Patients with sarcoidosis are characterized by lung predilection, and in some cases, the imaging features maybe similar to those of the patients with COVID-19 pneumonia. Sarcoidosis is a granulomatous disorder characterized by diffusion of noncaseating and non-necrotizing granulomas, lungs are the most affected site even though no organ is spared.
- sarcoidosis
- SARS-CoV-2 infection
1. Introduction
The recent COVID-19 pandemic has dramatically changed the world in the last months, leading to a serious global emergency related to a novel coronavirus infection, characterized by high infectivity and severe clinical pictures affecting mainly the respiratory system [1].
Symptoms can affect both sexes and all ages ubiquitously, but it is actually unknown how in some cases there is a significantly higher degree of severity, with the presence of extensive lung involvement, respiratory failure, and need of invasive ventilation. Advanced age, blood group predisposition, and viral load have been hypothesized as some of the risk factors. Moreover, comorbidity, such as cardiovascular disease (CVD) seems to play a role, but these data do not explain the severity observed sometimes in younger and otherwise healthy age groups [1,2][1][2].
The role in patients affected with many other diseases, in particular immune disorders, also remains unclear, and although the COVID-19 infection seems to interact with the immune system by leading to an energetic T-cell activation and B-cell antibody production, it remains unknown how the interaction differs in patients with an altered immune system, in particular in cases characterized by impairment of the T-cell immunity and granuloma formation, as in sarcoidosis [3].
Interestingly, patients with sarcoidosis are characterized by lung predilection, and in some cases, the imaging features maybe similar to those of the patients with COVID-19 pneumonia. Sarcoidosis is a granulomatous disorder characterized by diffusion of noncaseating and non-necrotizing granulomas, lungs are the most affected site even though no organ is spared [4,5][4][5]. The role of infection from the novel coronavirus of patients having sarcoidosis is still largely unknown [3], and some authors suggest high attention for these patients, that are at risk of serious complications and clinical deterioration.
2. Imaging Findings of Lung Involvement from Sarcoidosis and COVID-19 Pneumonia
2.1. Imaging Findings of Sarcoidosis.
2.1.1. The Scadding Classification at the Chest X-ray
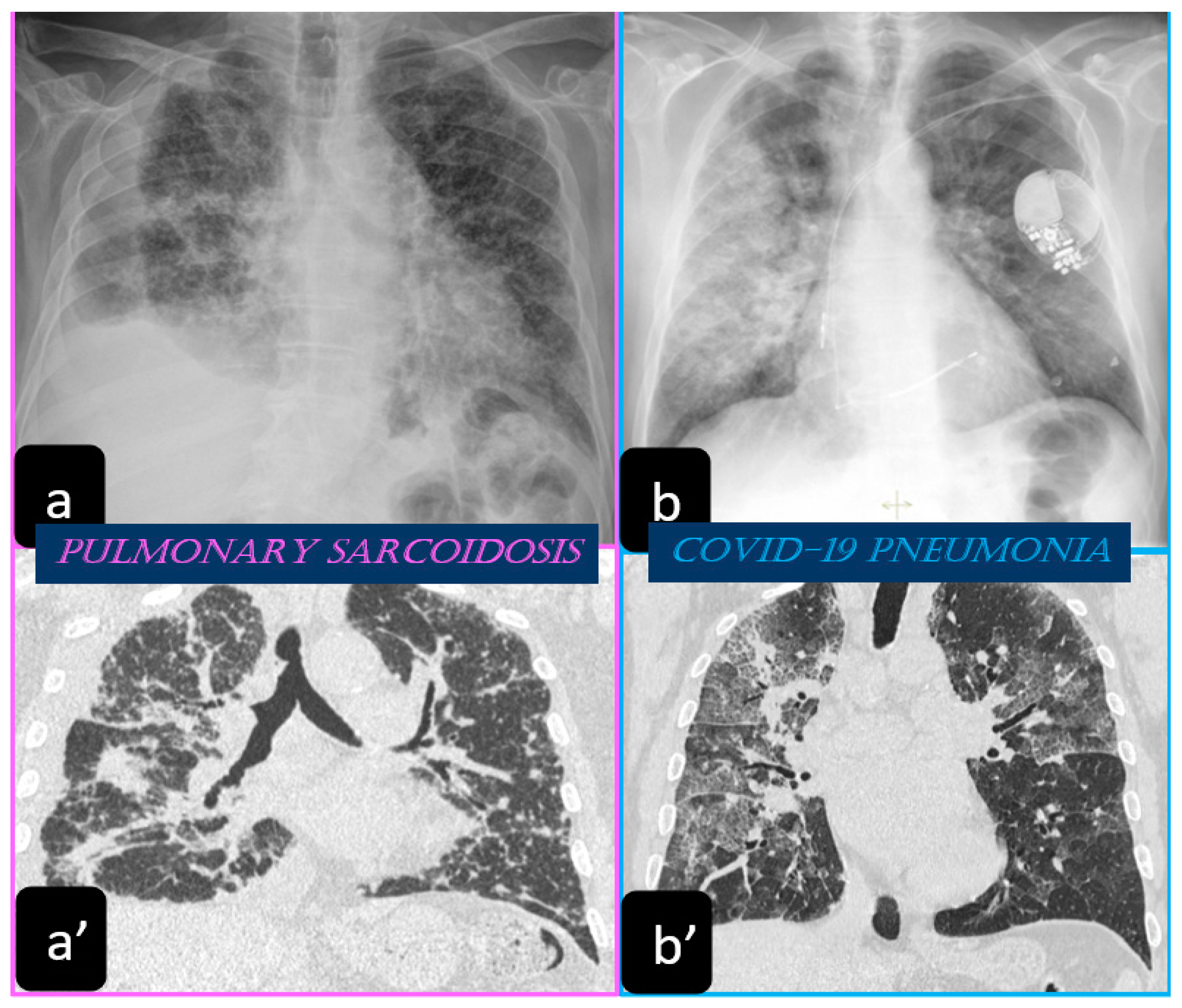
Traditionally, the chest X-ray has been used for several years to reveal the lung involvement of sarcoidosis. Four classes of involvement, according to the disease extension and severity, can be classified. Stage I defines the presence of bilateral hilar adenopathy, stage II shows the presence of bilateral hilar adenopathy with pulmonary infiltrates, while stage III shows the evidence of pulmonary infiltrates without the presence of overt hilar adenopathy (Figure 1a,a’), and stage IV shows the extensive lung involvement from pulmonary fibrosis. The chest X-ray has the limit of reduced resolution, and minimal parenchymal alterations can be missed at a routine lung examination [6].
