Curcumin is a principal bioactive phenolic compound of the spice turmeric, in regard to various human and animal herpesvirus infections and inflammation connected with these diseases. Curcumin was explored with potent antiherpetic actions against herpes simplex virus type 1 and type 2, human cytomegalovirus, Kaposi’s sarcoma-associated herpesvirus, Epstein–Barr virus, bovine herpesvirus 1, and pseudorabies virus.
- curcumin
- herpesviruses
- phenolics
- inflammation
- mechanisms and pathways
- viral infections
- Curcuma longa L.
1. Introduction
Infection with herpesviruses is frequently observed in humans and animals, inducing a range of diseases from moderate uncomplicated mucocutaneous infection to those that are life-threatening [1,2][1][2]. Herpesviruses belong to the family of Herpesviridae, which is a large family of DNA viruses with severely contagious properties that use a strategy of infection known as travel and hide [3,4][3][4]. These viruses share the feature of forming lifelong infections in a latent phase with the potential of periodic reactivation. During the life cycle, herpesviruses typically infect different cell types in various tissues, and therefore the sub-classification of these pathogens is partially based on their cell and tissue tropism [5,6,7][5][6][7]. So far, the herpesvirus family is divided into three subfamilies, the Alpha-, Beta-, and Gammaherpesvirinae, which include diverse types of herpesvirus known to infect humans. The morbidity and mortality of these viruses are mainly linked with the immunocompromised hosts. However, herpes simplex virus (HSV), varicella-zoster virus (VZV), and Epstein–Barr virus (EBV) were reported with a significant disease burden in people with normal immune function [8,9][8][9]. For decades, integrated management of herpesvirus infections remains the main challenge in virology research, where these infections are still incurable due to the viral latency, the problem of recurrent infections, and the resistance to antiherpetic drugs [10,11,12][10][11][12]. Antiviral natural products have gained immense consideration in herpesvirus research after the Nobel Prize in Physiology or Medicine (in 2015) was awarded for the discovery of the natural anti-malarial drug artemisinin. Thus, natural anti-infective agents might pave the road for defeating the problem of drug resistance as well as provide effective and safe treatment of infections caused by herpesviruses [13,14,15,16][13][14][15][16]. Curcumin, a natural phenolic compound found in the Curcuma longa L. (Zingiberaceae) (turmeric), could help eliminate certain viruses by various mechanisms of action (Figure 1).
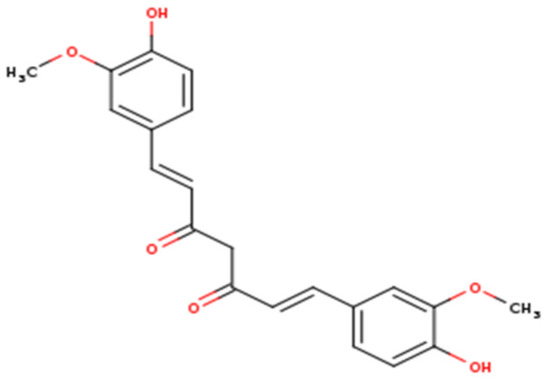
Figure 1. Chemical structure of curcumin.
2. Curcumin as an Antiviral Agent with Proven Health Benefits
Curcumin is the key component of the yellow pigment and the main bioactive molecule of turmeric [17]. Chemically, this compound belongs to the class of natural phenolic compounds and has been broadly identified in diverse Curcuma spp. In 1910, curcumin was characterized as a symmetrical molecule of two 4-hydroxy-3-methoxyphenyl rings fastened by α,β-unsaturated carbonyl groups [18[18][19],19], while its synthesis was defined in 1913 [20]. Curcumin has been employed widely in the traditional medicine systems of various countries and regions in the world [21,22][21][22]. Since the complete information about chemical structure and synthesis is acquired, curcumin has been extensively studied in various biological assays and has proven to induce numerous pharmacological and beneficial impacts on human health, including but not limited to the potential treatment of various viral infections such as human immunodeficiency virus, hepatitis B virus, hepatitis C virus, influenza A virus, human papillomavirus, respiratory syncytial virus, arboviruses, and noroviruses [23,24,25,26,27][23][24][25][26][27]. Unlike the notable antimicrobial actions, this biomolecule induces several biological effects including but not limited to antioxidant, anti-inflammatory, and anticancer properties [28,29][28][29].
