Blepharophimosis, ptosis, and epicanthus inversus syndrome (BPES) is a craniofacial disorder caused by heterozygous variants of the forkhead box L2 (FOXL2) gene. It shows autosomal dominant inheritance but can also occur sporadically. Depending on the mutation, two phenotypic subtypes have been described, both involving the same craniofacial features: type I, which is associated with premature ovarian failure (POF), and type II, which has no systemic features.
1. Introduction
Blepharophimosis, ptosis, and epicanthus inversus syndrome (BPES; OMIM #110100) is a rare autosomal dominant disease, with an estimated prevalence of 1 in 50,000 births, primarily affecting the development of the mid-face structures [1]. The four major clinical signs are dysplasia of the eyelids with shortening of the horizontal fissures (blepharophimosis), droopy upper lids reducing the vertical palpebral aperture (ptosis), bilateral skin fold arising from the medial lower eyelid ascending to the upper lid (epicanthus inversus), and an increased distance between the medial canthi (telecanthus). Two main phenotypes of BPES have emerged, and each harbour the four key ocular signs: (i) type I (BPES-I), which is also associated with premature ovarian failure (POF) involving secondary amenorrhoea before 40 years of age, leading to early menopause and infertility, and (ii) type II (BPES-II) with no systemic associations.
BPES can be caused by heterozygous variants involving the forkhead box L2 (
FOXL2
) gene, which encodes for a transcription factor expressed predominantly in the developing mesenchyme of eyelids and ovaries [2]. In mice, Foxl2
expression is localised to the protruding ridges of the developing eyelids and in ovarian follicular cells. Up to 75% of affected individuals may have detectable
FOXL2 mutation, leading to haploinsufficiency [3][4][5][6]. Transmission of BPES-I is usually by affected males as fertility in affected females is reduced due to ovarian dysfunction. BPES-II can have transmission occurring through both males and females.
mutation, leading to haploinsufficiency [3,4,5,6]. Transmission of BPES-I is usually by affected males as fertility in affected females is reduced due to ovarian dysfunction. BPES-II can have transmission occurring through both males and females.
2. Genetics of BPES
2.1. FOXL2 Gene
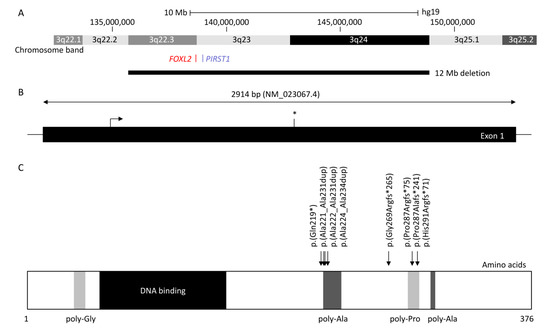
FOXL2 is a single-exon gene consisting of 2.9 kb (NM_023067.4) located on chromosome 3q22.3. The transcribed protein is 376 amino acids and belongs to the family of forkhead/winged helix transcription factors. FOXL2 regulates a number of genes that control cellular processes including inflammation, transcription, proteolysis, apoptosis, and steroidogenesis including gonadotrophins [7][8]. It is highly conserved among species, with 100% of homology for human, mouse, rat, cow, goat, pig, and rabbit, and consists of a 110 amino acid forkhead DNA-binding domain at position 54 to 148 [9]. It also contains a strictly conserved poly-alanine tract of 14 amino acids between position 221 and 234, whose role remains unknown; however, it is a hotspot for expansions from 14 to 24 alanine residues accounting for ≈30% of all intragenic
is a single-exon gene consisting of 2.9 kb (NM_023067.4) located on chromosome 3q22.3. The transcribed protein is 376 amino acids and belongs to the family of forkhead/winged helix transcription factors. FOXL2 regulates a number of genes that control cellular processes including inflammation, transcription, proteolysis, apoptosis, and steroidogenesis including gonadotrophins [7,8]. It is highly conserved among species, with 100% of homology for human, mouse, rat, cow, goat, pig, and rabbit, and consists of a 110 amino acid forkhead DNA-binding domain at position 54 to 148 [9]. It also contains a strictly conserved poly-alanine tract of 14 amino acids between position 221 and 234, whose role remains unknown; however, it is a hotspot for expansions from 14 to 24 alanine residues accounting for ≈30% of all intragenic FOXL2 pathogenic variants leading to predominantly BPES-II [6][9].