2.1.2. Typical and Atypical Manifestation of Pulmonary Sarcoidosis at HRCT
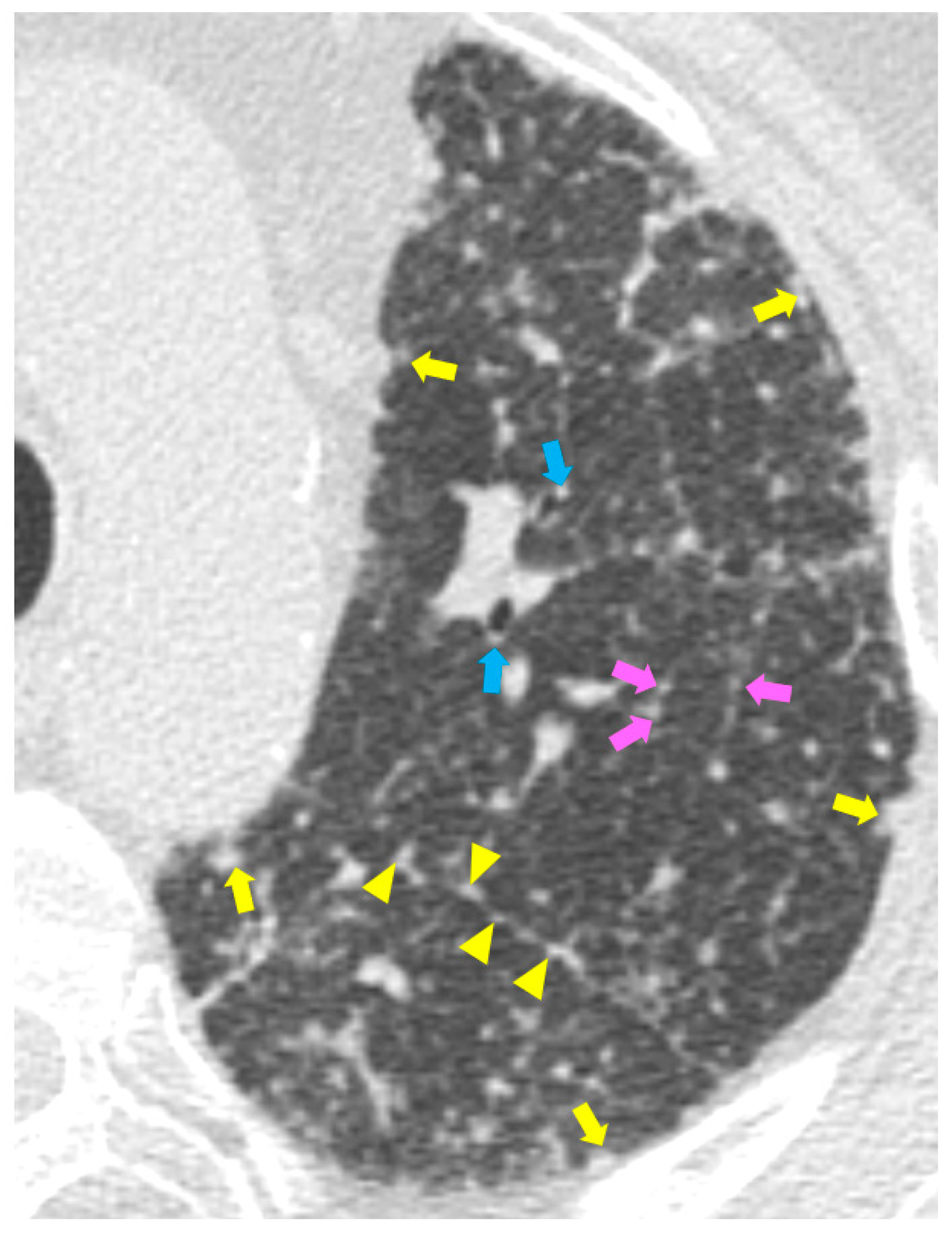
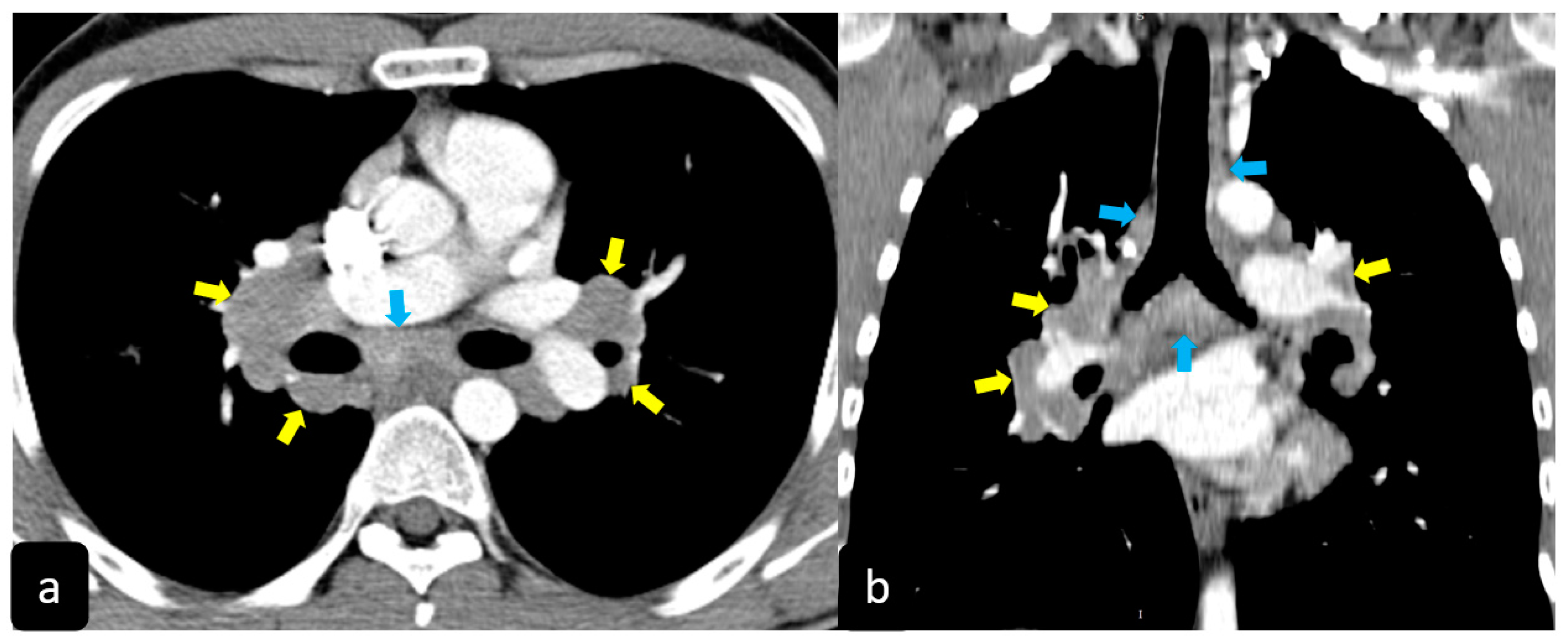
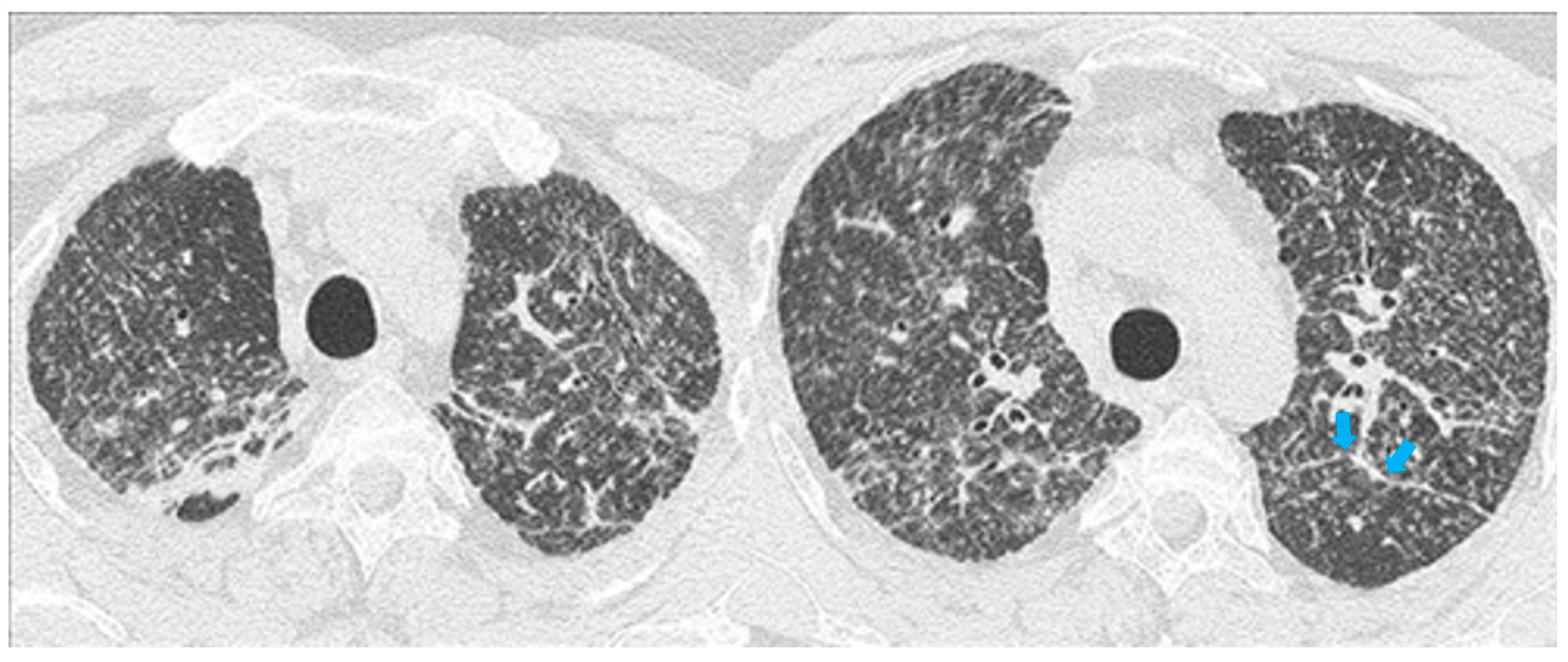
High resolution computed tomography (HRCT) has a higher sensitivity than the chest X-ray and lymph node enlargement, commonly with a size of 2–5 mm, and the specific impairment of lungs are visualized more in detail. Parenchymal involvement can be minimal or characterized by bilateral parenchymal infiltrates that tend to merge into large and irregular pulmonary opacities. The most common and important parenchymal finding is the presence of micronodules (granulomas; 2–4 mm in diameter; well defined and bilateral) with a typical perilymphatic distribution along the peribronchovascular and subpleural