Mesenchymal stromal/stem cells (MSCs) are multipotent adult stem cells that support homeostasis during tissue regeneration.
- MSCs
1. Introduction
Mesenchymal stromal/stem cells (MSCs) belong to the pool of adult stem cells that, in a specific microenvironment termed the “stem cells niche”, support tissue regeneration in both physiologic and pathologic conditions contributing to tissue homeostasis[1][2][3]. These cells can increase their own compound[4] and replace individual components of the microenvironment by differentiating or attracting supporting cells to a niche[1][2][3].
Mesenchymal stromal/stem cells (MSCs) belong to the pool of adult stem cells that, in a specific microenvironment termed the “stem cells niche”, support tissue regeneration in both physiologic and pathologic conditions contributing to tissue homeostasis [1–3]. These cells can increase their own compound [4] and replace individual components of the microenvironment by differentiating or attracting supporting cells to a niche [1–3].
2. Role
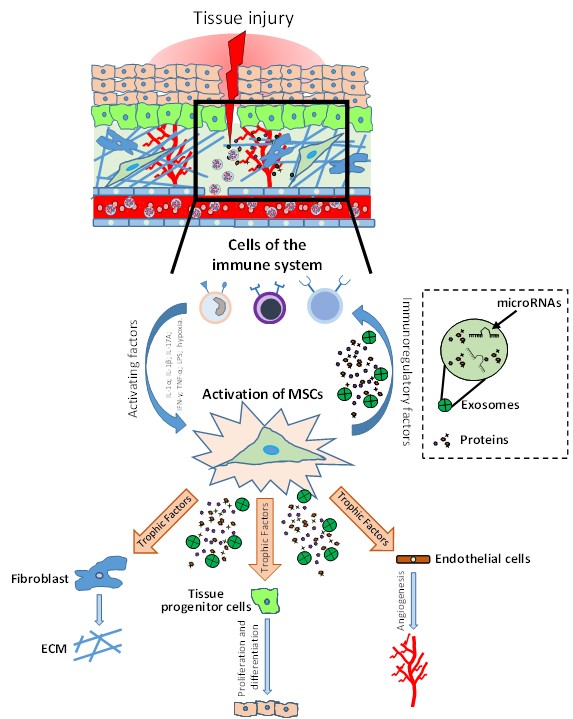
It has been shown that in tissues such as intestine and skin, MSCs support a high cellular turnover[5][6]. In contrast, in other tissues such as skeletal muscle, MSCs are considered adult stem cells that support regeneration after injury, even if they marginally contribute to myofiber renewal during physiologic turnover[7]. Indeed, MSC-like cells with adipogenic phenotype resident in the muscles are quiescent in intact tissue, but get activated in injured ones, providing a momentary source of key factors that induce proliferation of myogenic progenitor cells. Thus, these MSCs that normally have adipogenic potential, when injury-activated, can stimulate the differentiation of the myogenic progenitor’s cells supporting tissue repair[3][8]. A similar phenomenon has been shown in skin tissue, where MSC-like adipose precursor cells within the skin appear to be crucial for epithelial cell regulation[9]. Therefore, MSCs can be considered as key regulatory components in the regenerating stem cell niche (Figure 1).
It has been shown that in tissues such as intestine and skin, MSCs support a high cellular turnover [5,6]. In contrast, in other tissues such as skeletal muscle, MSCs are considered adult stem cells that support regeneration after injury, even if they marginally contribute to myofiber renewal during physiologic turnover [7]. Indeed, MSC-like cells with adipogenic phenotype resident in the muscles are quiescent in intact tissue, but get activated in injured ones, providing a momentary source of key factors that induce proliferation of myogenic progenitor cells. Thus, these MSCs that normally have adipogenic potential, when injury-activated, can stimulate the differentiation of the myogenic progenitor’s cells supporting tissue repair [3,8]. A similar phenomenon has been shown in skin tissue, where MSC-like adipose precursor cells within the skin appear to be crucial for epithelial cell regulation [9]. Therefore, MSCs can be considered as key regulatory components in the regenerating stem cell niche (Figure 1).
