The aryl hydrocarbon receptor (AhR) is a member of the family of basic helix-loop-helix transcription factors and well-known for its role in xenobiotic metabolism and detoxification.
- aryl hydrocarbon receptor
- aging
1. Introduction: AhR in Toxicology
Dioxins are generally anthropogenic compounds of industrial origin that, due to their high chemical stability, are persistent environmental pollutants.
Furthermore, since they are lipophilic, they tend to accumulate in the adipose tissue of animals, and in this way, they are incorporated into the food chain. These chemical compounds threaten not only animal life and ecological balances, but also human health. Mechanisms that regulate toxicity induced by dioxins through AhR activation are not fully understood at the molecular level and are a broad and relevant field of health study.
This receptor is considered an orphan receptor as it has a promiscuous ligand-binding site. It is capable of binding to a wide range of structurally different chemical compounds. This gives it great diversity in its functions and makes it a molecular receptor with high pharmacological potential [2][1]. AhR activity can also be modulated by food and even by endogenous ligands in humans [1][2].
The AhR is a ligand-activated receptor. Fukunaga and coworkers annotated the protein structure [3]. This activation, through heterodimer binding AhR / AhR nuclear translocator (ARNT) to short promoter elements called “dioxin responsive elements” (DRE), induces the transcription of enzymes responsible for xenobiotic metabolism, both phase I (cytochromes P450, (CYP450)) and phase II (transferases). In addition, the fact that enzymes induced by the binding of xenobiotics to AhR have degrading activity against these molecules has led to the idea that this pathway represents an adaptive metabolic response, which protects the body from exposure to a specific class of environmental pollutants [3]. However, the activation of this pathway does not always offer protection. In many cases, the induction of these enzymes leads to a metabolic activation/inactivation of their substrates and can convert them into compounds that are more potent carcinogens with adverse pathophysiological effects [4].
The binding of AhR to any of its ligands, both exogenous and endogenous, normally causes the activation of this receptor and its downstream signaling cascade. Before binding with ligands, AhR is in its latent form in the cytosol, associated with a protein complex, which stabilizes it and prevents its degradation by the proteasome in the absence of ligand. This protein complex is formed by two molecular chaperones in response to heat stress (HSP90), a chaperonin p23 and a HBV X-associated protein 2 (XAP2) also known as protein 9 associated with AhR (ARA9) or protein of interaction to AhR (AIP) [5] [5]. HSP90s maintain the AhR in the necessary conformation to expose the ligand-binding area and limit entry into the nucleus by blocking the nuclear localization signal (NLS) [6]. When the AhR is activated by the binding of a ligand, a change occurs in the cellular compartmentalization of the receptor, which rapidly accumulates in the nucleus (translocation), and XAP is released. Thus, the AhR, once in the nucleus, interacts with the ARNT protein and releases the HSP90/p23 complex to recycle it in the cytoplasm. The AhR/ARNT complex binds to response elements (DRE) in the promoter regions of target genes, where it recruits transcription cofactors and proteins that remodel chromatin, promoting the transcription of a multitude of genes.
When the agonist capable of activating AhR signaling disappears, AhR activity must be rapidly inactivated to maintain cellular homeostasis.
Mechanisms are known to terminate AhR signaling; both involve in its degradation through the 26S proteasome. For this purpose, AhR must be exported from the nucleus, ubiquitinated and degraded by the proteasome in the cytosol [7] or through the binding of AhR to its repressor AhRR, thus establishing a negative feedback inhibitor of AhR signaling [8].
Overall, since the biological activity of AhR is controlled by its association with proteins present in different cellular compartments, the control of its intracellular location may represent a mechanism for regulating its activity under physiological conditions, i.e., in the absence of interaction with xenobiotics [9].
The AhR arose much earlier in the evolutionary scale than the presence of environmental pollutants. Therefore, it must have a constitutive, physiological activity in an organism. Its constitutive expression is found in invertebrates and nearly all vertebrates, both aquatic and terrestrial, and in almost all cell types in mammals [10].
The highest expression levels of AhR mRNA are found in the placenta, heart, liver, lung, and pancreas, and the lowest are found in the kidney, brain, and skeletal muscle [9]. It is also present in multiple vascular beds and even in tumor cells. In addition to the differential expression of the receptor in each type of tissue, its regulation also depends on the stage of development of the organism [3].
We have performed a comprehensive literature review to underpin the role of AhR under physiological conditions independently of its response to toxins. Our aim was to bring together our current knowledge on the physiological functions of AhR and the changes that occur during aging.
2. Physiological Functions of AhR
The oldest physiological function of the AhR in terms of evolution is the regulation of developmental processes observed in Caenorhabditis elegans and Drosophila. [11].
The C. elegans ahr mutant develops slightly slower than the wild type [12] and shows a neuronal dysfunctional development [13–16][13][14][15][16]. Drosophila’s AhR homolog is necessary for development of the distal segments of the antennae [17] and sensory neurons [18].
Another conserved function is the regulation of fertility described in C. elegans and mice [12,19][12][19].
The importance of AhR in healthy development and in normal biochemical processes has been elucidated in studies using AhR gene knockout (KO) mice, which show numerous developmental abnormalities and dysfunctionalities [6]. However, other physiological functions require further investigation.
In 2014, Stockinger and his colleagues proposed that “the emphasis is shifting from AhR in the xenobiotic pathway to its mechanism action in response to its physiological ligands” [20].
