CD44 is a receptor described as a single span transmembrane glycoprotein without kinase activity whose ubiquitous and constitutive expression has been observed on many different cells.
- tumor-derived extracellular vesicles
- extracellular vesicles
- CD44
1. ‘Facts Check’—CD44 Receptor
CD44 can exist as an integral component of the extracellular matrix (ECM) or as a soluble protein found in body fluids [1][11]. Each of these protein forms plays biological roles, which may be shared or distinct [1][11].
The membrane form of CD44 is a receptor that binds ECM ligands, such as hyaluronan (HA), collagen, osteopontin, laminin, fibronectin, and others. The structure of CD44 in its standard (CD44s) and variant (CD44v) form is presented in Figure 1. CD44 is encoded by a single gene, which in humans is composed of 20 exons [2][12]. The schematic CD44 gene structure is presented in Figure 2. CD44s is composed of the homologous N-terminal and C-terminal domains only. The N-terminal domain is encoded by exons 1–5, whereas the C-terminal domain is encoded by exons 16–20. The molecular mass of CD44s is usually between 80–85 kDa. Multiple variant forms (CD44v, v1–v10) are generated by alternative splicing of exons 6–15, and are composed of both the N- and C-terminal domains and an additional variant domain(s). While CD44s is found in a wide variety of tissues, the CD44v isoform seems to have a much-restricted distribution and is observed to be present in keratinocytes, activated lymphocytes, macrophages, and some epithelial cells [3][13]. In both CD44 forms, the N-terminal domain binds the physiological ligands. CD44v isoforms are enriched in binding motifs, which promote the interactions with microenvironment components and may serve as co-receptors for growth factors, such as EGF and HGF [4][14]. Structural diversity of CD44 is also generated by posttranslational modification, such as phosphorylation, glycosylation, and attachment of glycosaminoglycans [1][5][11,15]. Resting cells show a low-affinity for binding CD44 ligands; however, cellular activation can induce a transition of CD44 to a high-affinity state that mediates binding to HA. The transition from the “inactive” low-affinity state to the “active” high-affinity state of CD44 can be induced by soluble factors, including cytokines [6][7][16,17]. Activation of CD44 leads to clustering and initiation of intracellular signaling pathways, such as ERK1, 2, AKT, FAK, Rho, Src, etc. (reviewed in [8][7]). Tumor cells express CD44 in a high-affinity state with the capacity for constitutive binding of HA [9][18]. The soluble and/or integral with ECM forms of CD44 are generated by metalloproteinase-inflicted (mainly MT1- and MT3-MMPs) shedding from a cell’s membrane [10][19]. The soluble CD44 protein can also be also produced by alternative splicing [11][20]. This form has been detected in the circulation and other body fluids, such as lymphatic, arthritic synovial, and bronchoalveolar fluids, as well as in cancer patients’ serum [12][13][14][21,22,23]. Soluble CD44 overexpression blocks cancer cell adhesion to HA [15][24] and is associated with aggressive growth and unfavorable prognosis for a patient.
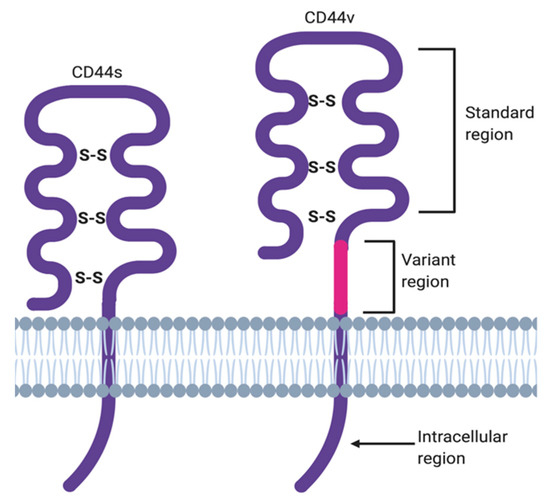
Figure 1. CD44 structure in its standard (CD44s) and variant (CD44v) forms.
