- placental polyps
- retained products of conception
- arteriovenous malformations
- neo-vascular lesion
1. Introduction
The sudden onset of critical vaginal bleeding 12 years after pregnancy was reported in 1884 in Philadelphia [1]. The disease, so-called placental polyps, was first recognized in this case report. Further details of this critical bleeding disease after delivery or miscarriage, the criteria for its diagnosis, and its etiology have gradually accumulated [2][3][4][5].
Placental polyps occur in <0.25% of pregnancies [3][5]. Furthermore, only 6% of placental polyps are hypervascular and cause severe hemorrhage, and the term hypervascular placental polypoid mass (HPPM) is sometimes used [6]. Placental polyps are strictly defined pathologically only by the examination of resected specimens. A placental polyp contains predominantly ghost villi that are hyalinized and necrotic, without lining trophoblasts. Some of the chorionic villi show a rim of viable syncytiotrophoblasts. The syncytiotrophoblasts may contribute to stimulating neovascularization in the myometrium. Partial hyalinization of the vascularization around the villi may lead to hemorrhage [6].
Remnants of placenta or a membrane attached to the uterine wall and fibrin deposition around the remnants are usually considered pathognomonic of the formation of placental polyps. The development of these remnants, that is, the pathogenesis of the placental polyps, has been explained by two theories. According to the first theory, the cornual or fundal site of the uterine myometrium is thin and atonic, so that the placental tissue attached to this region is easily retained. The second theory is that of placenta accreta, in which the villi are directly attached to the underlying myometrium due to the defective decidua, easily leading to the retention of the placental tissue combined with uterine atony [3][5]. Placental tissue attaches at the fundus or cornu, and then the trophoblastic villi invade the myometrium at the site of attachment. Placental polyps are of two types. The first type occurs in the first four weeks after the postparturition period, and the other one occurs months or years later [5]. The factors responsible for the survival of these villi are still unclear.
2. Relationship between RPOC and Placental Polyps
RPOC is a condition that starts at miscarriage or delivery, and the residual placenta pieces or villi change their pathophysiology over time. RPOC is more than just remnants: it is a condition that develops secondary changes with the passage of time. RPOC initially consists of retained villi. In the next stage, the residual villi undergo necrosis with the deposition of fibrin, producing the pathological condition called placental polyps. Placental polyps may thus be viewed as a specific form of RPOC constituting an extreme expression of this condition. The patient presents with severe hemorrhaging when the polyp is detached [7].
3. Neo-Vascular Lesions of RPOC
Neo-vascular lesions may arise from remnant placenta or villi. Lesions appear and are accompanied by frequent abundant vascularization in the myometrium attached to the remnant. One characteristic of neo-vascular lesions in RPOC is that they grow toward the myometrium. These phenomena can involve residual villi undergoing necrosis, the formation of arteriovenous fistulas, and continuing development of arteriovenous communication [7]. These neo-vascular lesions were not originally present but developed over time after miscarriage or delivery. They are benign neoplasms and go through proliferating and involuting phases. Many of these vascular lesions have been reported to resolve spontaneously within a few months [8]. Although careful monitoring for sudden hemorrhage is required, their potential for spontaneous regression makes them candidates for elective treatment [9].
According to a report [10], MRI findings of RPOC basically include a combination of three parts in varying degrees: presence of a remnant, breaking of the junctional zone in contact with the remnant, and vascularization. The absence of the junctional zone on MRI suggests that placenta accreta is the basis of the pathogenesis. At the defective portion of the decidua, the villi and the myometrium directly contact each other, resulting in various morbid conditions arising in the villi of this direct adhesion site [4][5]. How vascularization develops from the defective part in the decidua basalis may form the basis for a clinically useful classification system. With accurate classification of the vascular lesions, it is possible to develop treatment protocols tailored to each type of RPOC.
4. Differentiation between RPOC with Vascularization and an AVM (Case Report)
We treated a patient with suspected RPOC with vascularization that was, in fact, an AVM. We describe this case below. This case involved a 28-year-old, gravida 1 para 0 woman whose pregnancy progressed without any particular complications. On day 281 of pregnancy, the spontaneous delivery of a 3175-g girl occurred. The placenta was expelled and showed no apparent signs of a defect 13 min postpartum. Massive bleeding occurred suddenly 2 h postpartum, with blood loss since delivery totaling 1271 g. The administration of a uterotonic in combination with intravaginal gauze packing successfully stopped the bleeding. Transabdominal ultrasonography showed an intrauterine mass measuring 45 × 35 mm2
, raising suspicion of RPOC. Ultrasound showed a hypervascular appearance with turbulent flow on color Doppler, and this was initially thought to be typical RPOC with vascularization. On close observation, however, the hypervascularity was limited to the point of contact between the remnant and the myometrium, and there was no pulsatile perfusion in the remnant itself () [19,21].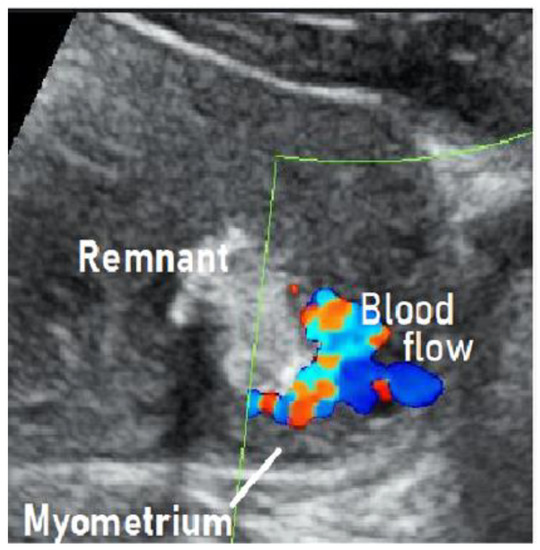
Figure 1.