interstitial space and interlobular septa (Figure 1a’ and Figure 2). CT is more sensitive than the chest x-ray in the identification of characteristic hilar (Figure 3a) and mediastinal (Figure 3b) lymphadenopathy [3]. A wide variety of less specific alterations can be found, such as unilateral or isolated lymphadenopathy, solitary nodules, confluent alveolar opacities, (the so-called alveolar sarcoid pattern), linear opacities, conglomerate masses, thickened interlobar septa, cysts, blebs, isolated bullae, or diffuse emphysema (Figure 4). Another typical imaging finding of sarcoidosis is the galaxy sign, a mass-like lesion, composed of numerous smaller coalescing granulomatous nodules, more concentrated in the center of the lesion (Figure 4c). The appearance of a central core with peripheral nodules is reminiscent of a globular cluster or galaxy [7,8][7][8].
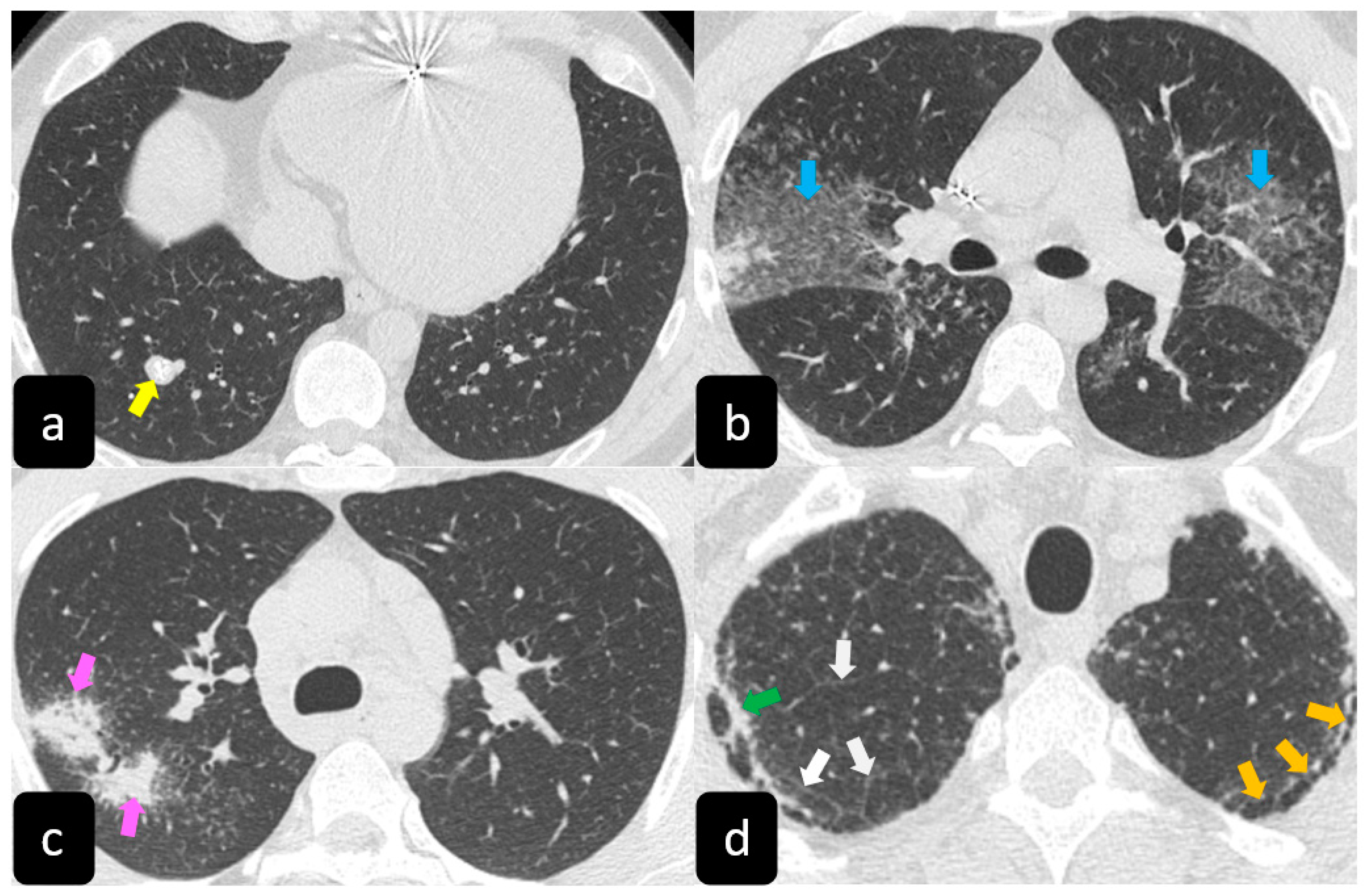
The presence of tracheobronchial abnormalities, atelectasis, or pleural involvement with thickening, effusions, calcification, pneumothorax, or plaque-like opacities such as aspergilloma and mycetoma can also be found [7,9,10][7][9][10]. Another atypical finding is represented by the ubiquitous presence of miliary opacities, that can mimic the parenchymal alterations observed in advanced tuberculosis [11,12][11][12]. Imaging findings can also be dominated, in an advanced stage, by architectural distortion, honeycombing-like opacities, traction bronchiectasis, and extended fibrosis (Figure 5). This group of findings represents a marker of poor outcome, being the evidence