Radiotherapy is involved in the treatment of many cancers, but damage induced to the surrounding normal tissue is often inevitable. Evidence suggests that the maintenance of homeostasis and regeneration of normal tissues is driven by specific adult tissue stem/progenitor cells. These tasks involve the input from several signaling pathways. Irradiation also targets these stem/progenitor cells, triggering a cellular response aimed at achieving tissue regeneration.
- Radiotherapy
- stem cells
- signaling pathways
- regeneration
Note: The following contents are extracted from your paper. The entry will be online only after author check and submit it.
1. Introduction
One of the main limitations of radiotherapy (RT) is the damage induced to the healthy tissue positioned unavoidably in the radiation field. Radiation-induced side effects can be linked to the loss of tissue stem cells (SCs) and damage accumulation in the remaining stem/progenitor cells. This may result in acute or late adverse effects depending on the number of surviving stem/progenitor cells. A better understanding of SC response and the pathways that orchestrate the regenerative response of the stem/progenitor pool in tissues to RT can help to predict unavoidable toxicity and aid to prevent or repair radiation-induced damage. In this review, we summarize our current understanding of the pathways that may promote solid tissue SC response to RT and the current models used to characterize RT response.
2. Models to Study SC Response to Radiation
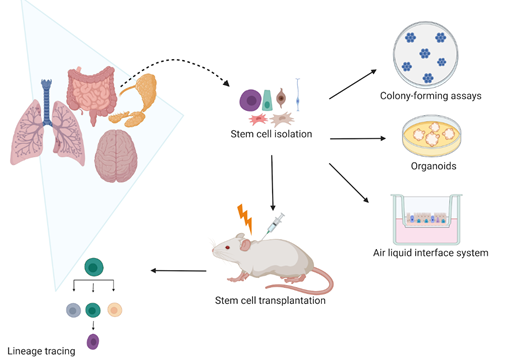
Many studies have assessed the self-renewal and differentiation potential of SCs upon irradiation (IR). These include two-dimensional (2D) and three-dimensional (3D) in vitro clonogenic studies of cell lines, spheroids, and organoids, replating assays, and in vivo lineage tracing (Figure 1). Although many IR studies have used submerged culture procedures, such as clonogenic and replating studies, they are unable to mimic the actual in vivo microenvironment and organ functionality [1][2]. The 3D models, such as spheroids, organoids, air–liquid interface (ALI) systems, and organ-on-chips recapitulate the organ structure, seem to better reflect the patient-specific response compared to in vitro 2D cell line models and enable assessment of in vitro SC responses to IR [3].
Figure 1. Current models use to assess stem cell radiation response in vivo and in vitro. In vitro, the self-renewal potential of stem cells is evaluated by assessing their colony-forming efficiency in clonogenic assays. The stem cell self-renewal potential is also studied in three-dimensional (3D) organoids and air–liquid interface (ALI) systems that not only allow stem cell radiation response studies but also their differentiation capacity upon irradiation. In vivo, the stem cell lineage tracing remains the most used model that enables to specifically mark stem cells and follow their cell fate. Therefore, it is possible to characterize how irradiation affects the stem cell self-renewal and differentiation capacity. Created with BioRender.com.
3. Signaling Pathways That Contribute to SC Radiation Response
3.1. Notch Signaling Pathway
The Notch pathway has been extensively studied in the SC field although only a few papers have investigated the effects of the Notch pathway in the radiation response of normal tissues. In the intestine, Notch pathway inhibition resulted in reduced expression of the intestinal SC (ISC) marker Olfm4, decreased numbers of Lgr5+ SCs, and reduced SC proliferation [4][5]. It has been reported that Notch signaling is crucial for the maintenance of the Lgr5+ crypt base columnar SC population upon IR [6] and Notch signaling activation stimulates Paneth cell plasticity during injury-induced regeneration [7][8]. Therefore, therapeutic inhibition of Notch to radiosensitize tumor cells should be approached with caution due to the important role it plays in homeostasis and repair.
