Factors secreted from adipose tissue may induce and/or maintain a local and systemic low-grade activation of the innate immune system. Attraction of macrophages into adipose tissue and altered crosstalk between macrophages, adipocytes, and other cells of adipose tissue are symptoms of metabolic inflammation. Among several secreted factors attracting immune cells to adipose tissue, chemotactic C-C motif chemokine ligand 2 (CCL2) (also described as monocyte chemoattractant protein-1 (MCP-1)) has been shown to play a crucial role in adipose tissue macrophage infiltration.
1. Introduction
Accumulation of adipose tissue (AT) is the major symptom of obesity. Until about 25 years ago, AT was regarded as an energy storage organ that additionally acts as isolation for the inner organs [1]. Due to the discovery of its endocrine function in the late 1980s, our understanding of AT changed fundamentally [2]. Since then, hundreds of bioactive components secreted by AT have been found [3][4]. Those AT-derived secretory factors including leptin, adiponectin, adipsin, plasminogen activator inhibitor-1 (PAI1), complement components, or cytokines such as tumor necrosis factors (e.g., TNF-α) or chemokines (e.g., CCL2) have been described with the term “adipokines” [5].
Accumulation of adipose tissue (AT) is the major symptom of obesity. Until about 25 years ago, AT was regarded as an energy storage organ that additionally acts as isolation for the inner organs [1]. Due to the discovery of its endocrine function in the late 1980s, our understanding of AT changed fundamentally [2]. Since then, hundreds of bioactive components secreted by AT have been found [3,4]. Those AT-derived secretory factors including leptin, adiponectin, adipsin, plasminogen activator inhibitor-1 (PAI1), complement components, or cytokines such as tumor necrosis factors (e.g., TNF-α) or chemokines (e.g., CCL2) have been described with the term “adipokines” [5].
In 1999, Funahashi et al. defined “
biologically active molecules secreted from adipose tissue
” as “adipocytokines” [6]. However, this term is potentially misleading because it suggests that all AT-secreted substances are cytokines. While this is true for some AT-secreted molecules (e.g., IL-6 or TNF-α), the majority is of non-cytokine origin. Although Trayhurn and Wood recommended to use the term “ ’adipokine’ […] to describe a protein that is secreted from […] adipocytes
”, commonly all AT-produced and -secreted substances are named “adipokines”, independent of whether they are secreted primarily from adipocytes or other AT cell types [7].
Adipokines are a heterogenous group of peptides including hormones, growth factors, and cytokines which differ in their physiological functions. Adipokines play an important role in the regulation of energy homeostasis, appetite, satiety, lipid metabolism and glucose homeostasis, blood pressure and vascular homeostasis, angiogenesis, and immune response [8]. Whereas adipocyte-secreted adiponectin and leptin circulate in the blood as endocrine factors, it was demonstrated that some adipokines mainly have a para or autocrine functions within AT without a contribution to inter-organ tissue crosstalk [9]. Serum concentrations of several adipokines reflect body energy stores, fat mass and distribution, systemic insulin sensitivity, glucose tolerance, a pro-inflammatory state, and other phenotype characteristics [4][10][11][12][13][14][15][16]. As examples, leptin serum concentrations are proportionally secreted to body fat mass [17], where circulating adiponectin are typically lower in individuals with obesity compared to those who are lean [18]. Additionally, several immune-modulating adipokines, such as IL-6, IL-8, CXCL5, or CCL2, are elevated in the obese state [9][19]. These changes in adipokines’ secretion pattern can be explained by AT remodeling, a process in which quantitative and qualitive changes in the cellular composition of AT occur in response to excessive weight gain [20].