Figure 1.
Role of mesenchymal stromal/stem cells (MSCs) in tissue injury and repair. After injury, the damaged tissue activates MSCs through different inflammatory signals (IL-1β, IFN-γ, TNF-α, LPS; hypoxia). MSC activation leads to coordination of the microenvironment by both the production of immunomodulatory factors (to modulate the progression of inflammation) and the production of growth factors which subsequently stimulate endothelial cells, fibroblasts cells and tissue progenitor cells. The physiological and orderly action of these factors allows tissue repair through angiogenesis, remodeling of the extracellular matrix (ECM) and functional tissue restoration through tissue progenitor cells differentiation.
These findings provide a robust rationale to investigate the role of MSCs as a therapeutic product to support tissue injury responses in different diseases. MSCs are multipotent cells with easy accessibility, few ethics-related issues, and higher adaptability to in vitro cultures for expansion. Unlike pluripotent stem cells, multipotent MSCs have a limited self-renewal capacity[10]. In light of this, in recent years the “stem” cell definition has been changed to “stromal” in order to give a more appropriate connotation to describe MSCs. Furthermore, these cells are immuno-privileged due to their low expression of
These findings provide a robust rationale to investigate the role of MSCs as a therapeutic product to support tissue injury responses in different diseases. MSCs are multipotent cells with easy accessibility, few ethics-related issues, and higher adaptability to in vitro cultures for expansion. Unlike pluripotent stem cells, multipotent MSCs have a limited self-renewal capacity [10]. In light of this, in recent years the “stem” cell definition has been changed to “stromal” in order to give a more appropriate connotation to describe MSCs. Furthermore, these cells are immuno-privileged due to their low expression of
CD40
,
CD80
,
CD86
and major histocompatibility complex I (
MHC I),
as well as the lack of
MHC II expression[11][12][13][14]. These features make these cells a very useful tool for cell therapy in the field of regenerative medicine.
expression [11–14]. These features make these cells a very useful tool for cell therapy in the field of regenerative medicine.
MSCs are found in several tissues, including bone marrow (BM)[15], adipose tissue (AT)[16], umbilical cord (UC)[17], dental pulp[18] and placenta[19], where these cells are surrounded by different cell types such as immune cells, epithelial cells, endothelial cells and stromal cells, and can exhibit immunomodulatory[20][21], angiogenic[22][23] and anti-oxidative properties[24]. Over the past decade the therapeutic action of MSCs has been investigated in several clinical trials for the treatment of many disorders including cardiovascular, neurodegenerative, immune, lung, liver, kidney and orthopedics diseases (clinicaltrials.gov). In these cases, MSCs have been shown to have moderate or poor efficacy, and the results from different clinical trials are controversial[25][26][27][28][29], indicating an urgent need to optimize the therapeutic use of MSCs or to enhance MSC potency. This inconsistent evidence is potentially related both to intrinsic differences in the use of cell-based products and to the lack of standardized methods for MSC production that affects their potency. MSC effects depend both on tissue source[30][31] and on how they are produced and administered. Indeed, it has been shown that the composition of MSCs secretome can be modulated by preconditioning of MSCs with hypoxia and cytokines treatments, as well as the growing of MSCs under specific culture systems, including three-dimensional (3D) culture conditions[32][33][34][35]. In response to MSC “priming”, the production of factors is switched towards an anti-inflammatory and pro-trophic phenotype that results in a homeostatic regulation of tissue regeneration/repair[36][37]. Currently, it is often stated that the efficacy of MSCs therapies is probably not related to cell engraftment and replacement but is linked to the production of crucial paracrine factors, such as cytokines, growth factors, and exosomes (EXOs), that regulate the cell niche for their regeneration. Indeed, in response to specific stimuli, MSCs are activated and can secrete a plethora of regulating factors that affect tissue injury responses in a transitory and paracrine manner to orchestrate the repairing tissue processes[20][38][39][40][41][42][43][44]. In a different model of injury it has been shown that MSCs, mainly triggered by inflammation processes, induce tissue regeneration/repair by cell niche empowerment/regulation[45][46][47]. In these cases, in an inflammatory-injured tissue, MSC effects were mediated by paracrine mechanisms that lead to regulation of fibrosis, immunomodulation, stimulation of angiogenesis and stimulation of resident cells to coordinate both tissue regeneration and function recovery[37][48][49][50][51][52]. Therefore, due to the regenerative potential and trophic properties of specific MSC-derived products, such as the conditioned medium (CM) and EXOs, these products have emerged as possible therapeutic tools with numerous applications and are consequently being extensively evaluated for medical use[53][54][55]. In addition, the clinical application of MSC-derived products must be considered for their advantages as opposed both to the lack of safety in the long-term use of MSCs and the risks related to transmission of infection diseases, such as some viruses found in the transplanted allogenic cells.
