; Nahmias, C.; Iwai, M.; Horiuchi, M. Angiotensin II-Induced Neural Differentiation via Angiotensin II Type 2 (AT 2 ) Receptor-MMS2 Cascade Involving Interaction between AT 2 Receptor-Interacting Protein and Src Homology 2 Domain-Containing Protein-Tyrosine Phosphatase 1. Molecular Endocrinology 2007, 21, 499–511, doi:10.1210/me.2006-0005.
- Di Benedetto, M.; Bièche, I.; Deshayes, F.; Vacher, S.; Nouet, S.; Collura, V.; Seitz, I.; Louis, S.; Pineau, P.; Amsellem-Ouazana, D.; et al. Structural Organization and Expression of Human MTUS1, a Candidate 8p22 Tumor Suppressor Gene Encoding a Family of Angiotensin II AT2 Receptor-Interacting Proteins, ATIP. Gene 2006, 380, 127–136, doi:10.1016/j.gene.2006.05.021.
- Nouet, S.; Amzallag, N.; Li, J.-M.; Louis, S.; Seitz, I.; Cui, T.-X.; Alleaume, A.-M.; Di Benedetto, M.; Boden, C.; Masson, M.; et al. Trans-Inactivation of Receptor Tyrosine Kinases by Novel Angiotensin II AT2 Receptor-Interacting Protein, ATIP. J Biol Chem 2004, 279, 28989–28997, doi:10.1074/jbc.M403880200.
- Wruck, C.J.; Funke-Kaiser, H.; Pufe, T.; Kusserow, H.; Menk, M.; Schefe, J.H.; Kruse, M.L.; Stoll, M.; Unger, T. Regulation of Transport of the Angiotensin AT2 Receptor by a Novel Membrane-Associated Golgi Protein. Arterioscler Thromb Vasc Biol 2005, 25, 57–64, doi:10.1161/01.ATV.0000150662.51436.14.
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev 2018, 98, 1627–1738, doi:10.1152/physrev.00038.2017.
- Porrello, E.R.; Delbridge, L.M.D.; Thomas, W.G. The Angiotensin II Type 2 (AT2) Receptor: An Enigmatic Seven Transmembrane Receptor. Frontiers in bioscience (Landmark edition) 2009, 14, 958–72.
- Sumners, C.; Fleegal, M.A.; Zhu, M. Angiotensin AT1 Receptor Signalling Pathways in Neurons. Clinical and experimental pharmacology & physiology 2002, 29, 483–490.