Adipokines are a heterogenous group of peptides including hormones, growth factors, and cytokines which differ in their physiological functions. Adipokines play an important role in the regulation of energy homeostasis, appetite, satiety, lipid metabolism and glucose homeostasis, blood pressure and vascular homeostasis, angiogenesis, and immune response [8]. Whereas adipocyte-secreted adiponectin and leptin circulate in the blood as endocrine factors, it was demonstrated that some adipokines mainly have a para or autocrine functions within AT without a contribution to inter-organ tissue crosstalk [9]. Serum concentrations of several adipokines reflect body energy stores, fat mass and distribution, systemic insulin sensitivity, glucose tolerance, a pro-inflammatory state, and other phenotype characteristics [4,10,11,12,13,14,15,16]. As examples, leptin serum concentrations are proportionally secreted to body fat mass [17], where circulating adiponectin are typically lower in individuals with obesity compared to those who are lean [18]. Additionally, several immune-modulating adipokines, such as IL-6, IL-8, CXCL5, or CCL2, are elevated in the obese state [9,19]. These changes in adipokines’ secretion pattern can be explained by AT remodeling, a process in which quantitative and qualitive changes in the cellular composition of AT occur in response to excessive weight gain [20].
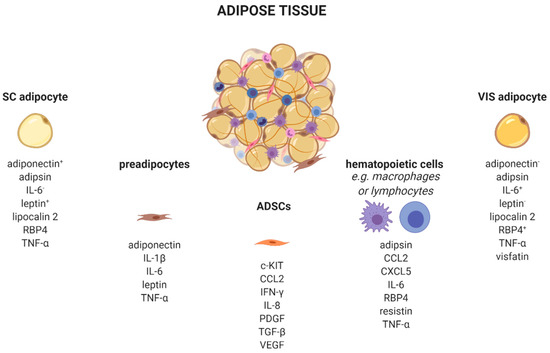
AT is a complex organ composed of several cell types (
). Adipocytes account for up to 80% of AT volume, but reflect only 20–40% of cell number. AT consists of adipose-derived stem cells (ADSCs), preadipocytes, endothelial cells, and leukocytes [21][22]. Very recently, single-nucleus RNA-sequencing (snRNA-seq) analysis of mouse and human adipose tissue revealed a subpopulation of adipocytes that regulates thermogenesis [23]. Depending on their type, different AT-cells produce distinct adipokine patterns. Therefore, knowing the cellular origin for adipokine production is important to dissect which cell type might be enriched and/or activated in AT. For example, adipocytes exhibit a quantitatively distinct adipokine pattern in the function of the fat-depot (subcutaneous (sc) and visceral (vis)) origin. Adiponectin and leptin are predominantly expressed in sc AT [24]. In contrast, IL-6 [25], omentin [26], visfatin [27], and RBP4 exhibit higher vis than sc production [28]. Adipsin [29], lipocalin 2 [30], and TNF-α [31] are secreted in both depots in comparable amounts. Using single-cell or single nuclei transcriptomics, it is now possible to discriminate adipocyte subpopulations within one depot, as well as more than 10 different AT cell types which differ in their metabolic and transcriptional properties including the identification of differences in cellular adipokine sources [23][32][33][34].
). Adipocytes account for up to 80% of AT volume, but reflect only 20–40% of cell number. AT consists of adipose-derived stem cells (ADSCs), preadipocytes, endothelial cells, and leukocytes [21,22]. Very recently, single-nucleus RNA-sequencing (snRNA-seq) analysis of mouse and human adipose tissue revealed a subpopulation of adipocytes that regulates thermogenesis [23]. Depending on their type, different AT-cells produce distinct adipokine patterns. Therefore, knowing the cellular origin for adipokine production is important to dissect which cell type might be enriched and/or activated in AT. For example, adipocytes exhibit a quantitatively distinct adipokine pattern in the function of the fat-depot (subcutaneous (sc) and visceral (vis)) origin. Adiponectin and leptin are predominantly expressed in sc AT [24]. In contrast, IL-6 [25], omentin [26], visfatin [27], and RBP4 exhibit higher vis than sc production [28]. Adipsin [29], lipocalin 2 [30], and TNF-α [31] are secreted in both depots in comparable amounts. Using single-cell or single nuclei transcriptomics, it is now possible to discriminate adipocyte subpopulations within one depot, as well as more than 10 different AT cell types which differ in their metabolic and transcriptional properties including the identification of differences in cellular adipokine sources [23,32,33,34].
Adipose tissue cells secrete distinct adipokines. Adipose tissue consists of a variety of cell types, such as adipocytes, preadipocytes, adipose tissue-derived stem cells, and several immune cells which produce and secrete cell-type-specific adipokines.