MSCs are found in several tissues, including bone marrow (BM) [15], adipose tissue (AT) [16], umbilical cord (UC) [17], dental pulp [18] and placenta [19], where these cells are surrounded by different cell types such as immune cells, epithelial cells, endothelial cells and stromal cells, and can exhibit immunomodulatory [20,21], angiogenic [22,23] and anti-oxidative properties [24]. Over the past decade the therapeutic action of MSCs has been investigated in several clinical trials for the treatment of many disorders including cardiovascular, neurodegenerative, immune, lung, liver, kidney and orthopedics diseases (clinicaltrials.gov). In these cases, MSCs have been shown to have moderate or poor efficacy, and the results from different clinical trials are controversial [25–29], indicating an urgent need to optimize the therapeutic use of MSCs or to enhance MSC potency. This inconsistent evidence is potentially related both to intrinsic differences in the use of cell-based products and to the lack of standardized methods for MSC production that affects their potency. MSC effects depend both on tissue source [30,31] and on how they are produced and administered. Indeed, it has been shown that the composition of MSCs secretome can be modulated by preconditioning of MSCs with hypoxia and cytokines treatments, as well as the growing of MSCs under specific culture systems, including three-dimensional (3D) culture conditions [32–35]. In response to MSC “priming”, the production of factors is switched towards an anti-inflammatory and pro-trophic phenotype that results in a homeostatic regulation of tissue regeneration/repair [36,37]. Currently, it is often stated that the efficacy of MSCs therapies is probably not related to cell engraftment and replacement but is linked to the production of crucial paracrine factors, such as cytokines, growth factors, and exosomes (EXOs), that regulate the cell niche for their regeneration. Indeed, in response to specific stimuli, MSCs are activated and can secrete a plethora of regulating factors that affect tissue injury responses in a transitory and paracrine manner to orchestrate the repairing tissue processes [20,38–44]. In a different model of injury it has been shown that MSCs, mainly triggered by inflammation processes, induce tissue regeneration/repair by cell niche empowerment/regulation [45–47]. In these cases, in an inflammatory-injured tissue, MSC effects were mediated by paracrine mechanisms that lead to regulation of fibrosis, immunomodulation, stimulation of angiogenesis and stimulation of resident cells to coordinate both tissue regeneration and function recovery [37,48–52]. Therefore, due to the regenerative potential and trophic properties of specific MSC-derived products, such as the conditioned medium (CM) and EXOs, these products have emerged as possible therapeutic tools with numerous applications and are consequently being extensively evaluated for medical use [53–55]. In addition, the clinical application of MSC-derived products must be considered for their advantages as opposed both to the lack of safety in the long-term use of MSCs and the risks related to transmission of infection diseases, such as some viruses found in the transplanted allogenic cells.