of irreversible alterations that are less likely to improve after therapy [13,14][13][14]. Clinical risk factors for fibrosis are illness severity, advanced age, history of smoking and alcoholism, prolonged ICU stay, and mechanical ventilation [15].
2.2. Imaging Findings of COVID-19 Pneumonia
2.2.1. Chest X-ray Findings
Chest radiographs show a limited value in the diagnosis of early stages mostly in the mild disease course. Rather, the HRCT findings may be present early even before the symptoms onset. Chest radiographs are useful in the intermediate to advanced stages of COVID-19 and for monitoring the rapid progression of lung abnormalities pneumonia, especially in critical patients admitted to intensive care units [16].
The most common chest X-ray features detected in COVID-19 cases were bilateral consolidation/ground glass haze (Figure 1b) and reticular interstitial thickening [16].
2.2.2. HRCT Findings of Lung Involvement from COVID-19
CT is now considered the main investigator for COVID-19, as it offers more sensitive results than the chest X-ray, especially in the initial assessment of the patients.
Lung involvement from COVID-19 is characterized by a broad spectrum of parenchymal alterations.
Typical findings that are hallmarks of the disease are represented by ground glass opacities (GGO) having a bilateral distribution, with or without posterior or peripheral lung consolidation (Figure 6) [17]. Usually, consolidations are an expression of disease progression after 1-3 weeks from the onset (Figure 7) [18].
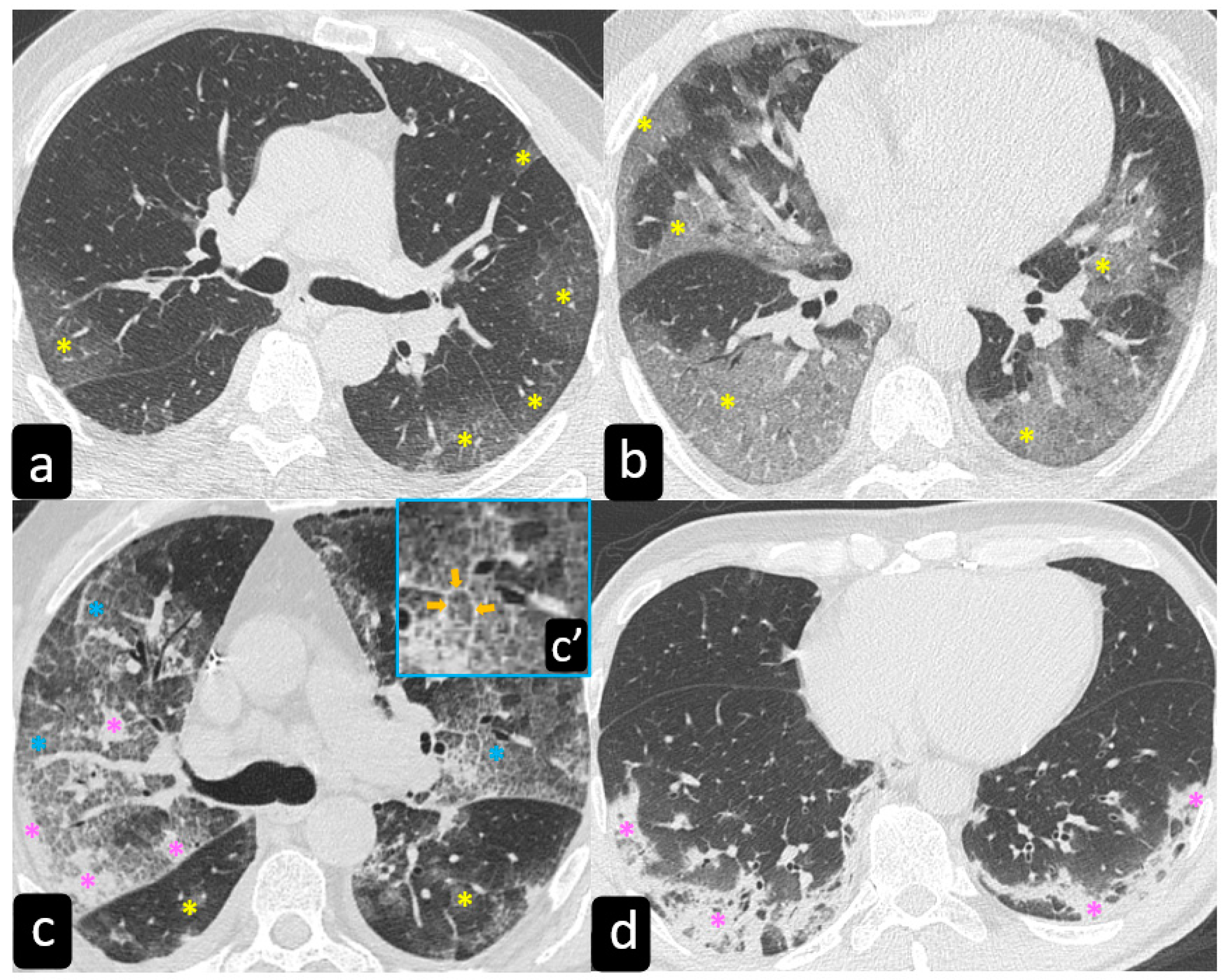