, higher/lower secreted in sc or vis. c-KIT, KIT proto-oncogene, receptor tyrosine kinase; CCL2, C-C motif chemokine ligand 2; CXCL5, C-X-C motif chemokine ligand 5; IFN-γ, interferon-γ; IL, interleukin; PDGF, platelet derived growth factor; RBP4, retinol binding protein 4; SC, subcutaneous; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor; VIS, visceral. Created with BioRender.com.
Besides adipocytes, ADSCs produce a variety of chemokines and growth factors such as the pro-angiogenic CCL2, IL-8, vascular-endothelial growth factor (VEGF) [35], platelet-derived growth factors (PDGF) [36], or c-kit which induces endothelial cell proliferation [37]. Furthermore, ADSCs secrete immune-modulating substances like interferon-γ (IFN-γ) or transforming growth factor-β (TGFβ) [38].
Depending on the fat depot, 15–50% of all resident AT-cells are preadipocytes [39]. Using a conditioned medium from obese murine epididymal AT, Renovato–Martins et al. demonstrated that 3T3-L1 preadipocytes secrete leptin and adiponectin as well as the pro-inflammatory factors IL-6, TNFα, and IL-1β [40].
In addition to those cell types of mesenchymal origin, there are various hematopoietic cells resident in AT. Nearly all known leukocytes such as macrophages, monocytes, dendritic, or natural killer cells, B-, and T-cells, as well as neutrophils or eosinophils, are of high importance in the adipokine context. The majority of immune cells express the leptin receptor on their cellular surface. Since circulating levels elevate proportional to the amount of white adipose tissue, leptin acts as a pro-inflammatory adipokine on immune cells. Subsequently, leptin receptor signaling via JAK2-STAT leads to a broad range production of pro-inflammatory adipokines, such as interleukins (IL-6, IL-8, IL-12, and IL-18), TNFα, or CCL2 [22][41]. Indeed, CCL2 (MCP-1) is a member of the small inducible gene family that plays a role in the recruitment of monocytes to sites of injury and infection, but also to AT under conditions of inflammation or adipocyte apoptosis [42][43][44]. Recently, CCL2 has been described as an important factor linking sc AT to altered glucose metabolism and body fat distribution [45].
In addition to those cell types of mesenchymal origin, there are various hematopoietic cells resident in AT. Nearly all known leukocytes such as macrophages, monocytes, dendritic, or natural killer cells, B-, and T-cells, as well as neutrophils or eosinophils, are of high importance in the adipokine context. The majority of immune cells express the leptin receptor on their cellular surface. Since circulating levels elevate proportional to the amount of white adipose tissue, leptin acts as a pro-inflammatory adipokine on immune cells. Subsequently, leptin receptor signaling via JAK2-STAT leads to a broad range production of pro-inflammatory adipokines, such as interleukins (IL-6, IL-8, IL-12, and IL-18), TNFα, or CCL2 [22,41]. Indeed, CCL2 (MCP-1) is a member of the small inducible gene family that plays a role in the recruitment of monocytes to sites of injury and infection, but also to AT under conditions of inflammation or adipocyte apoptosis [42,43,44]. Recently, CCL2 has been described as an important factor linking sc AT to altered glucose metabolism and body fat distribution [45].
2. C-C Motif Chemokine Ligand 2
2.1. Structure, Sources and Signaling
Chemokines are proteins with molecular weights ranging between 8 to 12 kDa that mediate cellular movement (chemotaxis), hematopoiesis, leukocyte degranulation, and angiogenesis [46]. Four chemokine subfamilies have been categorized based on the number and location of N-terminal cysteine residues: C, CC, CXC, and CX3C [47]. Chemokine sequences are highly conserved and share similar structures consisting of a flexible N-terminus followed by a loop containing three antiparallel β-sheets on to which is folded an α-helix [48]. Experiments which also defined the crystal structure of CCL2 revealed that it forms dimers in an anti-parallel β strand arrangement between the two flexible N-termini [49].
CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), was the first discovered human CC-family chemokine [50][51]. The gene is located on chromosome 17 (q11.2) [52] and is produced by endothelial cells, fibroblasts, epithelial, smooth muscle, mesangial, astrocytic, monocytic, and microglial cells [53][54][55][56], whereas monocytes and macrophages are major sources of CCL2 [57][58]. Additionally, (pre-)adipocytes express CCL2 [59].
CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), was the first discovered human CC-family chemokine [50,51]. The gene is located on chromosome 17 (q11.2) [52] and is produced by endothelial cells, fibroblasts, epithelial, smooth muscle, mesangial, astrocytic, monocytic, and microglial cells [53,54,55,56], whereas monocytes and macrophages are major sources of CCL2 [57,58]. Additionally, (pre-)adipocytes express CCL2 [59].
CCL2 expression is induced by inflammatory stimuli (IL-1, IL-4, IL-6, tumor necrosis factor α (TNFα)), transforming growth factor β (TGFβ), lipopolysaccharide (LPS), interferon γ (IFNγ), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), macrophage colony-stimulating factor (M-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) [60]. Human serum CCL2 has been associated with a chronic pro-inflammatory state and was suggested as a biomarker for malignant disease such as prostate and breast cancer [61][62]. High
CCL2 expression is induced by inflammatory stimuli (IL-1, IL-4, IL-6, tumor necrosis factor α (TNFα)), transforming growth factor β (TGFβ), lipopolysaccharide (LPS), interferon γ (IFNγ), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), macrophage colony-stimulating factor (M-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) [60]. Human serum CCL2 has been associated with a chronic pro-inflammatory state and was suggested as a biomarker for malignant disease such as prostate and breast cancer [61,62]. High CCL2
expression in tissues indicates chemo-attraction of monocytes in the context of local defense mechanism activation and repair of tissue damage [63].
Chemokines are secreted in response to pro-inflammatory signals, such as cytokines, to selectively recruit immune cells including monocytes, neutrophils, or lymphocytes to sites of inflammation or injuries. For CCL2, there are two activation pathways described. During the canonical pathway, inflammatory substances such as tumor-necrosis factor α (TNFα) binds to its receptor, resulting in the activation of the inhibitor of nuclear factor-κB kinase (IKK). Activated IKK then phosphorylates the NF-κB-bound inhibitor of NF-κB (IκB), whereby IκB is degraded. Consequently, released NF-κB homodimers translocate to the nucleus where they activate the transcription of inflammation-related genes e.g.,
CCL2
,
TNFα
, and
IL-6
[64]. Alternatively, CCL2 can be activated by the non-canonical pathway, i.e., NF-κB-independent CCL2 expression stimulated by PDGF [50] or insulin. In human aortic endothelial cells, physiological insulin concentrations were shown to suppress the expression of both NF-κB
and
CCL2
by more than 60% [65]. Nakatsumi et al. demonstrated that insulin activates the phosphatidylinositol 3-kinase (PI3K)-Akt pathway which leads to the inhibition mTORC1-repressor, the ras homolog enriched in brain (RHEB). In turn, mTORC1 induces forkhead box K1 (FOXK1) dephosphorylation via protein phosphatase 2A (PP2A), leading to CCL2
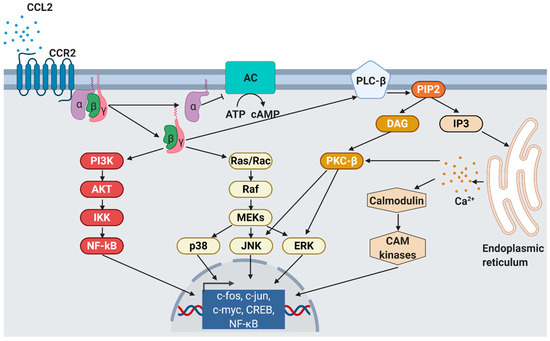
).
CCL2 signaling. As response to CCL2 binding at the N-terminus, extracellular loops and transmembrane bundle of CCR2, the intracellular G-protein α
subunit dissociates from the CCR2 and the βγ subunit. The α subunit then inhibits adenylyl cyclase (AC) function resulting in decreased cyclic adenosine monophosphate levels. In contrast, the βγ subunit signaling induces gene expression via several pathways. PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; IKK, inhibitor of NF-κB kinase; NF-κB, nuclear factor of kappa-light-chain-enhancer of activated B cells; Ras, rat sarcoma; Rac, ras-related C3 botulinum toxin substrate; Raf, rapidly accelerated fibrosarcoma; MEK, mitogen-activated protein kinase; p38, mitogen-activated protein kinase; JNK, c-jun N-terminal kinase; ERK, extracellular signal-regulated kinase; PLC-β, phospholipase C-β; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; IP3, inositol 1,4,5-triphosphate; PKC-β, protein kinase C-β; CAM, Ca
2+/calmodulin-dependent protein kinase; c-fos, proto-oncogene c-Fos; c-jun, proto-oncogene Jun; c-myc, proto-oncogene Myc; CREB, cAMP response element-binding protein. Modified from Bose S. & Cho J. [67] using BioRender.com.