Although the functions of the different endogenous ligands of the receptor are well characterized, none of these ligands acts as a high-affinity physiological activator. Further, there are differences in AhR activity and ligand reactivity between species. For instance, there are discrepancies in the experimental results between humans and mice [6]. In any case, AhR is involved in physiological processes such as embryogenesis, development, neurogenesis, circadian rhythm, metabolism, and hypoxia [21]. Furthermore, it also has a role in the proteasomal degradation of steroid hormone receptors, in the cellular response to stress caused by UVB radiation, and in the differentiation of specific T cell subtypes [22]. In Table 1, we summarize the functions known in which the receptor participates.
Table 1. The effect of AhR signaling in physiological functions.
Function | Effect on the AhR Pathway | References | ||||||
Cell regeneration | Activation |
[23] |
||||||
Immune reaction | Activation |
[24–26] | ||||||
Intestinal homeostasis | Activation |
[6,25] | ||||||
Hematopoietic stem cells (HSC) proliferation | Inhibition |
[27] |
||||||
Skin pathogenesis | Inhibition |
[28] |
||||||
Neurogenesis and neural precursor cells | Activation |
[2,27] | ||||||
Retinal homeostasis | Activation |
[29] |
||||||
Regulation of sex hormones and reproduction | Activation |
[19,30,31] | ||||||
Embryonic cardiac development | Activation |
[32] |
||||||
Insulin-glucose regulation | Activation |
[23,32] | ||||||
Adipocyte differentiation | Inhibition |
[33,34] | ||||||
Closure of ductus venous | Activation |
[35] |
||||||
Thickness aorta | Inhibition |
[36] |
||||||
Movement Regulation | Inhibition |
[37] |
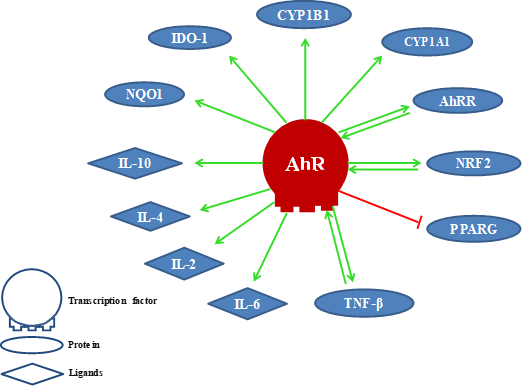
The activation of AhR induces cytochrome P450 enzymes, mainly CYP1A1 and CYP1B1, that detoxify xenobiotics and control the AhR signaling due to its ability to metabolize ligands. Therefore, it is presumed that metabolic clearance mediated by these enzymes for natural AhR ligands would affect various cell types that depend on AhR signaling for their survival [38,39]. In Figure 1, we show some proteins and ligands whose activity is modulated by AhR. Among them AhR’s own repressor AhRR [31], the transcription factor related to nuclear erythroid factor 2 (NRF2, alias NFE2L2) [40,41], the gamma activated receptor for peroxisome proliferation (PPARG) [33,34], the transforming growth factor-beta (TGF-β) [42], interleukins (IL6, IL2, IL4, IL10) [43], NAD(P)H dehydrogenase (quinone) 1 (NQO1) [44], and indolamine 2,3-dioxygenase (IDO1) [20], among others.
The activation of AhR induces cytochrome P450 enzymes, mainly CYP1A1 and CYP1B1, that detoxify xenobiotics and control the AhR signaling due to its ability to metabolize ligands. Therefore, it is presumed that metabolic clearance mediated by these enzymes for natural AhR ligands would affect various cell types that depend on AhR signaling for their survival [38][39]. In Figure 1, we show some proteins and ligands whose activity is modulated by AhR. Among them AhR’s own repressor AhRR [31], the transcription factor related to nuclear erythroid factor 2 (NRF2, alias NFE2L2) [40][41], the gamma activated receptor for peroxisome proliferation (PPARG) [33][34], the transforming growth factor-beta (TGF-β) [42], interleukins (IL6, IL2, IL4, IL10) [43], NAD(P)H dehydrogenase (quinone) 1 (NQO1) [44], and indolamine 2,3-dioxygenase (IDO1) [20], among others.
Figure 1.
Proteins and ligands modulated by the AhR. (Positive effect (link in green color) and negative effect (link in red color).
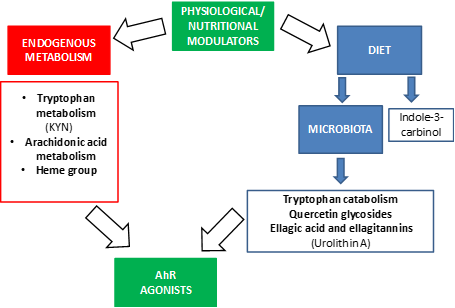
Physiological AhR activity can be changed by endogenous ligands and nutrition [1]. AhR is reported as a crucial regulator in maintenance of intraepithelial lymphocytes and microbial load composition in the intestine [45]. For this reason, AhR activity can be regulated by dietary components to improve the intestinal immune system and to avoid dysbiosis.
Physiological AhR activity can be changed by endogenous ligands and nutrition [2]. AhR is reported as a crucial regulator in maintenance of intraepithelial lymphocytes and microbial load composition in the intestine [45]. For this reason, AhR activity can be regulated by dietary components to improve the intestinal immune system and to avoid dysbiosis.
We summarize relevant modulators by AhR in Figure 2.
Figure 2.
Physiological and nutritional modulators of AhR.