3. Role of Curcumin in Inhibition of Herpes Simplex Virus Infections
It is known that nonclinical virology studies can assist in dose selection and study design to offer verification of concept and data validating an antiviral claim [39,40][30][31]. Consequently, in this section, we aim to embody all available data obtained from nonclinical investigations (in vitro and in vivo studies) that highlight the antiviral properties of curcumin, curcumin metal complexes, and curcumin formulations against the pathogenic herpes simplex virus (HSV) with an emphasis on the effective concentrations or doses and mechanisms of action (Table 1). The alpha-herpesvirus HSV is one of the most prevalent infections in humans, and therefore studies on this virus are intensively performed [41,42][32][33]. HSV is classified into two types, HSV-1 and HSV-2. HSV-1 is widely transmitted by oral-to-oral contact to induce oral herpes and in some cases, can also generate genital herpes. Genital herpes is a sexually transmitted disease triggered by HSV-2 [43,44][34][35]. Moreover, infection with HSV-2 enhances the risk of transmitting infection with the human immunodeficiency virus (HIV). Most oral and genital herpes infections are asymptomatic and persist lifelong in the host [45,46][36][37]. Considering the antiviral capacity of curcumin, defeating the viral infections is one of the main bio-functions reported for this compound via targeting of the viral entry and viral components that are crucial for viral replication as well as other cellular or molecular processes that are involved in the viral life/infectious cycle [47,48,49][38][39][40].
Table 1. Antiviral activities of curcumin, curcumin metal complexes, and curcumin formulations against herpes simplex virus 1 (HSV-1) and herpes simplex virus 2 (HSV-2) infections.
|
Type of Study, Test Performed, Virus, and Cells/Animal Model |
Results |
|---|
Antiviral properties of curcumin and curcumin formulations against human cytomegalovirus (HCMV), Kaposi’s sarcoma-associated herpesvirus (KSHV), Epstein–Barr virus (EBV), bovine herpesvirus 1 (BoHV-1), and pseudorabies virus (PRV) infections.
|
Herpesvirus and Type of Study |
Results |
Mechanism of Action and Pathway |
Reference |
||||
|---|---|---|---|---|---|---|---|
Mechanism of Action and Pathway |
Reference |
||||||
|
In vitro. Plaque assays, ChIP assay, and western blot analysis. HSV-1. HeLa and Vero cells. |
Inhibition of HSV-1 replication and suppression of IE gene expression. |
Curcumin was observed to utilize the mechanism independent of the transcriptional coactivator proteins p300/CBP histone acetyltransferase activity to affect the viral transactivator protein VP16-mediated enlistment of RNA polymerase II to IE gene promoters, leading to suppressing gene expression and blocking viral infection. |
|||||
|
HCMV (in vitro, in vivo, and in silico). |
At various concentrations in micromolar ranges, curcumin was detected with anti-HCMV properties. | ||||||
Inhibition of IEA and UL83A expressions and downregulation of Hsp90. Determination of anti-inflammatory and antioxidant effects as possible mechanisms underlying the anti-HCMV activity. |
In vitro and in vivo. Plaque reduction assay. HSV-2. Primary rabbit kidney cells (in vitro). Guinea pig model (in vivo). |
In an in vitro plaque reduction assay, curcumin suppressed the replication of HSV-2 with an ED50 value of 0.32 mg/mL, while at a concentration of 100 mg/mL, the in vivo inhibitory activity was confirmed using a mouse model of genital HSV-2 infection. |
The mechanism is unknown. |
||||
|
KSHV (in vitro). |
At various concentrations (in µM), curcumin efficiently inhibited KSHV replication and virus-associated pathogenic properties. |
Blocking APE1-mediated redox function. |
In vitro. Plaque assay. HSV-2. Human genital epithelial cells. |
||||
|