/calmodulin-dependent protein kinase; c-fos, proto-oncogene c-Fos; c-jun, proto-oncogene Jun; c-myc, proto-oncogene Myc; CREB, cAMP response element-binding protein. Modified from Bose S. & Cho J. [75] using BioRender.com.
The effects of CCL2 on target cells are mediated by a specific chemokine receptor. Cells that express the distinct CC chemokine receptor (CCR) are able to migrate along the chemokine gradient upon CCL2 activation [68]. CCRs are G-protein coupled receptors (GPCRs) belonging to the rhodopsin or serpentine receptor family [69] which are expressed on different types of leukocytes such as eosinophils, basophils, lymphocytes, macrophages, and dendritic cells [70]. Human CC chemokines bind to at least two different CCRs.
The effects of CCL2 on target cells are mediated by a specific chemokine receptor. Cells that express the distinct CC chemokine receptor (CCR) are able to migrate along the chemokine gradient upon CCL2 activation [67]. CCRs are G-protein coupled receptors (GPCRs) belonging to the rhodopsin or serpentine receptor family [68] which are expressed on different types of leukocytes such as eosinophils, basophils, lymphocytes, macrophages, and dendritic cells [69]. Human CC chemokines bind to at least two different CCRs.
CCL2 usually binds to CCR2 that exists in two different splice variants, CCR2A and CCR2B, which differ in their C-terminal tails [71]. In contrast to the widespread expression of CCL2, CCR2A is mainly expressed by vascular smooth muscle cells and mononuclear cells, whereas CCR2B is the predominant receptor on monocytes and natural killer cells [72]. In addition to CCL2, CCR2 binds another five pro-inflammatory cytokines, CCL7 [73], CCL8 [74], CCL12 [75], CCL13 [67], and CCL16 [76]. However, CCL2 has the highest activation potential that finally leads to monocyte migration into target tissues [77]. As a result of CCL2/CCR2 binding, cell migration is promoted by the activation of several signaling cascades such as JAK2/STAT3 [78], MAP kinase [79], and PI3K [80] pathways (
CCL2 usually binds to CCR2 that exists in two different splice variants, CCR2A and CCR2B, which differ in their C-terminal tails [70]. In contrast to the widespread expression of CCL2, CCR2A is mainly expressed by vascular smooth muscle cells and mononuclear cells, whereas CCR2B is the predominant receptor on monocytes and natural killer cells [71]. In addition to CCL2, CCR2 binds another five pro-inflammatory cytokines, CCL7 [72], CCL8 [73], CCL12 [74], CCL13 [75], and CCL16 [76]. However, CCL2 has the highest activation potential that finally leads to monocyte migration into target tissues [77]. As a result of CCL2/CCR2 binding, cell migration is promoted by the activation of several signaling cascades such as JAK2/STAT3 [78], MAP kinase [79], and PI3K [80] pathways ( ).
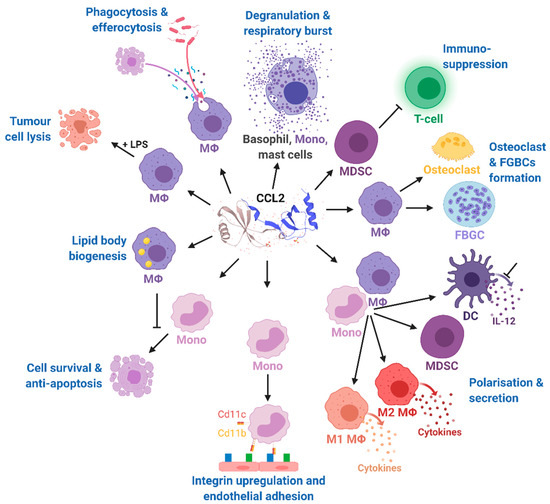
Figure 3. Effects of CCL2 on different immune cell types. Mono, monocytes; MΦ, macrophages; DC, dendritic cells; FBGCs, foreign body giant cells; MDSC, myeloid-derived suppressor cell; IL-12, interleukin 12; LPS, lipopolysaccharide; CD11b, cluster of differentiation molecule 11B; CD11c, cluster of differentiation molecule 11C. The CCL2 structure was taken from uniprot [81]. Modified from Gschwandtner M. et al. [60] using BioRender.com.