The most prominent endogenous ligands are those derived from tryptophan metabolism, but there are also ligands derived from arachidonic acid or the heme group (Figure 2). Of the catabolites derived from tryptophan, we would like to highlight the formation of n-formylquinurenine (KYN) by the enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) [46–49]. KYN is an AhR ligand that has been linked to the maturation of regulatory T cells and the suppression of inflammatory cytokines in dendritic cells. It also has a role in the response to microbial pathogens since lipopolysaccharides (LPS) stimulate the expression of IDO and TDO, leading KYN synthesis that in turn activates AhR. Thus, AhR decreases the expression of pro-inflammatory cytokines and regulates inflammation. Furthermore, in ahr gene KO mice, LPS induces higher concentrations of inflammatory cytokines and has a greater susceptibility to septic shock [50,51]. Under normal conditions, when LPS activates the Toll-like receptor, the enzyme TDO is expressed, leading to the formation of KYN that activates AhR in immune cells [52].
The most prominent endogenous ligands are those derived from tryptophan metabolism, but there are also ligands derived from arachidonic acid or the heme group (Figure 2). Of the catabolites derived from tryptophan, we would like to highlight the formation of n-formylquinurenine (KYN) by the enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) [46][47][48][49]. KYN is an AhR ligand that has been linked to the maturation of regulatory T cells and the suppression of inflammatory cytokines in dendritic cells. It also has a role in the response to microbial pathogens since lipopolysaccharides (LPS) stimulate the expression of IDO and TDO, leading KYN synthesis that in turn activates AhR. Thus, AhR decreases the expression of pro-inflammatory cytokines and regulates inflammation. Furthermore, in ahr gene KO mice, LPS induces higher concentrations of inflammatory cytokines and has a greater susceptibility to septic shock [50][51]. Under normal conditions, when LPS activates the Toll-like receptor, the enzyme TDO is expressed, leading to the formation of KYN that activates AhR in immune cells [52].
In addition, there are ligands for AhR that come from bacterial metabolism through the microbiota (Figure 2) [24], AhR is highly expressed in intestinal epithelium, AhR expression was found to decrease in germ-free mice, suggesting that it is related to the microbiota [47]. The bacterial tryptophan catabolites that act as AhR ligands are: indole, tryptamine, indoleethanol (IE), indolepropionic acid (IPA), indolelactic acid (ILA), indoleacetic acid (IAA), 3-methylindole (skatole), indolealdehyde (IAld) and indoleacrylic acid (IA) [48,53,54] (Figure 2). Thus, AhR mediates the regulation of intestinal immunity through both endogenous and bacterial tryptophan metabolites, having effects on the composition of the microbiota, the immune system and intestinal homeostasis [24]. One of the mechanisms by which these metabolites contribute to the maintenance of intestinal homeostasis is the production of IL-22 by type 3 innate lymphoid cells (ILC) and this is also mediated by the activation of AhR [38,42–45]. IL-22 is important for the protection of the epithelium against pathogens and promotes the expansion of intestinal stem cells, promoting the regeneration of the epithelium after intestinal injury [55].
In addition, there are ligands for AhR that come from bacterial metabolism through the microbiota (Figure 2) [24], AhR is highly expressed in intestinal epithelium, AhR expression was found to decrease in germ-free mice, suggesting that it is related to the microbiota [27]. The bacterial tryptophan catabolites that act as AhR ligands are: indole, tryptamine, indoleethanol (IE), indolepropionic acid (IPA), indolelactic acid (ILA), indoleacetic acid (IAA), 3-methylindole (skatole), indolealdehyde (IAld) and indoleacrylic acid (IA) [48][53][54] (Figure 2). Thus, AhR mediates the regulation of intestinal immunity through both endogenous and bacterial tryptophan metabolites, having effects on the composition of the microbiota, the immune system and intestinal homeostasis [24]. One of the mechanisms by which these metabolites contribute to the maintenance of intestinal homeostasis is the production of IL-22 by type 3 innate lymphoid cells (ILC) and this is also mediated by the activation of AhR [38][42][43][44][45]. IL-22 is important for the protection of the epithelium against pathogens and promotes the expansion of intestinal stem cells, promoting the regeneration of the epithelium after intestinal injury [55].
On the other hand, these bacterial tryptophan catabolites not only have consequences at the intestinal level but they can be absorbed at the systemic level and have anti-inflammatory effects, because, as mentioned above, the activation of AhR modifies the responses regulated by the Toll-Like receptor in dendritic cells and promotes the generation of T-helper cells that secrete IL-10 (anti-inflammatory) [56]. It should be noted that these studies are conducted in mice and that the results may differ in humans since the affinities of metabolites for AhR may be different. Recent studies suggest that the human AhR has a greater affinity for tryptophan catabolites than that of the mouse [54]. In addition, the diet can provide indole derivatives, like indole-3-carbinol (I3C), that directly activate the AhR signaling pathway [57].
An improved healthspan effect in C. elegans, Drosophila melanogaster and mice has also been attributed to indoles [58].
Other dietary components can also be metabolized by the microbiota giving rise to AhR agonists as shown in Table 2.
Table 2.
Dietary components and microbiota metabolites that are AhR ligands.
|
Dietary Component |
Microbiota Metabolites that Are AhR Ligands |
References |
|
Tryptophan |
indole, tryptamine, indoleethanol (IE), indolepropionic acid (IPA), indolelactic acid (ILA), indoleacetic acid (IAA), 3-methylindole (skatole), indolealdehyde (IAld) and indoleacrylic acid (IA) |
|
|
Indirubin |
[59] |
|
|
1,4-dihydroxy-2-naphthoic acid (DHNA) |
[60] |
|
|
Quercetin glycosides |
3,4-Dihydroxyphenylacetic acid (DOPAC) |
[61] |
|
Ellagic acid and ellagitannins |
Urolithin A |
[62] |
Urolithin A (UroA) is a derivative of elaginates: The successive hydrolysis and decarboxylation of elaginates by the intestinal microbiota form urolithins. Interindividual differences in urolithin formation depend on the composition of the microflora [63]. UroA shows anti-cancer, anti-inflammatory and anti-aging effects. This metabolite has the ability to reduce the production of IL-6 and TNF by macrophages and to bind to AhR in intestinal cells.