EBV (in vitro). | In primary human genital epithelial cells, pre-treatment of cells with curcumin (5 µM) decreased HSV-2 shedding by 1000-fold and at a concentration of 50 µM, entirely blocked HSV-2 production. |
Inhibition of EBV reactivation in Raji DR-CAT cells with curcumin treatment (15 µM). Investigation of the cellular pathways known to be regulated by curcumin involving the transcription factor NF-κB. |
Inhibition of BZLF1 gene transcription. |
||||
|
In vitro. Plaque assay and virus adsorption assay. HSV-1 and HSV-2. Vero cells. |
At a concentration of 30 µM, curcumin inhibited the replication of HSV-1 and HSV-2. |
Inhibition of adsorption and replication of HSV-1 and HSV-2. |
|||||
|
In vivo. Plaque assay. HSV-2. Genital epithelial cells of female C57BL/6 mice. |
Nanoparticle-containing curcumin (0.5 mg) reduced tissue inflammation and the severity of HSV-2 infection in an animal model. |
The mechanism of action was detected to be correlated with the anti-inflammatory properties of curcumin. |
|||||
|
In vitro. Cytopathic inhibition assay. HSV-1. Vero cells. |
Curcumin, gallium-curcumin, and copper-curcumin inhibited the replication of HSV-1 with IC50 values of 33.0, 13.9, and 23.1 µg/mL, respectively. |
The mechanisms of action of both gallium-curcumin and copper-curcumin have been suggested to be investigated in further studies. |
CBP, CREB-binding protein; ChIP, chromatin immunoprecipitation; ED50, the concentration of drug that decreased the plaque number by 50%; HSV-1, herpes simplex virus 1; HSV-2, herpes simplex virus 2; IC50, 50% inhibitory concentration; IE gene, immediate early gene; NF-κB, nuclear factor kappa B.
Consequently, the mechanisms by which curcumin induces anti-HSV actions were investigated in depth in various in vitro and in vivo studies (Figure 2). These mechanisms were found to relate to the ability of curcumin to hinder a range of cellular and molecular processes, which are essential for viral gene expression, replication, and pathogenesis. For instance, curcumin was detected to inhibit IE gene expression and hindering HSV-1 infection by employing a mechanism independent of the transcriptional coactivator proteins p300/CBP histone acetyltransferase activity to impact the viral transactivator protein VP16-mediated enlistment of RNA polymerase II to IE gene promoters [50][41]. In preclinical studies (in vitro and in vivo), Bourne et al. [42] [51] stated that curcumin has effectively inhibited the replication of HSV-2. Besides, curcumin was reported to inhibit the replication of HSV-2 through a mechanism involving the transcription factor NF-κB [52][43]. Additional mechanisms were also verified in an in vitro experiment via inhibiting HSV-1 and HSV-2 adsorption [53][44]. In an in vivo investigation, the anti-inflammatory properties of curcumin nanoparticles were claimed to be one of the mechanisms responsible for inhibiting the infectivity and replication of HSV-2 [54][45]. Curcumin metal complexes such as gallium-curcumin and copper-curcumin were studied by Zandi et al. [55][46], where both complexes were identified to inhibit the replication of HSV-1. However, the mechanism of action has not been revealed, and further investigation needs to be performed. Recently, Treml and colleagues [13] [13] have stated that the hydroxyl groups and phenyl rings of phenolic compounds, including curcumin, are responsible for the induced anti-HSV properties reported for these molecules. This has been probed in various structure-activity relationship analyses.
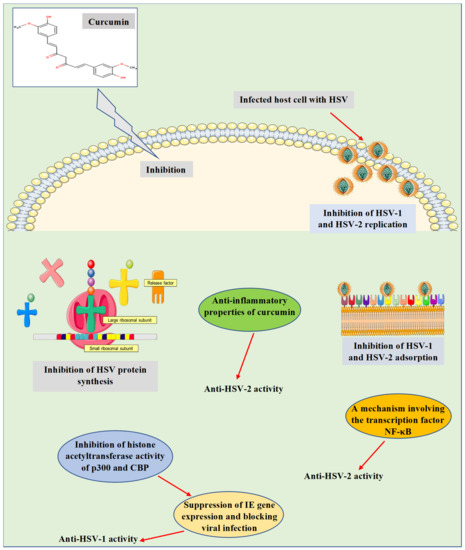
Figure 2. Antiviral properties and mechanisms of action of curcumin against herpes simplex virus infections. The presented mechanisms have been explored by in vitro and in vivo studies. CBP, CREB-binding protein; HSV-1, herpes simplex virus 1; HSV-2, herpes simplex virus 2; IE gene, immediate early gene; NF-κB, nuclear factor kappa B.