The role of the Notch pathway was investigated in the mammary SCs grown as mammosphere although the studies show controversial results. Dontu et al. [9] reported that Notch signaling activation resulted in a 10-fold increase of mammosphere-forming efficiency. On the other hand, Bouras et al. [10] showed that Notch inhibition by knockdown of Cbf1 in CD29hi/CD24+ cells resulted in increased transplantation efficiency, suggesting that the Notch pathway restricts the pool of mammary stem/progenitor cells. The discrepancy between these studies may be related to the models used for the studies (wild type versus genetically modified cells, pharmacological inhibition versus knockdown) and the population of SCs specifically targeted.
The role of the Notch pathway in the lung SC in response to RT has been investigated in only a few papers [11][12]. Giuranno et al. [11] showed that Notch inhibition increased the proliferation of the irradiated primary lung SCs, reduced DNA damage, and contributed to a more intact epithelium. However, more studies are needed to unravel the mechanism of action behind the protective effect of inhibiting Notch in the lung undergoing radiation damage.
3.2. Hedgehog Pathway
The Hedgehog (Hh) pathway is one of several cross-talking intercellular signaling pathways, which plays an important role in SC regeneration after RT and has a crucial role in salivary gland (SG), liver, and brain SC survival. Although the role of Hh in the adult SG is marginal, it promotes epithelial proliferation after damage. After RT, transient Hh activation significantly rescued SG function, promoting salivary stem progenitor cell maintenance, parasympathetic innervation [13][14], and rescued IR-induced hyposalivation [15]. Therefore, transient activation of the Hh represents a promising strategy to promote SG regeneration after RT and prevent xerostomia.
The Hh signaling pathway is activated in the damaged liver and regulates tissue reconstruction [16]. It has been suggested that the Hh pathway is associated with both acute and late response of the liver to IR [16][17]. In the small intestine, administration after IR of compound 5, which activates Hh signaling, promoted crypt regeneration. The same compound 5, when given after cranial IR, preserved the neural stem/progenitor cell population, inhibited microglial activation, mitigating radiation-induced neuroinflammation, and prevented radiation-induced cognitive impairment in mice without compromising the radiation antitumor effect, suggesting that this compound could be used to mitigate radiation side effects in brain tumor patients undergoing RT [18].
Given the significant role of Hh signaling in SC regeneration and repair after injury and its crucial role in cancer progression, more in-depth research is needed to develop more effective interventions that inhibit the Hh pathway in cancer while preserving its function in healthy tissue.
3.3. Wnt Canonical Signaling Pathway
After IR, the tight modulation of the Wnt signaling has been shown as crucial for the promotion of self-renewal and/or differentiation of stem/progenitor cells to drive regeneration. In the taste bud, the input from the Wnt pathway had a role in the reestablishment of taste cell differentiation upon fractionated IR [19]. Similarly, the Wnt pathway orchestrates SC-dependent-mammary tissue repair after IR [20]. In the intestine, studies describe the importance of Wnt signals in driving the response of different stem/progenitor populations to achieve regeneration after IR damage [21][22][23]. A role of the Wnt pathway in SG during regeneration after IR injury has been suggested. In human SGs, tissue atrophy, loss of acinar cells, and upregulation of Wnt1 and β‐catenin expression most prominently in the remaining viable acinar cells indicated that Wnt modulation might provide cues for SG remodeling.
Some studies have employed Wnt agonists, factors, and conditional medium containing Wnt ligands to mitigate radiation-induced damage by promoting tissue regeneration [24][25][26]. For the same purpose, temporally pharmacological Wnt signaling activation has been explored by the in vivo use of shRNA technology [27] or by inducible transient Wnt1 activation [28]. These approaches lead to the observation that the activation of the Wnt signaling pathway seems like a potential therapeutic approach to promote crypt regeneration of irradiated intestines.
In summary, the Wnt pathway plays a crucial role as a driver of tissue remodeling through the initiation of stem/progenitor proliferation. Strategies developed to modulate the Wnt signaling are a potential approach to promote tissue regeneration post-IR.
3.4. Hippo Signaling Pathway
The nuclear translocation of Yap1 is associated with regeneration after IR damage in many organs. Specifically, the involvement of Yap1 in the stem/progenitor cell response after radiation-induced injury has been described in the intestine [29][30][31], the SGs [32], and the brain [33]. In the intestine, Yap1 seems to maintain the ISC pool by inhibiting Wnt signaling and inducing regeneration with the participation of EGF signaling [30]. Moreover, it was shown that Yap1 upregulation led to increased intestinal regeneration [31]. The activation of Yap1 has been reported to be crucial for progenitor cells during the recovery of the adult cerebellum after IR. Yang and Joyner [33] highlighted the crucial role of Yap1 in Nestin-expressing progenitors for the orchestration of cerebellar recovery after IR damage during developmental stages. Interestingly, while Yap1 nuclear translocation is required for regeneration of the intestine and the brain, it seems to hamper tissue repair in the parotid gland [32].