3. The Implication of AhR in Aging
Population aging is a global demographic trend. The World Health Organization estimates that the proportion of the world population ≥65 years will reach 1500 million people (14% of the total) by 2050. Age-related changes contribute to the appearance of diseases that occur more frequently with aging [64]. This process significantly increases the prevalence of chronic age-related conditions such as heart and neurological diseases, cognitive decline, and dementia.
There is an interesting association between the AhR expression levels and longevity [27].
A pioneer study by Fernández-Salguero and his collaborators confirmed that AhR is affected in aging, leading to a deterioration of several physiological functions [21]. Thus, the detailed characterization of AhR functions in aging and age-related diseases could help discover this protein’s ancient functions and develop strategies for healthier aging. In this study, the role of AhR in the aging process was analyzed using a model Ahr gene KO mice. Almost 50% of the mice died or fell ill at 13 months of age (average half-life of mice is approximately 24 months). The most notorious changes were cardiovascular diseases (hypertrophy, focal inflammation, hypertrophic vessels, and vascular lesions), liver (portal vascular hypertrophy and hepatocellular tumors), hyperplasia gastric with aging to polyps, the immune system with deficiency of T and B cells, rectal prolapse, and severe epidermal hyperplasia. Age-related lesions in various organs appeared only at nine months of age, for instance, alterations in cell proliferation, fibrosis, collapse of the immune system, and alterations in vascular homeostasis. None of these complications was found in wild-type (WT) mice of similar ages [21]. Bravo-Ferrer and coworkers corroborated the accelerated aging phenotype in Ahr-deficient mice [43].
Singh and collaborators showed implications of the AhR in regulating the HSC [64]. Normally, HSCs are largely inactive in order to prevent premature exhaustion and limit genetic alterations. Singh et al. showed that the HSC of young adult Ahr KO mice had high rates of cell division. These KO mice showed higher numbers of bone marrow cells. A similar survival rate was also observed in WT mice until approximately 15 months of age. At 24 months, only 33% of deficient mice survived in comparison with 78% of WT mice. Therefore, the lack of expression of Ahr allows HSCs to escape inactivity and lose control over the signals that limit proliferation and differentiation, ultimately resulting in stem cell exhaustion and premature aging.
Biljes and collaborators carried out a study in young (from 2 to 5 months) and old (>18 months) mice, with or without Ahr gene deficiency [22]. In young KO mice, they found that postprandial TG, cholesterol, VLDL, and HDL levels were significantly lower than in the WT ones. LDL levels were not changed. Unexpectedly, there was no difference in any of these lipid markers between Ahr KO old and young; therefore, there were no age-associated changes in KO mice concerning these parameters. However, expression of lipoprotein lipase (LpL), which is the enzyme that degrades the lipid moiety of lipoproteins and therefore releases TG, increased with age in the livers of WT and KO mice, but it was higher in deficient mice than in wild-type mice. The LpL has an assumed DRE transcription motif in its promoter and therefore might need the AhR for constitutive expression. This could explain why the low TG levels in LpL KO mice are very similar to the human situation, making the mouse a relevant model for the study of lipid metabolism [22].
The prevalence of type 2 diabetes increases with age. In studies to evaluate the potential role of AhR in altering the metabolism of glucose, authors observed that in the KO mice the blood glucose levels were significantly higher in the old mice (>18 months) than in young (from 2 to 5 months), indicating that old Ahr KO mice cannot maintain blood glucose homeostasis [23].
At the vascular aging level, Eckers et al. observed that mice deficient in the Ahr gene showed a decrease in pulse wave velocity in young and old animals compared to wild types. This indicates a reduced stiffness of the arteries and, therefore, healthier vessels. However, overexpression of AhR alters eNOS activation and reduces S-NO content in human endothelial cells; moreover, pulse wave velocity is positively correlated with AhR expression in healthy human subjects [9].
The study by Bravo-Ferrer and collaborators revealed the participation of AhR in inflammation in aging. Its dysregulation could contribute to the loss of homeostasis that occurs during aging. The inflammation markers profile was compared in WT and Ahr KO mice at 2, 12, and 16 months. The Ahr KO mice showed higher plasma levels of IL6, TNFα, IL2, IL4, and IL10 than WT mice, and lower in IL6, IFNγ, IL17α, IL2, and IL4 with age in KO mice compared to WT. In both groups of aged mice there were signs of inflammation, but KO mice showed levels of specific cytokines higher than WT. These results indicate that the lack of AhR promotes an age-related increase in plasma cytokine levels and further supports the hypothesis that the loss of AhR promotes premature aging in mice.
Age causes changes in AhR expression levels in the mouse brain [43]. Indeed, AhR levels fall in the cortex and the hippocampus, but this is not reflected in its transcriptional activity as determined by levels of its target genes, CYP1A1 and CYP1B1. Moreover, the size of specific brain structures as determined by magnetic resonance imaging in WT and KO in 16-month-old mice was reduced when compared with WT animals. The results showed that the KO mice had a cortical volume reduction compared to WT animals. They also showed that the KO mice had a loss of the white matter integrity compared to WT.