4. Curcumin Targets Thymidine Kinase Encoded by Herpes Simplex Virus
HSV encodes a set of enzymes that are essential for viral replication along with numerous enzymes involved in nucleotide metabolism that are not necessary for viral replication [2,13][2][13]. Such enzymes have been employed in various therapeutic strategies as valuable drug targets useful for the therapy of HSV diseases [2,40,56][2][31][47]. For instance, thymidine kinase (TK) is an essential enzyme that catalyzes the transfer of the gamma-phospho group of adenosine triphosphate (ATP) to thymidine, leading to generate thymidine monophosphate (dTMP). Thus, TK is a critical enzyme in the salvage pathway of pyrimidine synthesis [57][48]. This enzyme has been detected in both human and HSV with crucial molecular functions [58][49]. It has been well recognized that HSV-TK, which plays an imperative function in HSV pathogenesis, has a broad substrate specificity including pyrimidines and pyrimidine analogs (deoxycytidine, thymidine, and zidovudine) as well as purine (guanosine) analogs (acyclovir, ganciclovir, penciclovir, and buciclovir) [59,60][50][51]. Antiviral drugs that inhibit HSV DNA synthesis (for instance, acyclovir) require HSV-TK to be phosphorylated to monophosphate form. Cellular kinase enzymes then further phosphorylate the drug to the triphosphate form (an active form of the drug). Therefore, HSV TK plays an important role in the design of antiherpetic drugs as a critical mediator protein [61][52].
Recently, El-Halim et al. [53] [62] have reported in an in silico assay the ability of curcumin to successfully bind to the active site of HSV-1 TK via forming hydrogen bonding and hydrophobic interactions with the amino acid residues of the enzyme active site. The results proposed hydroxyl and carbonyl groups along with phenyl rings of curcumin as functional groups that are accountable for the anti-HSV-1 TK activity. Indeed, the obtained results revealed the mechanism underlying the anti-HSV-1 activity of curcumin through the inhibition of HSV-1 TK. On the other hand, this study might pave the way towards additional investigations, where curcumin could act synergistically with acyclovir and with other nucleoside analogs, leading to improving the treatment efficacy of HSV infections. Unlike the crucial role of TK in HSV pathogenesis, HSV-TK in combination with ganciclovir has been detected to be a promising therapy for melanoma. This has been revealed in an in vivo study using the xenografted melanoma model, where curcumin combined with HSV-TK/ganciclovir efficiently impeded xenografted melanoma growth, which in turn, provided a potential therapeutic approach for improving gene therapy efficacy against skin cancer [63][54].
5. Role of Curcumin in Inhibition of Various Herpesviruses Infections
In addition to the remarkable antiviral properties against HSV infections, curcumin has been shown to inhibit other human and animal herpesviruses investigated in various assay systems. This section highlights all studies that have reported the antiviral properties of curcumin and curcumin formulations against human cytomegalovirus (HCMV), Kaposi’s sarcoma-associated herpesvirus (KSHV), Epstein–Barr virus (EBV), bovine herpesvirus 1 (BoHV-1), and pseudorabies virus (PRV) infections with a focus on concentrations or doses used along with the mechanisms of action (Table 2).
[ | |||
] | [ | 59] |
|
|
BoHV-1 (in vitro). |
At a concentration of 10 µM, curcumin reduced BoHV-1 titer, leading to inhibiting viral replication. Co-encapsulation of acyclovir and curcumin into three microparticle formulations noticeably reduced the BoVH-1 plaque formation at a concentration of 75 µg/mL. |
Inhibition of virus post-binding entry process by upregulating the lipid raft formation. |
|
|
PRV (in vitro). |
Treatment with curcumin (30 µM) blocked PRV infectivity in PK-15 cells by decreasing the viral plaque formation. |
No mechanism of action was revealed. |
APE1, apurinic/apyrimidinic endonuclease 1; BoHV-1, bovine herpesvirus 1; EBV, Epstein–Barr virus; HCMV, human cytomegalovirus; Hsp90, heat shock protein 90; IEA, immediate early antigen; KSHV, Kaposi’s sarcoma-associated herpesvirus; PK, porcine kidney; PRV, pseudorabies virus.