Figure 2. Findings of angiography: Abnormal engorgement in the arterial phase, with a thick venous phase visualized only 2 s later. This indicated the presence of an arteriovenous shunt. There was no pulsatile arterial perfusion in the remnant itself, where the contrast agent simply appeared to be leaking.
Currently, in clinical practice, a hypervascular appearance with turbulent flow on color Doppler ultrasound after delivery or miscarriage is sometimes observed roughly, and RPOC with vascularization is not clearly distinguished from AVM. This may result in the performance of unnecessary interventions for RPOC when hemorrhage is unlikely to occur or the mistaken choice of elective therapy for AVMs. Reaching a clear differential diagnosis leads to the right treatment strategy. It enables a clear policy to be decided: should a patient be treated electively and conservatively, or would proactive intervention be better [9]? Careful observation of the state of perfusion is required to make the differential diagnosis.
Currently, in clinical practice, a hypervascular appearance with turbulent flow on color Doppler ultrasound after delivery or miscarriage is sometimes observed roughly, and RPOC with vascularization is not clearly distinguished from AVM. This may result in the performance of unnecessary interventions for RPOC when hemorrhage is unlikely to occur or the mistaken choice of elective therapy for AVMs. Reaching a clear differential diagnosis leads to the right treatment strategy. It enables a clear policy to be decided: should a patient be treated electively and conservatively, or would proactive intervention be better [12]? Careful observation of the state of perfusion is required to make the differential diagnosis.References
- Baer, B.F. Placental Polypus which simulated malignant disease of the uterus. Phila. Med. Times 1884, 15, 175.
- Hagstrom, H.T. Late puerperal hemorrhages due to placental polyp. Am. J. Obstet. Gynecol. 1940, 39, 879–881.
- Hoberman, L.K.; Hawkinson, J.A.; Beecham, C.T. Placental polyps: Report of three cases. Obstet. Gynecol. 1963, 22, 25–29.
- Swan, R.W.; Woodruff, J.D. Retained products of conception: Histologic viability of placental polyps. Obstet. Gynecol. 1969, 34, 506–514.
- Dyer, I.; Bradbum, D.M. An inquiry into the etiology of placental polyp. Am. J. Obstet. Gynecol. 1971, 109, 858–867.
- Milovanov, A.P.; Kirsanov, I.N. The pathogenesis of uterine hemorrhages in the so-called placental polyps. Arkh. Pathol. 2008, 70, 34–37.
- Kido, A.; Togashi, K.; Koyama, T.; Ito, H.; Tatsumi, K.; Fujii, S.; Konishi, J. Retained products of conception masquerading as acquired arteriovenous malformation. J. Comput. Assist. Tomogr. 2003, 27, 88–92.
- Mulliken, J.B.; Glowacki, J. Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast. Reconstr. Surg. 1982, 69, 412–422.
- Timmerman, D.; Wauters, J.; Van Celenbergh, S. Color Doppler imaging is a valuable tool for diagnosis and management of uterine vascular malformations. Ultrasound Obstet. Gynecol. 2003, 21, 570–577.
- Shiina, Y.; Itagaki, T.; Ohtake, H. Hypervascular retained product of conception: Characteristic magnetic resonance imaging and possible relationship to placental polyp and pseudoaneurysm. J. Obstet. Gynecol. Res. 2018, 44, 165–170.
- Kole, M.; Keerthy, M.; Fotouhi, A.; Sangha, R. Incidence of pregnancy following uterine artery embolization performed for uterine arteriovenous malformations [22N]. Obstet. Gynecol. 2020, 135, 151S.
- Roach, M.K.; Thomassee, M.S. Acquired uterine arteriovenous malformation and retained placenta increta. Obstet. Gynecol. 2015, 126, 642–644.
- Brown, J.V.; Asrat, T.; Epstein, H.D.; Oglevie, S.; Goldstein, B. Contemporary diagnosis and management of uterine arteriovenous malformation. Obstet. Gynecol. 2008, 112, 467–470.