The discovery that AhR protein levels decrease during aging in specific brain structures suggests the role of the AhR in brain aging. Moreover, because brain aging is associated with cognitive impairment, researchers have also assessed cognitive function throughout aging. This age-associated loss of cognition is increased in Ahr KO mice. These early-aging characteristics were not present in WT or young KO mice. Furthermore, administration of a diet rich in xenobiotics, as commonly occurs in the aging population, accelerates the appearance of β-amyloid plaques in the mouse brain and deficits in working memory in aged mice [43].
The processes related to AhR dysregulation in aging are summarized in the following Table 3.
Table 3.
AhR dysregulation in aging.
|
Processes |
References |
|
Dysregulation of hematopoietic stem cells (HSC) |
[64] |
|
Dysregulation of lipid metabolism |
[22] |
|
Loss of glucose homeostasis |
[22] |
|
Increased inflammaging |
[43] |
|
Impaired motor ability |
[43] |
|
Brain and vascular deterioration |
In addition to these processes, kynurenine (KYN) levels increase in aging [43]. These high levels are associated with more significant inflammation and seems to contribute directly to various age-related conditions’ development and progression. When KYN interacts with AhR, reactive oxygen species’ levels are increased, leading to loss of characteristic muscle mass (sarcopenia) in old age. Lamas and coworkers’ studies argue that the concentration of KYN required to triggering AhR activity casts doubt on its relevance as an AhR activator in physiological conditions [6]. Furthermore, IDO, which is the enzyme that metabolizes tryptophan giving rise to KYN, may represent a new approach therapy for the prevention of sarcopenia and possibly other age-associated conditions [65]. In
In addition to these processes, kynurenine (KYN) levels increase in aging [43]. These high levels are associated with more significant inflammation and seems to contribute directly to various age-related conditions’ development and progression. When KYN interacts with AhR, reactive oxygen species’ levels are increased, leading to loss of characteristic muscle mass (sarcopenia) in old age. Lamas and coworkers’ studies argue that the concentration of KYN required to triggering AhR activity casts doubt on its relevance as an AhR activator in physiological conditions [6]. Furthermore, IDO, which is the enzyme that metabolizes tryptophan giving rise to KYN, may represent a new approach therapy for the prevention of sarcopenia and possibly other age-associated conditions[65]. In
C. elegans
, indoles induce a transcriptomic profile in aged animals similar to that of young ones, but which is different from that associated with normal aging [58].
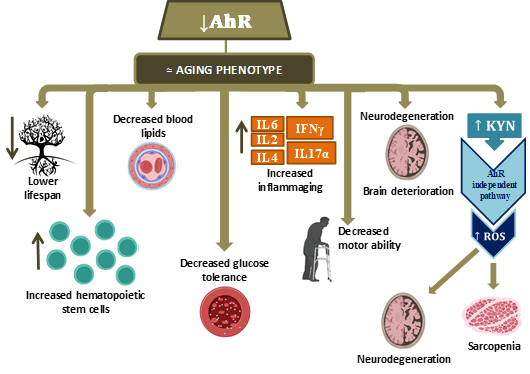
The available literature data are summarized in Figure 3.
Figure 3.
Pathophysiological processes observed when AhR decreases in aging. (Hematopoietic stem cells (HSC); Interleukin (IL); Interferon (INF); Kynurenine (KYN); Reactive oxygen species (ROS)).
References
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenousWu, P.Y.; Chuang, P.Y.; Chang, G.D.; Chan, Y.Y.; Tsai, T.C.; Wang, B.J.; Lin, K.H.; Hsu, W.M.; Liao, Y.F.; Lee, H. Novel Endogenous Ligands of Aryl Hydrocarbon Receptor Mediate Neural Development and Differentiation of Neuroblastoma», ACS Chem. Neurosci. 2019, 10, 4031–4042, doi:10.1021/acschemneuro.9b00273.
- chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334, doi:10.1146/annurev.pharmtox.43.100901.135828.
- Fukunaga, B.N.; Probst, M.R.; Reisz-Porszasz, S.; Hankinson, O. Identification of functional domains of the aryl hydrocarbon receptor. J. Biol. Chem. 1995, 270, 29270–29278, doi:10.1074/jbc.270.49.29270.
- Rowlands, J.C.; McEwan, I.J.; Gustafsson, J.A. Trans-activation by the human aryl hydrocarbon receptor and aryl hydrocar-bon receptor nuclear translocator proteins: Direct interactions with basal transcription factors. Mol. Pharmacol. 1996, 50, 538–548.
- Enan, E.; Matsumura, F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem. Pharmacol. 1996, 52, 1599–1612, doi:10.1016/S0006-2952(96)00566-7.
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038, doi:10.1038/s41385-018-0019-2.
- Pollenz, R.S.; Davarinos, N.A.; Shearer, T.P. Analysis of aryl hydrocarbon receptor-mediated signaling during physiological hypoxia reveals lack of competition for the aryl hydrocarbon nuclear translocator transcription factor. Mol. Pharmacol. 1999, 56, 1127–1137, doi:10.1124/mol.56.6.1127.
- Haarmann-Stemmann, T.; Bothe, H.; Kohli, A.; Sydlik, U.; Abel, J.; Fritsche, E. Analysis of the transcriptional regulation and molecular function of the aryl hydrocarbon receptor repressor in human cell lines. Drug Metab. Dispos. Biol. Fate Chem. 2007, 35, 2262–2269, doi:10.1124/dmd.107.016253.