Overall, the presented studies describe the tissue-dependent role of Yap1 nuclear localization as a key regulator in the stem/progenitor cell niche to drive regeneration after IR injury during adulthood.
3.5. Autophagy Pathway
Some studies have described a crucial involvement of autophagy in radiation-induced regeneration of SGs [34][35], intestine [36][37], and kidney [38]. Morgan-Bathke et al. [34] explored the role of autophagy in parotid gland IR-triggered repair of acinar cells, coming to the postulation that autophagy is necessary for parotid gland regeneration. Moreover, it was explored the potential induction of autophagy to reduce SG radiation damage by the use of the rapamycin analog CCI-779 [35]. Similarly, the administration of Rapamycin induced renal protection against radiation damage by promoting the density of SCs that could support regeneration [38]. Furthermore, Levy described in vivo and in vitro beneficial effects of using an initiator of autophagy as a radioprotector of ISCs [37]. The observed intestinal regeneration after IR was attributed to the mediated Atg16L1 activation of mitophagy and the control of intracellular reactive oxygen species (ROS) levels in ISCs. Asano and colleagues [36] further supported the protection against ROS intestinal cytotoxicity through mitophagy stimulation, considering that autophagy plays an important role in driving intestinal regeneration by balancing the intracellular ROS levels in the SC population.
To conclude, these studies describe that the loss of autophagy impairs the regeneration of different adult tissues after IR damage and that the activation of autophagy seems to be a beneficial approach to induce the SC repair response to radiation.
4. Concluding remarks
Advances in the complexity of the current models employed to study the stem/progenitor response to IR should be considered, to obtain data that more accurately describe the mechanisms that govern normal tissue response to IR. Concisely, Notch, Hh, Wnt, Hippo, and autophagy constituted the most important signaling cascades that are crucial for driving sustained stem/progenitor regeneration after RT in different solid adult tissues. These signaling pathways offer potential genes/protein as therapeutic targets, which modulation must be carefully spatiotemporal regulated to achieve stem/progenitor-driven regeneration after RT, without compromising the antitumoral effects of RT.
References
- Kaushik, G.; Ponnusamy, M. P.; Batra, S. K. Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models. Stem Cells 2018, 36 (9), 1329–1340. https://doi.org/10.1002/stem.2852.
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D. D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G. D.; et al. Long-Term Expanding Human Airway Organoids for Disease Modeling. EMBO J. 2019, 38 (4), e100300. https://doi.org/https://doi.org/10.15252/embj.2018100300.
- Nagle, P. W.; Hosper, N. A.; Ploeg, E. M.; Van Goethem, M. J.; Brandenburg, S.; Langendijk, J. A.; Chiu, R. K.; Coppes, R. P. The in Vitro Response of Tissue Stem Cells to Irradiation with Different Linear Energy Transfers. Int. J. Radiat. Oncol. Biol. Phys. 2016. https://doi.org/10.1016/j.ijrobp.2016.02.020.
- Demitrack, E. S.; Gifford, G. B.; Keeley, T. M.; Carulli, A. J.; VanDussen, K. L.; Thomas, D.; Giordano, T. J.; Liu, Z.; Kopan, R.; Samuelson, L. C. Notch Signaling Regulates Gastric Antral LGR5 Stem Cell Function. EMBO J. 2015, 34 (20), 2522–2536. https://doi.org/https://doi.org/10.15252/embj.201490583.
- Demitrack, E. S.; Samuelson, L. C. Notch Regulation of Gastrointestinal Stem Cells. J. Physiol. 2016, 594 (17), 4791–4803. https://doi.org/10.1113/JP271667.
- Carulli, A. J.; Keeley, T. M.; Demitrack, E. S.; Chung, J.; Maillard, I.; Samuelson, L. C. Notch Receptor Regulation of Intestinal Stem Cell Homeostasis and Crypt Regeneration. Dev. Biol. 2015. https://doi.org/10.1016/j.ydbio.2015.03.012.