Effects of CCL2 on different immune cell types. Mono, monocytes; MΦ, macrophages; DC, dendritic cells; FBGCs, foreign body giant cells; MDSC, myeloid-derived suppressor cell; IL-12, interleukin 12; LPS, lipopolysaccharide; CD11b, cluster of differentiation molecule 11B; CD11c, cluster of differentiation molecule 11C. The CCL2 structure was taken from uniprot [86]. Modified from Gschwandtner M. et al. [60] using BioRender.com.
CCL2’s binding at CCR2 results in the dissociation of GDP from the G
αi
subunit which associates with intracellular GTP and inhibits membrane-bound adenylyl cyclases, finally leading to decreased intracellular cAMP levels. In contrast, the released G-protein βγ heterodimer activates phospholipase C which then hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP
2
) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP
3) [67]. IP
3 diffuses in the cytosol and stimulates calcium release from the endoplasmic reticulum [82]. The released Ca
diffuses in the cytosol and stimulates calcium release from the endoplasmic reticulum [81]. The released Ca 2+ is further bound by calmodulin (CaM), an essential modulator of various processes like immune response, inflammation [83], apoptosis, or metabolism [84]. Elevated Ca
is further bound by calmodulin (CaM), an essential modulator of various processes like immune response, inflammation [82], apoptosis, or metabolism [83]. Elevated Ca 2+ levels as well as DAG activate protein kinase C-β (PKC-β) that mediates gene expression via c-Jun N-terminal kinases (JNK) and extracellular signal-regulated kinases (ERK) activation [85]. Monocyte migration is regulated via G
levels as well as DAG activate protein kinase C-β (PKC-β) that mediates gene expression via c-Jun N-terminal kinases (JNK) and extracellular signal-regulated kinases (ERK) activation [84]. Monocyte migration is regulated via G βγ activation of PI3K/Akt, which in turn polymerizes actin for pseudopod formation [86].
activation of PI3K/Akt, which in turn polymerizes actin for pseudopod formation [85].
The multiple and pleiotropic effects of CCL2 on multiple cells of the myeloid lineage are summarized in
and are more extensively discussed in a recent comprehensive review highlighting the role of CCL2 on immune cell behavior and tumor immunity [60]. In our review, we focused on the role of CCL2 in the context of obesity-related diseases.
Figure 4.
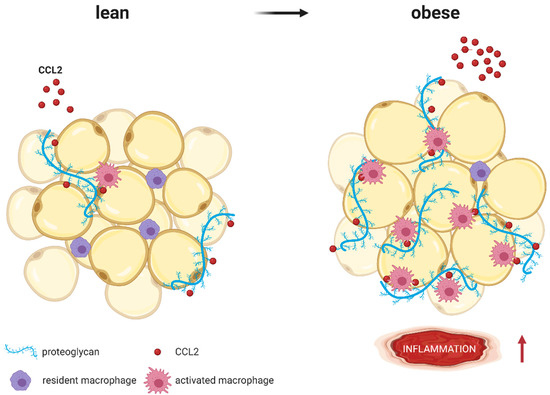
CCL2 (red molecules) binds to proteoglycans (blue strands) such as heparan sulfate or heparin, which are part of the extracellular matrix surrounding adipocytes. Whereas lean AT expresses low proteoglycan levels, expression increases with obesity. As coreceptors, proteoglycans immobilize CCL2 and present it to macrophages, resulting in higher AT inflammation (represented by
↑). Modified from Pessentheiner A. et al. [87] using BioRender.com.
). Modified from Pessentheiner A. et al. [140] using BioRender.com.