- Eckers, A.; Jakob, S.; Heiss, C.; Haarmann-Stemmann, T.; Goy, C.; Brinkmann, V.; Cortese-Krott, M.M.; Sansone, R.; Esser, C.; Ale-Agha, N.; et al. The aryl hydrocarbon receptor promotes aging phenotypes across species. Sci. Rep. 2016, 6, 19618, doi:10.1038/srep19618.
- Hahn, M.E. Aryl hydrocarbon receptors: Diversity and evolution11Invited review for Chemico-Biological Interactions. Chem. Biol. Interact. 2002, 141, 131–160, doi:10.1016/S0009-2797(02)00070-4.
- Hahn, M.E.; Karchner, S.I.; Evans, B.R.; Franks, D.G.; Merson, R.R.; Lapseritis, J.M. Unexpected diversity of aryl hydrocar-bon receptors in non-mammalian vertebrates: Insights from comparative genomics. J. Exp. Zoolog. A Comp. Exp. Biol. 2006, 305A, 693–706, doi:10.1002/jez.a.323.
- Aarnio, V.; Storvik, M.; Lehtonen, M.; Asikainen, S.; Reisner, K.; Callaway, J.; Rudgalvyte, M.; Lakso, M.; Wong, G. Fatty acid composition and gene expression profiles are altered in aryl hydrocarbon receptor-1 mutant Caenorhabditis elegans. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2010, 151, 318–324, doi:10.1016/j.cbpc.2009.12.006.
- Huang, X.; Powell-Coffman, J.A.; Jin, Y. The AHR-1 aryl hydrocarbon receptor and its co-factor the AHA-1 aryl hydrocar-bon receptor nuclear translocator specify GABAergic neuron cell fate in C. elegans. Development 2004, 131, 819–828, doi:10.1242/dev.00959.
- Qin, H. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev. Biol. 2004, 270, 64–75, doi:10.1016/S0012-1606(04)00119-8.
- Qin, H.; Zhai, Z.; Powell-Coffman, J. The Caenorhabditis elegans AHR-1 transcription complex controls expression of soluble guanylate cyclase genes in the URX neurons and regulates aggregation behavior. Dev. Biol. 2006, 298, 606–615, doi:10.1016/j.ydbio.2006.07.017.
- Smith, C.J.; O’Brien, T.; Chatzigeorgiou, M.; Spencer, W.C.; Feingold-Link, E.; Husson, S.J.; Hori, S.; Mitani, S.; Gottschalk, A.; Schafer, W.R.; et al. Sensory Neuron Fates Are Distinguished by a Transcriptional Switch that Regulates Dendrite Branch Stabilization. Neuron 2013, 79, 266–280, doi:10.1016/j.neuron.2013.05.009.
- Burgess, E.A.; Duncan, I. Direct control of antennal identity by the spineless-aristapedia gene of Drosophila. Mol. Gen. Genet. 1990, 221, 347–357, doi:10.1007/BF00259398.
- Kim, M.D.; Jan, L.Y.; Jan, Y.N. The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev. 2006, 20, 2806–2819, doi:10.1101/gad.1459706.
- Baba, T.; Shima, Y.; Owaki, A.; Mimura, J.; Oshima, M.; Fujii-Kuriyama, Y.; Morohashi, K.I. Disruption of aryl hydrocarbon receptor (AhR) induces regression of the seminal vesicle in aged male mice. Sex. Dev. Genet. Mol. Biol. Evol. Endocrinol. Em-bryol. Pathol. Sex. Determ. Differ. 2008, 2, 1–11, doi:10.1159/000117714.
- Stockinger, B.; di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432, doi:10.1146/annurev-immunol-032713-120245.
- Fernandez-Salguero, P.M.; Ward, J.M.; Sundberg, J.P.; Gonzalez, F.J. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 1997, 34, 605–614, doi:10.1177/030098589703400609.
- Biljes, D.; Hammerschmidt-Kamper, C.; Kadow, S.; Diel, P.; Weigt, C.; Burkart, V.; Esser, C. Impaired glucose and lipid me-tabolism in ageing aryl hydrocarbon receptor deficient mice. EXCLI J. 2015, 14, 1153–1163, doi:10.17179/excli2015-638.
- Casado, F.L. The Aryl Hydrocarbon Receptor Relays Metabolic Signals to Promote Cellular Regeneration. Stem Cells Int. 2016, 2016, 4389802, doi:10.1155/2016/4389802.
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediat-ed by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13, doi:10.3389/fcimb.2018.00013.
- Hao, N.; Whitelaw, M.L. The emerging roles of AhR in physiology and immunity. Biochem. Pharmacol. 2013, 86, 561–570, doi:10.1016/j.bcp.2013.07.004.
- Rael, L.T.; Bar-Or, R.; Banton, K.L.; Mains, C.W.; Roshon, M.; Tanner, A.H.; Lieser, M.J.; Acuna, D.L.; Bar-Or, D. The an-ti-inflammatory effect of LMWF5A and N-acetyl kynurenine on macrophages: Involvement of aryl hydrocarbon receptor in mechanism of action. Biochem. Biophys. Rep. 2018, 15, 61–67, doi:10.1016/j.bbrep.2018.06.006.
- Singh, K.P.; Casado, F.L.; Opanashuk, L.A.; Gasiewicz, T.A. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem. Pharmacol. 2009, 77, 577–587, doi:10.1016/j.bcp.2008.10.001.