Haploinsufficiency of
pathogenic variants leading to predominantly BPES-II [6,9].
Haploinsufficiency of FOXL2
remains the only reported cause of BPES, and the first autosomal gene implicated in syndromic POF [2]. FOXL2
can be disrupted by intragenic mutations as well as larger genomic deletions involving the gene locus [10]. More than 250 variants are associated with BPES [11]: intragenic mutations of FOXL2
account for 81% and can be subdivided into indel frameshift (44%), in-frame deletions (33%), nonsense (12%), missense (11%), and duplications [11]. Whole-gene deletions and larger sub-microscopic deletions encompassing FOXL2
and neighbouring genes represent 12% and 5% of molecularly confirmed cases, respectively [11].
At least 460 patients have been reported with FOXL2-related BPES [1][6][10][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62]. The most common variants affect two intragenic regions (
-related BPES [1,6,10,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. The most common variants affect two intragenic regions (): (i) the poly-alanine region with c.663_692dup p.(Ala221_Ala231dup) reported in 12 patients (four BPES-II cases and eight with undefined type) [6][14][16][30][31][32], c.664_693dup p.(Ala222_Ala231dup) reported in five patients (two BPES-II, two BPES-I, and one with undefined type), and c.672_701dup p.(Ala225_Ala234dup), which was reported in at least 80 patients (24 BPES-II, 2 BPES-I, and 54 with undefined type) [6][14][16][22][26][30][31][32][54][61][63], and (ii) the poly-proline region, which encompasses amino acids at positions 284 to 292, has two duplications variants: c.843_859dup p.(Pro287Argfs*75) reported in 46 patients (3 BPES-I, 2 BPES-II, and 41 with undefined type) [6][14][16][31][32][63] and c.855_871dup p.(His291Argfs*71) in 15 patients (3 BPES-I and 12 undefined type) [6][14][16][24][38][63], and the deletion c.855_871del p.(Pro287Alafs*71) in 5 patients (3 BPES-I, 1 BPES-II, and 1 with undefined type) [6][14][34][40][63].
): (i) the poly-alanine region with c.663_692dup p.(Ala221_Ala231dup) reported in 12 patients (four BPES-II cases and eight with undefined type) [6,14,16,30,31,32], c.664_693dup p.(Ala222_Ala231dup) reported in five patients (two BPES-II, two BPES-I, and one with undefined type), and c.672_701dup p.(Ala225_Ala234dup), which was reported in at least 80 patients (24 BPES-II, 2 BPES-I, and 54 with undefined type) [6,14,16,22,26,30,31,32,54,61,63], and (ii) the poly-proline region, which encompasses amino acids at positions 284 to 292, has two duplications variants: c.843_859dup p.(Pro287Argfs*75) reported in 46 patients (3 BPES-I, 2 BPES-II, and 41 with undefined type) [6,14,16,31,32,63] and c.855_871dup p.(His291Argfs*71) in 15 patients (3 BPES-I and 12 undefined type) [6,14,16,24,38,63], and the deletion c.855_871del p.(Pro287Alafs*71) in 5 patients (3 BPES-I, 1 BPES-II, and 1 with undefined type) [6,14,34,40,63].