- Jones, J. C.; Brindley, C. D.; Elder, N. H.; Myers, M. G.; Rajala, M. W.; Dekaney, C. M.; McNamee, E. N.; Frey, M. R.; Shroyer, N. F.; Dempsey, P. J. Cellular Plasticity of Defa4 Cre -Expressing Paneth Cells in Response to Notch Activation and Intestinal Injury. CMGH 2019. https://doi.org/10.1016/j.jcmgh.2018.11.004.
- Yu, S.; Tong, K.; Zhao, Y.; Balasubramanian, I.; Yap, G. S.; Ferraris, R. P.; Bonder, E. M.; Verzi, M. P.; Gao, N. Paneth Cell Multipotency Induced by Notch Activation Following Injury. Cell Stem Cell 2018. https://doi.org/10.1016/j.stem.2018.05.002.
- Dontu, G.; Jackson, K. W.; McNicholas, E.; Kawamura, M. J.; Abdallah, W. M.; Wicha, M. S. Role of Notch Signaling in Cell-Fate Determination of Human Mammary Stem/Progenitor Cells. Breast Cancer Res. 2004. https://doi.org/10.1186/bcr920.
- Bouras, T.; Pal, B.; Vaillant, F.; Harburg, G.; Asselin-Labat, M. L.; Oakes, S. R.; Lindeman, G. J.; Visvader, J. E. Notch Signaling Regulates Mammary Stem Cell Function and Luminal Cell-Fate Commitment. Cell Stem Cell 2008. https://doi.org/10.1016/j.stem.2008.08.001.
- Giuranno, L.; Roig, E. M.; Wansleeben, C.; van den Berg, A.; Groot, A. J.; Dubois, L.; Vooijs, M. NOTCH Inhibition Promotes Bronchial Stem Cell Renewal and Epithelial Barrier Integrity after Irradiation. Stem Cells Transl. Med. 2020, 9 (7), 799–812. https://doi.org/https://doi.org/10.1002/sctm.19-0278.
- Giuranno, L.; Wansleeben, C.; Iannone, R.; Arathoon, L.; Hounjet, J.; Groot, A. J.; Vooijs, M. NOTCH Signaling Promotes the Survival of Irradiated Basal Airway Stem Cells. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317 (3), L414–L423. https://doi.org/10.1152/ajplung.00197.2019.
- B., H.; L., Q.; Z., Y.; Q., Z.; L., S.; X., T.; Y., Z.; S., K.; D., R.; F., L. Transient Activation of Hedgehog Pathway Rescued Irradiation-Induced Hyposalivation by Preserving Salivary Stem/Progenitor Cells and Parasympathetic Innervation. Clin. Cancer Res. 2014.
- Hai, B.; Zhao, Q.; Qin, L.; Rangaraj, D.; Gutti, V. R.; Liu, F. Rescue Effects and Underlying Mechanisms of Intragland Shh Gene Delivery on Irradiation-Induced Hyposalivation. Hum. Gene Ther. 2016. https://doi.org/10.1089/hum.2016.005.
- Hu, L.; Zhu, Z.; Hai, B.; Chang, S.; Ma, L.; Xu, Y.; Li, X.; Feng, X.; Wu, X.; Zhao, Q.; et al. Intragland Shh Gene Delivery Mitigated Irradiation-Induced Hyposalivation in a Miniature Pig Model. Theranostics 2018. https://doi.org/10.7150/thno.26509.
- Wang, S.; Lee, K.; Hyun, J.; Lee, Y.; Kim, Y.; Jung, Y. Hedgehog Signaling Influences Gender-Specific Response of Liver to Radiation in Mice. Hepatol. Int. 2013. https://doi.org/10.1007/s12072-013-9461-0.
- Wang, S.; Lee, Y.; Kim, J.; Hyun, J.; Lee, K.; Kim, Y.; Jung, Y. Potential Role of Hedgehog Pathway in Liver Response to Radiation. PLoS One 2013, 8 (9). https://doi.org/10.1371/journal.pone.0074141.