- Zhu, Z.; Chen, J.; Lin, Y.; Zhang, C.; Li, W.; Qiao, H.; Fu, M.; Dang, E.; Wang, G. Aryl Hydrocarbon Receptor in Cutaneous Vascular Endothelial Cells Restricts Psoriasis Development by Negatively Regulating Neutrophil Recruitment. J. Invest. Der-matol. 2019, doi:10.1016/j.jid.2019.11.022.
- Gutierrez, M.A.; Davis, S.S.; Rosko, A.; Nguyen, S.M.; Mitchell, K.P.; Mateen, S.; Neves, J.; Garcia, T.Y.; Mooney, S.; Perdew, G.H.; et al. A novel AhR ligand, 2AI, protects the retina from environmental stress. Sci. Rep. 2016, 6, 29025, doi:10.1038/srep29025.
- Hernandez-Ochoa, I.; Barnett-Ringgold, K.R.; Dehlinger, S.L.; Gupta, R.K.; Leslie, T.C.; Roby, K.F.; Flaws, J.A. The ability of the aryl hydrocarbon receptor to regulate ovarian follicle growth and estradiol biosynthesis in mice depends on stage of sexu-al maturity. Biol. Reprod. 2010, 83, 698–706, doi:10.1095/biolreprod.110.087015.
- Matvere, A.; Teino, I.; Varik, I.; Kuuse, S.; Tiido, T.; Kristjuhan, A.; Maimets, T. FSH/LH-Dependent Upregulation of Ahr in Murine Granulosa Cells Is Controlled by PKA Signaling and Involves Epigenetic Regulation. Int. J. Mol. Sci. 2019, 20, doi:10.3390/ijms20123068.
- Thackaberry, E.A.; Bedrick, E.J.; Goens, M.B.; Danielson, L.; Lund, A.K.; Gabaldon, D.; Smith, S.M.; Walker, M.K. Insulin regulation in AhR-null mice: Embryonic cardiac enlargement, neonatal macrosomia, and altered insulin regulation and re-sponse in pregnant and aging AhR-null females. Toxicol. Sci. Off. J. Soc. Toxicol. 2003, 76, 407–417, doi:10.1093/toxsci/kfg229.
- Hu, T., Wang, D., Yu, Q., Li, L., Mo, X., Pan, Z., Zouboulis, C.C., Peng, L., Xia, L.; Ju, Q. Aryl hydrocarbon receptor nega-tively regulates lipid synthesis and involves in cell differentiation of SZ95 sebocytes in vitro. Chem. Biol. Interact. 2016, 258, 52–58, doi:10.1016/j.cbi.2016.08.012.
- Shimba, S.; Wada, T.; Tezuka, M. Arylhydrocarbon receptor (AhR) is involved in negative regulation of adipose differentia-tion in 3T3-L1 cells: AhR inhibits adipose differentiation independently of dioxin. J. Cell Sci. 2001, 114 Pt 15, 2809–2817.
- Lahvis, G.P.; Pyzalski, R.W.; Glover, E.; Pitot, H.C.; McElwee, M.K.; Bradfield, C.A. The aryl hydrocarbon receptor is re-quired for developmental closure of the ductus venosus in the neonatal mouse. Mol. Pharmacol. 2005, 67, 714–720, doi:10.1124/mol.104.008888.
- Sauzeau, V.; Carvajal-González, J.M.; Riolobos, A.S.; Sevilla, M.A.; Menacho-Márquez, M.; Román, Á.C.; Abad, A.; Montero, M.J.; Fernández-Salguero, P.; Bustelo, X.R. Transcriptional factor aryl hydrocarbon receptor (Ahr) controls cardiovascular and respiratory functions by regulating the expression of the Vav3 proto-oncogene. J. Biol. Chem. 2011, 286, 2896–2909, doi:10.1074/jbc.M110.187534.
- Williams, E.G.; Mouchiroud, L.; Frochaux, M.; Pandey, A.; Andreux, P.A.; Deplancke, B.; Auwerx, J. An evolutionarily con-served role for the aryl hydrocarbon receptor in the regulation of movement. PLoS Genet. 2014, 10, e1004673, doi:10.1371/journal.pgen.1004673.
- Schiering, C.; Wincent, E.; Metidji, A.; Iseppon, A.; Li, Y.; Potocnik, A.J.; Omenetti, S.; Henderson, C.J.; Wolf, C.R.; Nebert, D.W.; et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 2017, 542, 242–245, doi:10.1038/nature21080.
- Bergander, L.V.; Cai, W.; Klocke, B.; Seifert, M.; Pongratz, I. Tryptamine serves as a proligand of the AhR transcriptional pathway whose activation is dependent of monoamine oxidases. Mol. Endocrinol. Baltim. Md. 2012, 26, 1542–1551, doi:10.1210/me.2011-1351.
- Kaiser, H.; Parker, E.; Hamrick, M.W. Kynurenine signaling through the aryl hydrocarbon receptor: Implications for aging and healthspan. Exp. Gerontol. 2020, 130, 110797, doi:10.1016/j.exger.2019.110797.
- Perepechaeva, M.L.; Kolosova, N.G.; Stefanova, N.A.; Fursova, A.Z.; Grishanova, A.Y. The influence of changes in expres-sion of redox-sensitive genes on the development of retinopathy in rats. Exp. Mol. Pathol. 2016, 101, 124–132, doi:10.1016/j.yexmp.2016.07.008.
- Wolff, S.; Harper, P.A.; Wong, J.M.; Mostert, V.; Wang, Y.; Abel, J. Cell-specific regulation of human aryl hydrocarbon re-ceptor expression by transforming growth factor-beta(1). Mol. Pharmacol. 2001, 59, 716–724, doi:10.1124/mol.59.4.716.