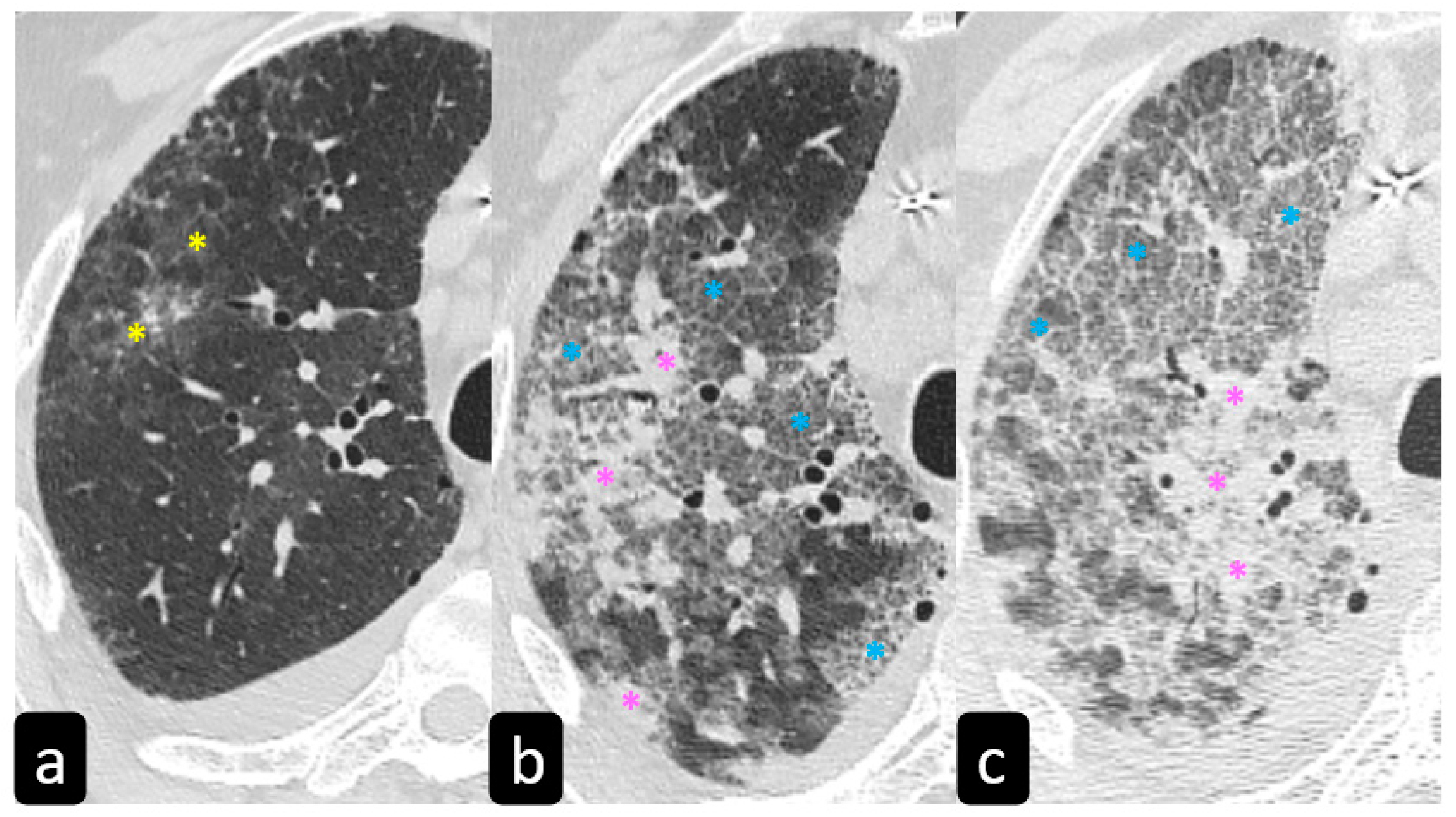
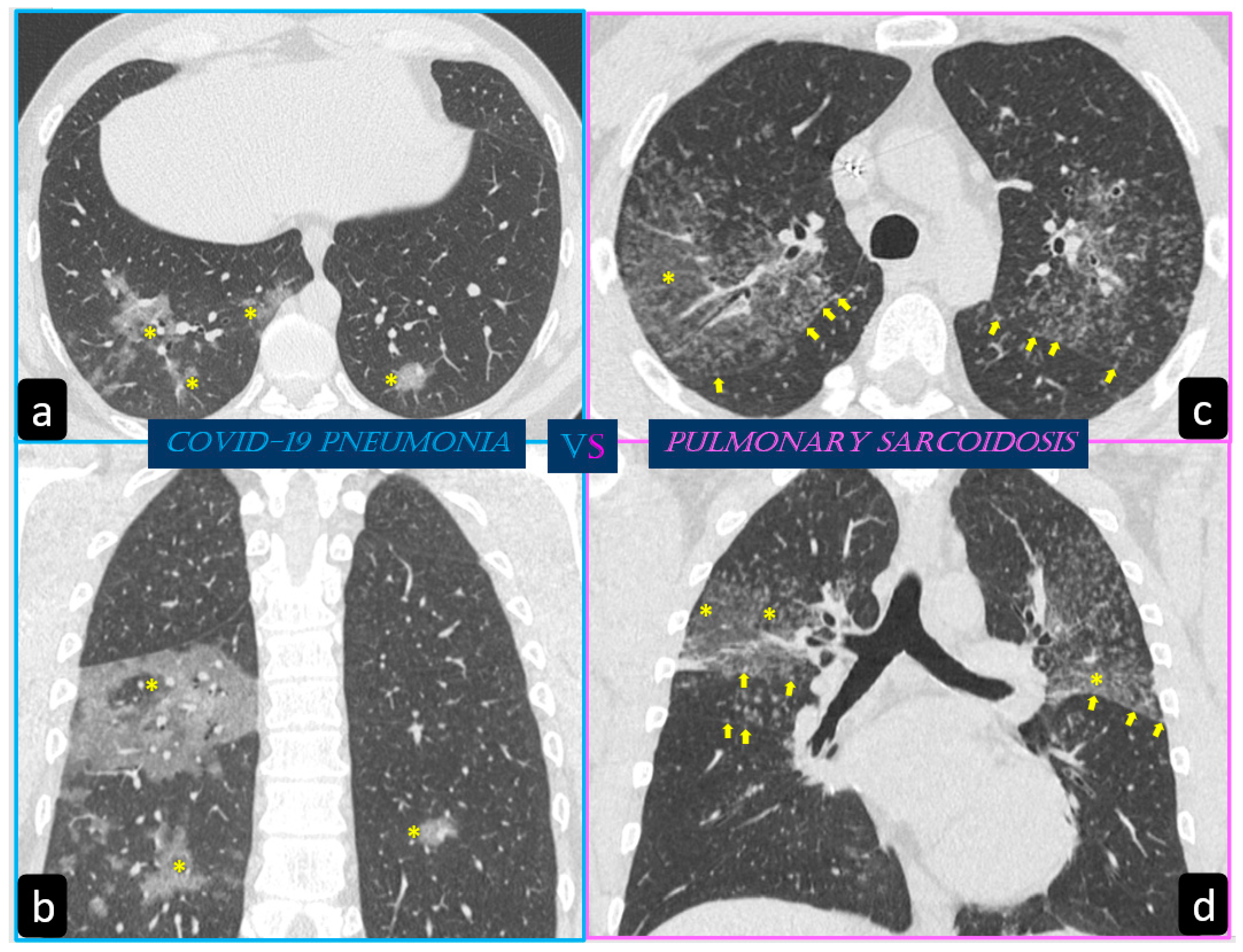
By contrast, predominant GGO are rare and nonspecific findings in pulmonary sarcoidosis, being most typical in other diseases such as infections, pulmonary alveolar proteinosis, nonspecific interstitial pneumonia, alveolar hemorrhage, and also lung carcinoma [19]. In sarcoidosis, opacification is variably reversible, predicting the presence of alveolitis (Figure 8) [20][21]. As mentioned before, however, alveolar sarcoidosis is an atypical CT finding in sarcoidosis and without a specific pattern. In this setting, a hallmark of lung sarcoidosis can be the upper lobes preference, differently from COVID-19 pneumonia, which is characterized by a predominantly peripheral distribution and a lower lobes preference (Figure 8).