HCMV is a human beta-herpesvirus that causes lifelong infection in the host and has been involved in the development of several diseases in humans, generating severe complications in immunocompromised individuals [64,65]. At various micromolar concentrations, the anti-HCMV activity of curcumin was explored in vitro, in an animal model (Balb/c mice) and in silico experiments. The underlying mechanisms were clarified through the capacity of curcumin to impede HCMV immediate early antigen (IEA) and UL83A expressions [55] [66] and downregulate the heat shock protein 90 (Hsp90) [56] [67] (Figure 3). Through a molecular docking analysis, curcumin was also found to accurately bind to the binding pocket of Hsp90 by establishing crucial contacts such as hydrogen bonds and hydrophobic interactions [67][56]. Moreover, the anti-inflammatory and antioxidant effects of curcumin were identified as possible mechanisms behind the antiviral action against HCMV [68][57].
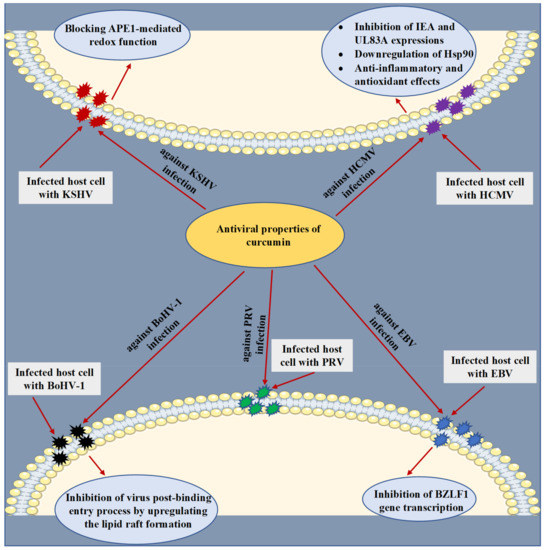
Antiviral properties and mechanisms of action of curcumin against human cytomegalovirus (HCMV), Kaposi’s sarcoma-associated herpesvirus (KSHV), Epstein–Barr virus (EBV), bovine herpesvirus 1 (BoHV-1), and pseudorabies virus (PRV) infections. The displayed mechanisms have been investigated by in vitro, in vivo, and in silico studies. APE1, apurinic/apyrimidinic endonuclease 1; Hsp90, heat shock protein 90; IEA, immediate early antigen.
KSHV is a gamma-herpesvirus (human herpesvirus 8) known to be one of the DNA tumor viruses involved in the etiology of human cancers [69,70]. It has been reported that the redox function of apurinic/apyrimidinic endonuclease 1 (APE1) plays a crucial role in the replication of KSHV [71,72]. Accordingly, Li et al. [58] [73] examined the antiviral action of curcumin against the replication of KSHV. To reactivate the virus, primary effusive lymphoma (PEL)-BCBL-1 cells were latently infected with the virus and further were treated with 12-O-Tetradecanoyl-phorbol-13-acetate (TPA) and curcumin. The results indicated that treatment with curcumin at a concentration of 30 µM has led to significant blocking of KSHV reactivation by reducing expression of the switch gene replication and transcription activator (RTA) and the delayed-early gene K8. Additionally, with an IC50 value of 8.76 µM and an EC50 value of 6.68 µM, curcumin has notably reduced intracellular and extracellular KSHV genomic DNA levels, respectively. Eventually, the outcome of the study indicated that curcumin diminished the replication of KSHV by blocking APE1-mediated redox function.
EBV is a gamma-herpesvirus known to infect humans that induces mononucleosis and is critically linked with multiple malignancies, including nasopharyngeal carcinoma, Burkitt lymphoma, Hodgkin lymphoma, gastric carcinoma, and posttransplant lymphoproliferative illnesses. Moreover, EBV was detected to be associated with some autoimmune diseases such as systemic sclerosis, multiple sclerosis, and systemic lupus erythematosus [74,75,76]. Hergenhahn and colleagues [77][59] have proved that curcumin (15 µM) inhibited the EBV reactivation in Raji DR-CAT cells by a mechanism of suppressing BZLF1 gene transcription.