- Bravo‐Ferrer, I.; Cuartero, M.I.; Medina, V.; Ahedo‐Quero, D.; Peña‐Martinez, C.; Pérez‐Ruíz, A.; Fernández‐Valle, M.E.; Hernández‐Sánchez, C.; Fernández‐Salguero, P.M.; Lizasoain, I.; et al. Lack of the aryl hydrocarbon receptor accelerates ag-ing in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 12644–12654, doi:10.1096/fj.201901333R.
- Ross, D.; Siegel, D. NQO1 in protection against oxidative stress. Curr. Opin. Toxicol. 2018, 7, 67–72, doi:10.1016/j.cotox.2017.10.005.
- Li, Y.; Innocentin, S.; Withers, D.R.; Roberts, N.A.; Gallagher, A.R.; Grigorieva, E.F.; Wilhelm, C.; Veldhoen, M. Exogenous Stimuli Maintain Intraepithelial Lymphocytes via Aryl Hydrocarbon Receptor Activation. Cell 2011, 147, 629–640, doi:10.1016/j.cell.2011.09.025.
- Tilg, H.; Moschen, A.R. Alimentos, inmunidad y el microbioma. Gastroenterology 2015, 148, 1107–1119, doi:10.1053/j.gastro.2014.12.036.
- Lee, H.U.; McPherson, Z.E.; Tan, B.; Korecka, A.; Pettersson, S. Host-microbiome interactions: The aryl hydrocarbon receptor and the central nervous system. J. Mol. Med. 2017, 95, 29–39, doi:10.1007/s00109-016-1486-0.
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33, doi:10.1016/j.immuni.2017.12.012.
- Shinde, R.; McGaha, T.L. The Aryl Hydrocarbon Receptor: Connecting Immunity to the Microenvironment. Trends Immunol. 2018, 39, 1005–1020, doi:10.1016/j.it.2018.10.010.
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and In-flammatory Diseases. Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19123851.
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2018, 8, doi:10.3389/fimmu.2017.01957.
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451, doi:10.1007/s12035-016-0004-2.
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in Autoimmune and Inflammatory Disorders. Nutrients 2018, 10, doi:10.3390/nu10101537.
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, doi:10.1038/s41467-018-05470-4.
- Li, S.; Bostick, J.W.; Zhou, L. Regulation of Innate Lymphoid Cells by Aryl Hydrocarbon Receptor. Front. Immunol. 2018, 8, doi:10.3389/fimmu.2017.01909.
- Longhi, M.S.; Moss, A.; Jiang, Z.G.; Robson, S.C. Purinergic signaling during intestinal inflammation. J. Mol. Med. 2017, 95, 915–925, doi:10.1007/s00109-017-1545-1.
- Megna, B.W.; Carney, P.R.; Kennedy, G.D. Intestinal inflammation and the diet: Is food friend or foe? World J. Gastrointest. Surg. 2016, 8, 115–123, doi:10.4240/wjgs.v8.i2.115.
- Sonowal, R.; Swimm, A.; Sahoo, A.; Luo, L.; Matsunaga, Y.; Wu, Z.; Bhingarde, J.A.; Ejzak, E.A.; Ranawade, A.; Qadota, H.; et al. Indoles from commensal bacteria extend healthspan. Proc. Natl. Acad. Sci. USA 2017, 114, E7506–E7515, doi:10.1073/pnas.1706464114.
- Liang, Y.N.; Yu, J.G.; Zhang, D.B.; Zhang, Z.; Ren, L.L.; Li, L.H.; Wang, Z.; Tang, Z.S. Indigo Naturalis Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating the Intestinal Microbiota Community. Molecules 2019, 24, doi:10.3390/molecules24224086.
- Langan, D.; Kim, E.Y.; Moudgil, K.D. Modulation of autoimmune arthritis by environmental ‘hygiene’ and commensal mi-crobiota. Cell. Immunol. 2019, 339, 59–67, doi:10.1016/j.cellimm.2018.12.005.
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Bio-technol. Biochem. 2018, 82, 600–610, doi:10.1080/09168451.2018.1444467.
- Pernomian, L.; Duarte-Silva, M.; De Cardoso, C.R. The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Con-trol of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor. Clin. Rev. Allergy Immunol. 2020, doi:10.1007/s12016-020-08789-3.
- Xia, B.; Shi, X.C.; Xie, B.C.; Zhu, M.Q.; Chen, Y.; Chu, X.Y.; Cai, G.H.; Liu, M.; Yang, S.Z.; Mitchell, G.A.; et al. Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLoS Biol. 2020, 18, e3000688, doi:10.1371/journal.pbio.3000688.
- Singh, K.P.; Bennett, J.A.; Casado, F.L.; Walrath, J.L.; Welle, S.L.; Gasiewicz, T.A. Loss of aryl hydrocarbon receptor promotes gene changes associated with premature hematopoietic stem cell exhaustion and development of a myeloproliferative disor-der in aging mice. Stem Cells Dev. 2014, 23, 95–106, doi:10.1089/scd.2013.0346.
- Kaiser, H.; Yu, K.; Pandya, C.; Mendhe, B.; Isales, C.M.; McGee-Lawrence, M.E.; Johnson, M.; Fulzele, S.; Hamrick, M.W. Kynurenine, a Tryptophan Metabolite That Increases with Age, Induces Muscle Atrophy and Lipid Peroxidation. Oxid. Med. Cell. Longev. 2019, 2019, 9894238, doi:10.1155/2019/9894238.