Figure 1. Diagram showing forkhead box L2 (FOXL2), up- and downstream genomic position, protein structure, and hotspot located in the poly-alanine domain. (A) Chromosomal deletions were reported in at least 84 blepharophimosis, ptosis, and epicanthus inversus syndrome (BPES) patients, with the largest encompassing 3q22.3 to 3q24 (12 Mb) and the smallest encompassing FOXL2 or PIRST1 genes (UCSC: hg19:chr3:133,064,629-153,716,375). (B) FOXL2 is composed of one 2.9 kb exon (NM_02367.4). The stop codon is indicated with an asterisk (*). (C) FOXL2 is a 376 amino acid protein, composed of poly-glycine (amino acid position 35–43) depicted in light grey, a DNA binding protein or forkhead (amino acid 54–148) in dark, two poly-alanine (poly-Ala) region (amino acid 221–234 and 301–304) in grey, and a poly-proline region (amino acid 284–292) in light grey (Uniprot: P58012). The most common variants (more than five patients were reported) are represented herein, which affect the highly conserved poly-alanine domain (amino acid 221–234) with c.672_701dup p.(Ala224_Ala234dup) (n = 80), c.663_692dup p.(Ala221_Ala231dup) (n = 12), and c.664_693dup p.(Ala222_Ala231) (n = 5), or the poly-proline domain (amino acid 284–292) with c.843_859dup p.(Pro287Argfs*241) (n = 46), c.855_871del p.(Pro287Alafs*241) (n = 5), and c.855_871dup p.(His291Argfs*71) (n = 15). The nonsense variant c.655C>T p.(Gln219*) and the frameshift variant c.804dup p.(Gly269Argfs*265) were reported in 6 and 10 patients, respectively.
A classification (groups A-H) of intragenic variants in ≈500 FOXL2 cases was previously proposed by De Baere et al. in order to determine a genotype–phenotype correlation [6]. However, as ovarian function was largely unavailable due to the young age or sex of the patient, this was not possible. A tendency was observed with variants leading to an expanded poly-alanine region, such as c.663_692dup p.(Ala221_Ala231dup), c.664_693dup p.(Ala222_Ala231dup), and c.672_701dup p.(Ala225_Ala234dup), which are mostly associated with BPES-II [6][14][16][22][26][30][31][32][43][45][51][54][61][63]. Interfamilial variability was observed for these variants, where some cases are reported to be associated with BPES-I. Of note, one family reported a BPES-II-affected mother and her BPES-I-affected daughter, both carrying c.822C>G p.(Tyr274*), revealing possible intrafamilial phenotypic variability [6].
cases was previously proposed by De Baere et al. in order to determine a genotype–phenotype correlation [6]. However, as ovarian function was largely unavailable due to the young age or sex of the patient, this was not possible. A tendency was observed with variants leading to an expanded poly-alanine region, such as c.663_692dup p.(Ala221_Ala231dup), c.664_693dup p.(Ala222_Ala231dup), and c.672_701dup p.(Ala225_Ala234dup), which are mostly associated with BPES-II [6,14,16,22,26,30,31,32,43,45,51,54,61,63]. Interfamilial variability was observed for these variants, where some cases are reported to be associated with BPES-I. Of note, one family reported a BPES-II-affected mother and her BPES-I-affected daughter, both carrying c.822C>G p.(Tyr274*), revealing possible intrafamilial phenotypic variability [6].
2.2. Intragenic Variants in FOXL2
As FOXL2
is composed of one exon, it may be resistant to nonsense mediated decay (NMD) as in other cases [64]. Thus, null variants in FOXL2 will result in a truncated protein leading to partial or complete loss of the forkhead domain and poly-alanine tract [6] or in a shorter protein without the N-terminal region, due to a re-initiation of translation, as observed in COS-7 cell lines transfected with the c.157C>T p.(Gln53*) construct [65]. Whereas duplications within or downstream of the forkhead domain can be predicted to result in an extended protein, mutations involving only part of the forkhead domain may give rise to haploinsufficiency and BPES-II by reducing the transactivation activity of the gene without affecting its DNA binding [66]. The effect of missense mutations can vary depending on their location, which may be in a functionally important region as the gene is highly conserved [66]. Most are mapped to the forkhead DNA binding domain, and these are likely to be pathogenic. However, as missense mutations have been reported in both BPES-I and -II, no prediction can be made regarding the genotype–phenotype correlation [6].
will result in a truncated protein leading to partial or complete loss of the forkhead domain and poly-alanine tract [6] or in a shorter protein without the N-terminal region, due to a re-initiation of translation, as observed in COS-7 cell lines transfected with the c.157C>T p.(Gln53*) construct [65]. Whereas duplications within or downstream of the forkhead domain can be predicted to result in an extended protein, mutations involving only part of the forkhead domain may give rise to haploinsufficiency and BPES-II by reducing the transactivation activity of the gene without affecting its DNA binding [66]. The effect of missense mutations can vary depending on their location, which may be in a functionally important region as the gene is highly conserved [66]. Most are mapped to the forkhead DNA binding domain, and these are likely to be pathogenic. However, as missense mutations have been reported in both BPES-I and -II, no prediction can be made regarding the genotype–phenotype correlation [6].