BoHV-1 is an enveloped DNA herpesvirus that infects cattle and causes a severe respiratory infection, leading to significant economic losses for the cattle industry [78,79]. In an in vitro experiment using Madin-Darby Bovine Kidney (MDBK) cells, curcumin (10 µM) substantially reduced viral titer, leading to inhibiting viral replication. The authors suggested that the anti-BoHV-1 activity of curcumin could be related to its capability to upregulate lipid raft formation [80][60]. On the other hand, co-encapsulation of acyclovir and curcumin into three microparticle formulations composed of the polymers hydroxypropyl methylcellulose, Eudragit® RS100, or both were recently developed by Reolon et al. [81] [61] for improving the antiviral efficacy against BoVH-1 infection. All three microparticle formulations were tested in infected MDBK cells with BoVH-1 and were observed to reduce the viral plaque formation at a concentration of 75 µg/mL.
PRV is an infectious herpesvirus that belongs to the Alphaherpesvirinae subfamily. This virus is an etiological agent of Aujeszky’s disease, a viral infection that primarily affects pigs, causing reproductive and severe neurological complications in affected animals [82,83]. By employing an in vitro assay, the anti-PRV activity of curcumin was studied by infecting the porcine kidney (PK-15) cells with PRV and then treating them with curcumin at a concentration of 30 µM. The research demonstrated that curcumin effectively inhibited the infectivity of PRV by reducing viral plaque formation. On the other hand, the results did not report any mechanism of action [84][62].
References
- Brezáni, V.; Leláková, V.; Hassan, S.T.S.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360.
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Šmejkal, K.; Echeverría, J. Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties. Molecules 2019, 24, 2912.
- Hassan, S.T.S.; Šudomová, M.; Masarčíková, R. Herpes simplex virus infection: An overview of the problem, pharmacologic therapy and dietary measures. Ceska Slov. Farm. 2017, 66, 95–102.
- Ho, D.Y.; Enriquez, K.; Multani, A. Herpesvirus Infections Potentiated by Biologics. Infect. Dis. Clin. N. Am. 2020, 34, 311–339.
- Roizman, B.; Pellett, P.E. The family Herpesviridae: A brief introduction. In Fields—Virology, 4th ed.; Knipe, D.M., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 2381–2397.
- Johnston, B.P.; McCormick, C. Herpesviruses and the Unfolded Protein Response. Viruses 2019, 12, 17.
- Cohen, J.I. Herpesvirus latency. J Clin Investig. 2020, 130, 3361–3369.
- Pellett, P.; Roizman, B. Herpesviridae. In Fields Virology; Knipe, D., Howley, P., Eds.; Lippincott, Wil-liams and Wilkins: Philadelphia, PA, USA, 2013; pp. 1802–1822.
- Savva, R. The Essential Co-Option of Uracil-DNA Glycosylases by Herpesviruses Invites Novel Antiviral Design. Microorganisms 2020, 8, 461.
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336.
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple In vitro biological effects of phenolic compounds from Morus alba root bark. J. Ethnopharmacol. 2020, 248, 112296.
- Zheng, W.; Xu, Q.; Zhang, Y.; Xiaofei, E.; Gao, W.; Zhang, M.; Zhai, W.; Rajkumar, R.S.; Liu, Z. Toll-like receptor-mediated innate immunity against herpesviridae infection: A current perspective on viral infection signaling pathways. Virol. J. 2020, 17, 192.
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154.
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B interacts synergistically with antibiotics against Staphylococcus aureus clinical isolates and exhibits antiviral activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94.
- Andreu, S.; Ripa, I.; Bello-Morales, R.; López-Guerrero, J.A. Valproic Acid and Its Amidic Derivatives as New Antivirals against Alphaherpesviruses. Viruses 2020, 12, 1356.
- Hassan, S.T.S. Shedding Light on the Effect of Natural Anti-Herpesvirus Alkaloids on SARS-CoV-2: A Treatment Option for COVID-19. Viruses 2020, 12, 476.
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398.
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714.