3. Conclusions
In a situation where the spread of COVID-19 is progressively affecting a large portion of the population, it remains necessary to investigate the effects and interactions of this disease in patients suffering from other pathologies. Since sarcoidosis is a disease with pulmonary predilection and has characteristics which are sometimes similar to those observed in COVID-19 patients, it is important to distinguish what aspects can be distinctive of one disease or the other. HRCT, and the related recent evolution of deep learning techniques, could provide additional key points for diagnosing which condition is prevalent, being important for the treatment and outcome of patients that can have both disorders in an acute phase of the disease.
References
- Stawicki, S.P.; Jeanmonod, R.; Miller, A.C.; Paladino, L.; Gaieski, D.F.; Yaffee, A.Q.; De Wulf, A.; Grover, J.; Papadimos, T.J.; Bloem, C.; et al. The 2019–2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: A joint american college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group consensus paper. J. Glob. Infect. Dis. 2020, 12, 47–93.
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Gómez-Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020, 98, 115094.
- Tana, C.; Schiavone, C.; Cipollone, F.; Giamberardino, M.A. Management issues of sarcoidosis in the time of COVID-19. Chest 2021, 159. in press.
- Chokoeva, A.A.; Tchernev, G.; Tana, M.; Tana, C. Exclusion criteria for sarcoidosis: A novel approach for an ancient disease? Eur. J. Intern. Med. 2014, 25, E120.
- Ricci, F.; Mantini, C.; Grigoratos, C.; Bianco, F.; Bucciarelli, V.; Tana, C.; Mastrodicasa, D.; Caulo, M.; Aquaro, G.D.; Cotroneo, A.R.; et al. The Multi-modality Cardiac Imaging Approach to Cardiac Sarcoidosis. Curr. Med. Imaging Formerly Curr. Med. Imaging Rev. 2018, 15, 10–20.
- Scadding, J.G. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br. Med. J. 1961, 2, 1165–1172.
- Criado, E.; Sánchez, M.; Ramírez, J.; Arguis, P.; De Caralt, T.M.; Perea, R.J.; Xaubet, A. Pulmonary Sarcoidosis: Typical and Atypical Manifestations at High-Resolution CT with Pathologic Correlation. Radiographics 2010, 30, 1567–1586.
- Tchernev, G.; Chokoeva, A.A.; Schiavone, C.; D Erme, A.M.; Tana, C.; Darling, M.; Kaley, J.; Gianfaldoni, S.; Wollina, U.; Lotti, T.; et al. Sarcoidosis exclusion criteria: The simple truth for a “complicated diagnosis. J. Biol. Regul. Homeost. Agents 2015, 29, 5–9.
- Cozzi, D.; Bargagli, E.; Calabrò, A.G.; Torricelli, E.; Giannelli, F.; Cavigli, E.; Miele, V. Atypical HRCT manifestations of pulmonary sarcoidosis. La Radiol. Med. 2018, 123, 174–184.
- Tana, C.; Tchernev, G.; Chokoeva, A.A.; Wollina, U.; Lotti, T.; Fioranelli, M.; Roccia, M.G.; Maximov, G.K.; Silingardi, M. Pulmonary and abdominal sarcoidosis, the great imitators on imaging? J. Biol. Regul. Homeost Agents. 2016, 30, 45–48.
- Arar, O.; Boni, F.; Meschi, T.; Tana, C. Pulmonary Sarcoidosis Presenting with Miliary Opacities. Curr. Med. Imaging Formerly Curr. Med. Imaging Rev. 2019, 15, 81–83.
- Rajagopala, S.; Sankari, S.; Kancherla, R.; Ramanathan, R.P.; Balalakshmoji, D. Miliary Sarcoidosis: Does it exist? A case series and systematic review of literature. Sarcoidosis Vasc. Diffuse Lung Dis. 2020, 37, 53–65.
- Patel, D.C.; Valeyre, D. Advanced pulmonary sarcoidosis. Curr. Opin. Pulm. Med. 2020, 26, 574–581.
- Tchernev, G.; Chokoeva, A.A.; Tana, C.; Patterson, J.W.; Wollina, U.; Lotti, T. Sarcoid sine sarcoidosis? A classificative, semantic and therapeutic dilemma. J. Boil. Regul. Homeost. agents 2015, 29, 33–34.
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 1–10.
- Borghesi, A.; Maroldi, R. COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. La Radiol. Med. 2020, 125, 509–513.
- Ye, Z.; Zhang, Y.; Wang, Y.; Huang, Z.; Song, B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur. Radiol. 2020, 30, 4381–4389.
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721.
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434.
- Infante, M.; Lutman, R.F.; Imparato, S.; Di Rocco, M.; Ceresoli, G.L.; Torri, V.; Morenghi, E.; Minuti, F.; Cavuto, S.; Bottoni, E.; et al. Differential diagnosis and management of focal ground-glass opacities. Eur. Respir. J. 2009, 33, 821–827.
- Wells, A. High resolution computed tomography in sarcoidosis: A clinical perspective. Sarcoidosis Vasc. Diffus. lung Dis. Off. J. WASOG 1998, 15, 140–146.