2.3. Poly-Alanine Tract Expansion Variants
Studies have confirmed the existence of a mutational hotspot in the poly-alanine tract of FOXL2 in families of different ethnicities [6][67]. This highly conserved region consists of 14 Alanine residues, and the secondary protein structure is predicted to be an α helix, which may become distorted when mutated and disrupt an essential function [6]. Poly-alanine tract expansion is the most common mutation to have been described in BPES-II [67]. Eight different alanine tract expansions (c.663_692dup30 p.(Ala221_Ala231dup), c.664_693dup30 p.(Ala222_Ala231dup), c.664_701dup p.(Ala222_Ala234dup), c.667_702dup p.(Ala223_Ala234), c.672_701dup30 p.(Ala225_Ala234dup), c.684_698dup p.(Ala228_Ala232dup), c.684_698trip15 p.(Ala228_Ala232trip), c.696_728dup p.(Ala232_Ala243dup)) have been described, with the most common consisting of a repeat of 30 bases [6][14][16][22][24][30][31][32][41][54][55][61][63]. These expansions may be caused by slippage of DNA polymerase when duplicating trinucleotide repeats, accounting for about 33% of all intragenic mutations in BPES overall [6][11][41][67]. Whilst expansions are more likely to be associated with BPES-II (without ovarian involvement) and truncated proteins are correlated with BPES-I (with ovarian involvement) [68], some poly-alanine expansions such as c.664_693dup30 p.(Ala222_Ala231dup) and c.672_701dup p.(Ala224_Ala234dup) have resulted in some degree of ovarian dysfunction [14][45]. There has only been one autosomal recessive consanguineous Indian family with evidence of a homozygous poly-alanine tract expansion, c.684_698dup p.(Ala228_Ala232dup), with ovarian failure; segregated carrier parents and siblings were not affected. As such, BPES is mainly regarded as an autosomal dominant disorder [41].
Further functional analyses have been performed to study mutation consequences at a cellular level. Luciferase assays revealed some nonsense variants, such as p.(Glu19*), produced a shorter protein with an alternative initiation codon and formed nuclear aggregates, while wild-type protein was diffuse in the nucleus [65]. Caburet et al. showed protein mislocalisation from the nucleus to the cytoplasm with mutated FOXL2 poly-alanine tract expansions leading to its cytoplasmic aggregation [69]. Moreover, these poly-alanine tract expansions lead to decreased expression of several genes involved in apoptosis, transcriptional regulation, mediation of inflammation, cholesterol metabolism, and reactive oxygen species detoxification [70]. Two models were suggested to explain BPES phenotype with or without POF: (1) a higher dose of functional FOXL2 might be required to target promoters in the developing eyelid than in ovarian follicular cells, or (2) the number of FOXL2 binding sites in promoters is the same in both tissues, but aggregation and mislocalisation of mutant protein are stronger in the eyelids than ovaries, due to different tissue-specific proteomics [70]. Some missense variants, such as c.931C>T p.(His311Tyr) associated with BPES-I [6] or with undefined type due to the young age of female patients [62], were also reported to affect the expression of targeted genes, such as the steroidogenic acute regulatory gene (
in families of different ethnicities [6,67]. This highly conserved region consists of 14 Alanine residues, and the secondary protein structure is predicted to be an α helix, which may become distorted when mutated and disrupt an essential function [6]. Poly-alanine tract expansion is the most common mutation to have been described in BPES-II [67]. Eight different alanine tract expansions (c.663_692dup30 p.(Ala221_Ala231dup), c.664_693dup30 p.(Ala222_Ala231dup), c.664_701dup p.(Ala222_Ala234dup), c.667_702dup p.(Ala223_Ala234), c.672_701dup30 p.(Ala225_Ala234dup), c.684_698dup p.(Ala228_Ala232dup), c.684_698trip15 p.(Ala228_Ala232trip), c.696_728dup p.(Ala232_Ala243dup)) have been described, with the most common consisting of a repeat of 30 bases [6,14,16,22,24,30,31,32,41,54,55,61,63]. These expansions may be caused by slippage of DNA polymerase when duplicating trinucleotide repeats, accounting for about 33% of all intragenic mutations in BPES overall [6,11,41,67]. Whilst expansions are more likely to be associated with BPES-II (without ovarian involvement) and truncated proteins are correlated with BPES-I (with ovarian involvement) [68], some poly-alanine expansions such as c.664_693dup30 p.(Ala222_Ala231dup) and c.672_701dup p.(Ala224_Ala234dup) have resulted in some degree of ovarian dysfunction [14,45]. There has only been one autosomal recessive consanguineous Indian family with evidence of a homozygous poly-alanine tract expansion, c.684_698dup p.(Ala228_Ala232dup), with ovarian failure; segregated carrier parents and siblings were not affected. As such, BPES is mainly regarded as an autosomal dominant disorder [41].