- Milobedeska, J.; Kostanecki, S.; Lampe, V. Zur kenntnis des curcumins. Ber. Deut. Chem. Ges. 1910, 43, 2163–2170.
- Lampe, V.; Milobedeska, J. Studien über curcumin. Eur. J. Pharm. Biopharm. 1913, 46, 2235–2240.
- Zhou, Y.; Xie, M.; Song, Y.; Wang, W.; Zhao, H.; Tian, Y.; Wang, Y.; Bai, S.; Zhao, Y.; Chen, X.; et al. Two traditional Chinese medicines Curcumae radix and Curcumae Rhizoma: An ethnopharmacology, phytochemistry, and pharmacology review. Evid. Based Complement. Altern. Med. 2016, 2016, 4973128.
- Seidi Damyeh, M.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759.
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995.
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front Microbiol. 2019, 10, 912.
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92.
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930.
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014, 2014, 186864.
- Jha, A.; Mohapatra, P.P.; AlHarbi, S.A.; Jahan, N. Curcumin: Not So Spicy After All. Mini Rev. Med. Chem. 2017, 17, 1425–1434.
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426.
- Adler, B.; Sattler, C.; Adler, H. Herpesviruses and Their Host Cells: A Successful Liaison. Trends Microbiol. 2017, 25, 229–241.
- Hassan, S.T.S. Brassicasterol with Dual Anti-Infective Properties against HSV-1 and Mycobacterium tuberculosis, and Cardiovascular Protective Effect: Nonclinical in Vitro and In Silico Assessments. Biomedicines 2020, 8, 132.
- Memish, Z.A.; Almasri, M.; Chentoufi, A.A.; Al-Tawfiq, J.A.; Al-Shangiti, A.M.; Al-Kabbani, K.M.; Otaibi, B.; Assirri, A.; Yezli, S. Seroprevalence of Herpes Simplex Virus Type 1 and Type 2 and Coinfection with HIV and Syphilis: The First National Seroprevalence Survey in Saudi Arabia. Sex. Trans. Dis. 2015, 42, 526–532.
- Ma, W.; He, H.; Wang, H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018, 19, 40.
- Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722.
- Johnston, C.; Corey, L. Current Concepts for Genital Herpes Simplex Virus Infection: Diagnostics and Pathogenesis of Genital Tract Shedding. Clin. Microbiol. Rev. 2016, 29, 149–161.
- Widener, R.W.; Whitley, R.J. Herpes simplex virus. Handb. Clin. Neurol. 2014, 123, 251–263.
- Goins, W.F.; Hall, B.; Cohen, J.B.; Glorioso, J.C. Retargeting of herpes simplex virus (HSV) vectors. Curr. Opin. Virol. 2016, 21, 93–101.
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111.
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968.
- Dai, J.; Gu, L.; Su, Y.; Wang, Q.; Zhao, Y.; Chen, X.; Deng, H.; Li, W.; Wang, G.; Li, K. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int. Immunopharmacol. 2018, 54, 177–187.
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247.
- Bourne, K.Z.; Bourne, N.; Reising, S.F.; Stanberry, L.R. Plant products as topical microbicide candidates: Assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 1999, 42, 219–226.
- Ferreira, V.H.; Nazli, A.; Dizzell, S.E.; Mueller, K.; Kaushic, C. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PLoS ONE 2015, 10, e0124903.
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of Curcumin-Treated Herpes Simplex Virus 1 and 2 in Vero Cells. Adv. Microbiol. 2016, 6, 276–287.
- Vitali, D.; Bagri, P.; Wessels, J.M.; Arora, M.; Ganugula, R.; Parikh, A.; Mandur, T.; Felker, A.; Garg, S.; Kumar, M.R.; et al. Curcumin Can Decrease Tissue Inflammation and the Severity of HSV-2 Infection in the Female Reproductive Mucosa. Int. J. Mol. Sci. 2020, 21, 337.
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat. Prod. Commun. 2010, 5, 1935–1938.
- Poole, C.L.; James, S.H. Antiviral Therapies for Herpesviruses: Current Agents and New Directions. Clin. Ther. 2018, 40, 1282–1298.