- Bhat, K.; Medina, P.; He, L.; Zhang, L.; Saki, M.; Ioannidis, A.; Nguyen, N. T.; Sodhi, S. S.; Sung, D.; Magyar, C. E.; Liau, L. M.; Kornblum, H. I.; Pajonk, F. 1-[(4-Nitrophenyl)Sulfonyl]-4-Phenylpiperazine Treatment after Brain Irradiation Preserves Cognitive Function in Mice. Neuro. Oncol. 2020. https://doi.org/10.1093/neuonc/noaa095.
- Gaillard, D.; Shechtman, L. A.; Millar, S. E.; Barlow, L. A. Fractionated Head and Neck Irradiation Impacts Taste Progenitors, Differentiated Taste Cells, and Wnt/β-Catenin Signaling in Adult Mice. Sci. Rep. 2019, 9 (1), 17934. https://doi.org/10.1038/s41598-019-54216-9.
- Woodward, W. A.; Chen, M. S.; Behbod, F.; Alfaro, M. P.; Buchholz, T. A.; Rosen, J. M. WNT/β-Catenin Mediates Radiation Resistance of Mouse Mammary Progenitor Cells. Proc. Natl. Acad. Sci. 2007, 104 (2), 618 LP – 623. https://doi.org/10.1073/pnas.0606599104.
- Yan, K. S.; Chia, L. A.; Li, X.; Ootani, A.; Su, J.; Lee, J. Y.; Su, N.; Luo, Y.; Heilshorn, S. C.; Amieva, M. R.; et al. The Intestinal Stem Cell Markers Bmi1 and Lgr5 Identify Two Functionally Distinct Populations. Proc. Natl. Acad. Sci. 2012, 109 (2), 466 LP – 471. https://doi.org/10.1073/pnas.1118857109.
- Yamauchi, M.; Otsuka, K.; Kondo, H.; Hamada, N.; Tomita, M.; Takahashi, M.; Nakasono, S.; Iwasaki, T.; Yoshida, K. A Novel in Vitro Survival Assay of Small Intestinal Stem Cells after Exposure to Ionizing Radiation. J. Radiat. Res. 2014, 55 (2), 381–390. https://doi.org/10.1093/jrr/rrt123.
- Hua, G.; Wang, C.; Pan, Y.; Zeng, Z.; Lee, S. G.; Martin, M. L.; Haimovitz-Friedman, A.; Fuks, Z.; Paty, P. B.; Kolesnick, R. Distinct Levels of Radioresistance in Lgr5+ Colonic Epithelial Stem Cells versus Lgr5+ Small Intestinal Stem Cells. Cancer Res. 2017, 77 (8), 2124 LP – 2133. https://doi.org/10.1158/0008-5472.CAN-15-2870.
- Zhou, W.-J.; Geng, Z. H.; Spence, J. R.; Geng, J.-G. Induction of Intestinal Stem Cells by R-Spondin 1 and Slit2 Augments Chemoradioprotection. Nature 2013, 501 (7465), 107–111. https://doi.org/10.1038/nature12416.
- Abo, H.; Chassaing, B.; Harusato, A.; Quiros, M.; Brazil, J. C.; Ngo, V. L.; Viennois, E.; Merlin, D.; Gewirtz, A. T.; Nusrat, A.; et al. Erythroid Differentiation Regulator-1 Induced by Microbiota in Early Life Drives Intestinal Stem Cell Proliferation and Regeneration. Nat. Commun. 2020, 11 (1), 513. https://doi.org/10.1038/s41467-019-14258-z.
- Saha, S.; Aranda, E.; Hayakawa, Y.; Bhanja, P.; Atay, S.; Brodin, N. P.; Li, J.; Asfaha, S.; Liu, L.; Tailor, Y.; et al. Macrophage-Derived Extracellular Vesicle-Packaged WNTs Rescue Intestinal Stem Cells and Enhance Survival after Radiation Injury. Nat. Commun. 2016, 7, 13096. https://doi.org/10.1038/ncomms13096.
- Romesser, P. B.; Kim, A. S.; Jeong, J.; Mayle, A.; Dow, L. E.; Lowe, S. W. Preclinical Murine Platform to Evaluate Therapeutic Countermeasures against Radiation-Induced Gastrointestinal Syndrome. Proc. Natl. Acad. Sci. 2019, 116 (41), 20672 LP – 20678. https://doi.org/10.1073/pnas.1906611116.