Further functional analyses have been performed to study mutation consequences at a cellular level. Luciferase assays revealed some nonsense variants, such as p.(Glu19*), produced a shorter protein with an alternative initiation codon and formed nuclear aggregates, while wild-type protein was diffuse in the nucleus [65]. Caburet et al. showed protein mislocalisation from the nucleus to the cytoplasm with mutated FOXL2 poly-alanine tract expansions leading to its cytoplasmic aggregation [69]. Moreover, these poly-alanine tract expansions lead to decreased expression of several genes involved in apoptosis, transcriptional regulation, mediation of inflammation, cholesterol metabolism, and reactive oxygen species detoxification [70]. Two models were suggested to explain BPES phenotype with or without POF: (1) a higher dose of functional FOXL2 might be required to target promoters in the developing eyelid than in ovarian follicular cells, or (2) the number of FOXL2 binding sites in promoters is the same in both tissues, but aggregation and mislocalisation of mutant protein are stronger in the eyelids than ovaries, due to different tissue-specific proteomics [70]. Some missense variants, such as c.931C>T p.(His311Tyr) associated with BPES-I [6] or with undefined type due to the young age of female patients [62], were also reported to affect the expression of targeted genes, such as the steroidogenic acute regulatory gene (STAR, OMIM 600617) [62].
OMIM 600617) [62].
2.4. Chromosomal Translocations and Involvement of FOXL2 Regulatory Genes
Translocation breakpoints in chromosome 3 close to the FOXL2
gene and FOXL2
regulatory genes such as PISRT1
in nine patients carrying t(1;3)(p21;q22), t(1;3) associated with a 1.2 Mb deletion in 3q23 upstream of the FOXL2 transcription unit, t(2;3)(q33;q23), t(3;4)(q23;p15), t(3;7)(q23;q32), t(3;11)(q22.3;q14.1), t(3;15)(q23;q25), t(3;20)(q22;q13), and t(3;21)(q23;q22.1) were shown to be correlated with BPES [6][12][25][48][52][59][71][72]. Only the patient with t(3;11)(q22.3;q14.1) was known to have BPES-I [48]. Although the
transcription unit, t(2;3)(q33;q23), t(3;4)(q23;p15), t(3;7)(q23;q32), t(3;11)(q22.3;q14.1), t(3;15)(q23;q25), t(3;20)(q22;q13), and t(3;21)(q23;q22.1) were shown to be correlated with BPES [6,12,25,48,52,59,71,72]. Only the patient with t(3;11)(q22.3;q14.1) was known to have BPES-I [48]. Although the FOXL2
gene itself carried no mutation, positional effects are prevalent in human genetic diseases involving transcriptional factors, for example, PAX6
and PITX2
genes, which are involved in aniridia and Axenfeld–Rieger syndrome, respectively [73].