- Hannigan, B.M.; Barnett, Y.A.; Armstrong, D.B.; McKelvey-Martin, V.J.; McKenna, P.G. Thymidine kinases: The enzymes and their clinical usefulness. Cancer Biother. 1993, 8, 189–197.
- Fujii, H.; Harada, S.; Yoshikawa, T.; Yamada, S.; Omura, N.; Shibamura, M.; Inagaki, T.; Kato, H.; Fukushi, S.; Saijo, M. Differences in the Likelihood of Acyclovir Resistance-Associated Mutations in the Thymidine Kinase Genes of Herpes Simplex Virus 1 and Varicella-Zoster Virus. Antimicrob. Agents Chemother. 2019, 63, e00017–e00019.
- Coen, N.; Duraffour, S.; Haraguchi, K.; Balzarini, J.; van den Oord, J.J.; Snoeck, R.; Andrei, G. Antiherpesvirus activities of two novel 4′-thiothymidine derivatives, KAY-2-41 and KAH-39-149, are dependent on viral and cellular thymidine kinases. Antimicrob. Agents Chemother. 2014, 58, 4328–4340.
- Topalis, D.; Gillemot, S.; Snoeck, R.; Andrei, G. Thymidine kinase and protein kinase in drug-resistant herpesviruses: Heads of a Lernaean Hydra. Drug Resist. Updat. 2018, 37, 1–16.
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941.
- El-Halim, S.M.A.; Mamdouh, M.A.; El-Haddad, A.E.; Soliman, S.M. Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Sci. Pharm. 2020, 88, 9.
- Li, H.; Du, H.; Zhang, G.; Wu, Y.; Qiu, P.; Liu, J.; Guo, J.; Liu, X.; Sun, L.; Du, B.; et al. Curcumin plays a synergistic role in combination with HSV-TK/GCV in inhibiting growth of murine B16 melanoma cells and melanoma xenografts. PeerJ 2019, 7, e7760.
- Lv, Y.; An, Z.; Chen, H.; Wang, Z.; and Liu, L. Mechanism of curcumin resistance to human cytomegalovirus in HELF cells. BMC Complement. Altern. Med. 2014, 14, 284.
- Lv, Y.; Gong, L.; Wang, Z.; Han, F.; Liu, H.; Lu, X.; Liu, L. Curcumin inhibits human cytomegalovirus by downregulating heat shock protein 90. Mol. Med. Rep. 2015, 12, 4789–4793.
- Lv, Y.; Lei, N.; Wang, D.; An, Z.; Li, G.; Han, F.; Liu, H.; Liu, L. Protective effect of curcumin against cytomegalovirus infection in Balb/c mice. Environ. Toxicol. Pharmacol. 2014, 37, 140–147.
- Li, H.; Zhong, C.; Wang, Q.; Chen, W.; Yuan, Y. Curcumin is an APE1 redox inhibitor and exhibits an antiviral activity against KSHV replication and pathogenesis. Antivir. Res. 2019, 167, 98–103.
- Hergenhahn, M.; Soto, U.; Weninger, A.; Polack, A.; Hsu, C.H.; Cheng, A.L.; Rosl, F. The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BZLF1 transcription in Raji DR-LUC cells. Mol. Carcinog. 2002, 33, 137–145.
- Zhu, L.; Ding, X.; Zhang, D.; Yuan, C.; Wang, J.; Ndegwa, E.; Zhu, G. Curcumin inhibits bovine herpesvirus type 1 entry into MDBK cells. Acta Virol. 2015, 59, 221–227.
- Reolon, J.B.; Brustolin, M.; Accarini, T.; Viçozzi, G.P.; Sari, M.H.M.; Bender, E.A.; Haas, S.E.; Brum, M.C.S.; Gündel, A.; Colomé, L.M. Co-encapsulation of acyclovir and curcumin into microparticles improves the physicochemical characteristics and potentiates in vitro antiviral action: Influence of the polymeric composition. Eur. J. Pharm. Sci. 2019, 131, 167–176.
- Chen, T.Y.; Chen, D.Y.; Wen, H.W.; Ou, J.L.; Chiou, S.S.; Chen, J.M.; Wong, M.L.; Hsu, W.L. Inhibition of enveloped viruses infectivity by curcumin. PLoS ONE 2013, 8, e62482.