- Hai, B.; Yang, Z.; Shangguan, L.; Zhao, Y.; Boyer, A.; Liu, F. Concurrent Transient Activation of Wnt/β-Catenin Pathway Prevents Radiation Damage to Salivary Glands. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83 (1), e109–e116. https://doi.org/10.1016/j.ijrobp.2011.11.062.
- Barry, E. R.; Morikawa, T.; Butler, B. L.; Shrestha, K.; de la Rosa, R.; Yan, K. S.; Fuchs, C. S.; Magness, S. T.; Smits, R.; Ogino, S.; et al. Restriction of Intestinal Stem Cell Expansion and the Regenerative Response by YAP. Nature 2013, 493 (7430), 106–110. https://doi.org/10.1038/nature11693.
- Gregorieff, A.; Liu, Y.; Inanlou, M. R.; Khomchuk, Y.; Wrana, J. L. Yap-Dependent Reprogramming of Lgr5+ Stem Cells Drives Intestinal Regeneration and Cancer. Nature 2015, 526 (7575), 715–718. https://doi.org/10.1038/nature15382.
- Llado, V.; Nakanishi, Y.; Duran, A.; Reina-Campos, M.; Shelton, P. M.; Linares, J. F.; Yajima, T.; Campos, A.; Aza-Blanc, P.; Leitges, M.; et al. Repression of Intestinal Stem Cell Function and Tumorigenesis through Direct Phosphorylation of β-Catenin and Yap by PKCζ Cell Rep. 2015, 10 (5), 740–754. https://doi.org/10.1016/j.celrep.2015.01.007.
- Chibly, A. M.; Wong, W. Y.; Pier, M.; Cheng, H.; Mu, Y.; Chen, J.; Ghosh, S.; Limesand, K. H. APKCζ-Dependent Repression of Yap Is Necessary for Functional Restoration of Irradiated Salivary Glands with IGF-1. Sci. Rep. 2018, 8 (1), 6347. https://doi.org/10.1038/s41598-018-24678-4.
- Yang, Z.; Joyner, A. L. YAP1 Is Involved in Replenishment of Granule Cell Precursors Following Injury to the Neonatal Cerebellum. Dev. Biol. 2019, 455 (2), 458–472. https://doi.org/https://doi.org/10.1016/j.ydbio.2019.07.018.
- Morgan-Bathke, M.; Hill, G. A.; Harris, Z. I.; Lin, H. H.; Chibly, A. M.; Klein, R. R.; Burd, R.; Ann, D. K.; Limesand, K. H. Autophagy Correlates with Maintenance of Salivary Gland Function Following Radiation. Sci. Rep. 2014, 4, 5206. https://doi.org/10.1038/srep05206.
- Morgan-Bathke, M.; Harris, Z. I.; Arnett, D. G.; Klein, R. R.; Burd, R.; Ann, D. K.; Limesand, K. H. The Rapalogue, CCI-779, Improves Salivary Gland Function Following Radiation. PLoS One 2014, 9 (12), e113183.
- Asano, J.; Sato, T.; Ichinose, S.; Kajita, M.; Onai, N.; Shimizu, S.; Ohteki, T. Intrinsic Autophagy Is Required for the Maintenance of Intestinal Stem Cells and for Irradiation-Induced Intestinal Regeneration. Cell Rep. 2017, 20 (5), 1050–1060. https://doi.org/10.1016/j.celrep.2017.07.019.
- Levy, A.; Stedman, A.; Deutsch, E.; Donnadieu, F.; Virgin, H. W.; Sansonetti, P. J.; Nigro, G. Innate Immune Receptor NOD2 Mediates LGR5(+) Intestinal Stem Cell Protection against ROS Cytotoxicity via Mitophagy Stimulation. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (4), 1994–2003. https://doi.org/10.1073/pnas.1902788117.
- Shao, L.; Yang, W.; Xu, R.; Zhu, S.; Huang, Y.; Li, H.; Wu, X.; Yue, M.; Xiong, X.; Chen, X.; et al. Inhibition of MTORC1 Signaling Protects Kidney from Irradiation-Induced Toxicity via Accelerating Recovery of Renal Stem-like Cells. Stem Cell Res. Ther. 2018, 9 (1), 219. https://doi.org/10.1186/s13287-018-0963-5.