Genomic alterations in loci outside of the FOXL2
region such as deletion of upstream or downstream regulatory regions close to FOXL2
or PISTR1
deletion have also been found to account for about 5% of BPES [53]. Larger deletions encompassing 3q22.3-3q24 are associated with an undefined type of BPES, Dandy–Walker malformation, and Wisconsin syndrome [47], whereas smaller deletions of FOXL2
or PISTR1
gene lead to just undefined BPES [63]. Upstream regions of FOXL2
and PISTR1
genes are highly conserved in goat, mouse, and human [74], and their deletion leads to polled goats due to PIS (polled intersex syndrome) mutation [75]. This model is characterised by cranio-facial defects, female sterility, and XX sex reversal, associated with decreased expression level of FOXL2
and PISTR1
in the ovaries [75]. Luciferase assay revealed the identified genomic deletions in loci outside of the FOXL2
region affect gene expression in ovarian cell lines [21]. Rearrangements, such as large chromosomal deletion or translocation occurring in these regions, can dissociate the transcription unit from its regulatory elements, resulting in the same phenotype as intragenic mutations [5]. Total and partial gene deletions as well as microdeletions mapping upstream and downstream of FOXL2
have been found in cases of sporadic and familial cases of BPES [5]. These deletion points are scattered and lie in transcription factor-binding sites and the goat PIS
locus requiring further investigation to fully understand the intergenic regulatory elements [5]. Deletions were shown to be conserved between different generations of affected family members, revealing meiotic stability. A number of microdeletions, such as a 197 kb deletion upstream of FOXL2, have been correlated with BPES-like disorders associated with microcephaly and intellectual disability [13].
, have been correlated with BPES-like disorders associated with microcephaly and intellectual disability [13].
2.5. FOXL2 and Primary Ovarian Failure (POF)
FOXL2 is the earliest known but not the sole regulator of sex differentiation in mammals [76]. It is involved in foetal development as well as maintenance of the mature ovary. In the postnatal ovary, FOXL2 supports follicular growth. Ablation of FOXL2 in mice led to atresia of the oocytes with no maturation of secondary follicles. It has been shown that the STAR protein, which is a marker of granulosa cell differentiation, is a direct target of FOXL2, acting as a repressor of STAR
. It was concluded that the entire alanine/proline-rich carboxyl terminus is important for the repressor activity of FOXL2 and that truncating variants may preferentially lead to BPES and ovarian dysfunction by accelerated differentiation of granulosa cells and secondary depletion of the primordial follicle pool. The identification of a considerable number of ovarian FOXL2 targets may be essential to reveal more insights into phenotypic effects of FOXL2
pathogenic variants in the adult ovary. Digenic inheritance might contribute to POF associated with BPES through a synergistic effect of FOXL2
mutations and other genes involved in ovarian function. This may also explain the apparent pleiotropism of FOXL2
mutations.
The phenomena of waxing and waning gonadotrophin levels which suggest infertility may be partially reversible in BPES type I. Varying gonadotrophin levels in female patients with BPES type I may not necessarily meet the diagnostic criteria for POF. POF is commonly defined as the presence of four or more months of secondary amenorrhea, postmenopausal levels of follicle-stimulating hormone (FSH; >40 IU/L) all before the age of 40 years [77]. However, there is no universal definition of POF, and coupled with the large normal variation in ovarian reserve, it can be difficult to make a diagnosis of a POF [78][79]. Moreover, spontaneous pregnancies and pregnancies post-stimulation with gonadotrophins have been reported in individuals with
The phenomena of waxing and waning gonadotrophin levels which suggest infertility may be partially reversible in BPES type I. Varying gonadotrophin levels in female patients with BPES type I may not necessarily meet the diagnostic criteria for POF. POF is commonly defined as the presence of four or more months of secondary amenorrhea, postmenopausal levels of follicle-stimulating hormone (FSH; >40 IU/L) all before the age of 40 years [77]. However, there is no universal definition of POF, and coupled with the large normal variation in ovarian reserve, it can be difficult to make a diagnosis of a POF [78,79]. Moreover, spontaneous pregnancies and pregnancies post-stimulation with gonadotrophins have been reported in individuals with FOXL2 mutations and in women with POF alone [22][80].
mutations and in women with POF alone [22,80].