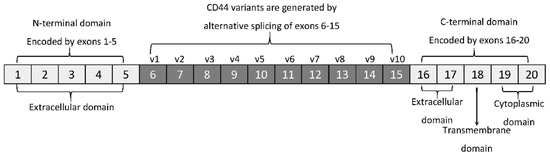
Figure 2. CD44 gene structure. CD44s form is encoded by exons 1–5 and 16–20 only, whereas CD44v form undergoes alternative splicing (exons 6–15).
2. Hyaluronan—The Major Ligand of CD44
As mentioned above, CD44 binds to a number of ligands; however, hyaluronan (hyaluronic acid, HA) is its most specific ligand that is recognizable by both standard and variant forms of this receptor [8][7]. HA is an anionic, non-sulfated polysaccharide that belongs to glycosaminoglycans. Its basic disaccharide monomer is a D-glucuronic acid linked with N-acetyl-D-glucosamine by glycosidic bonds (β1–4, β1–3). The schematic structure of HA is presented in Figure 3. The molecular weight of HA varies from 103 to 107 Da and depends on its tissue origin. In contrast to other glycosaminoglycans, HA is synthesized by three HA synthases (HAS 1–3) at the cell surface (not in Golgi apparatus) [16][25] and is released into the extracellular space. HA is a major component of ECM, which supports tissue organization and contributes to crucial processes, such as cell migration, proliferation, survival, etc. [17][26].
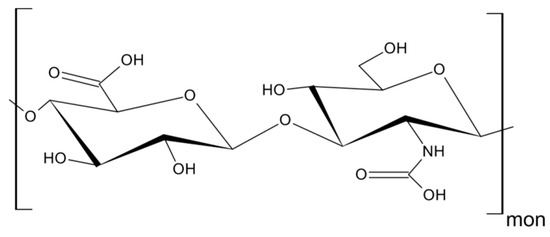
Figure 3. Structure of hyaluronan (HA).
The downstream effects of HA binding depend on its molecular weight. Under normal circumstances, HA, in its “native state”, represents a high-molecular weight protein of about 1000 kDa [18][27] with anti-angiogenic, anti-inflammatory, and proliferation inhibiting activities [19][28]. On the other hand, HA in its ‘fragmented state’, with a molecular weight below 500 kDa, stimulates proliferation and migration of cells, induces an immune response, e.g., by increasing expression of inflammatory genes in human macrophages [18][20][27,29], the formation of immunosuppressive macrophages [21][30], death of activated T cells [22][31], and augments angiogenesis [19][28].
A number of studies have shown that HA is produced in abundance by malignant and stromal cells supporting the growth of tumors (breast [23][32], bladder [24][33], prostate [25][34] colorectal cancer [26][35]), and correlates with tumor aggressiveness in humans [21][26][30,35]. HA facilitates tumor cell proliferation, cell-to-cell adhesion and protects them from the immune cell response [27][36]. The balance between HA contradictory activities is governed by a complex system of HA synthases and hyaluronidases (Hyal), which regulate its degradation. Tumor cells have been reported to secrete excess amounts of hyaluronidases with the ability to cleave high molecular weight HA into smaller fragments (10–40 oligomers) [28][37], which, in turn, promoted CD44 cleavage [29][38]. Enhanced cleavage of CD44 was shown to facilitate tumor cell motility, which has been observed in many human tumors, including breast, colon, gastric, ovarian, lung, and others [28][37]. It has been shown that HA is involved in cancer cell, lymphocyte, and neutrophil motility in a CD44-dependent manner, however, the expression of this receptor alone was insufficient for chemotaxis towards HA [30][31][39,40]. Data on the binding efficiency of HA to CD44s and CD44v are conflicting. Bennett et al. reported that CD44s binds HA more efficiently than CD44v [32][41], however, other authors presented convincing data showing HA’s affinity to both receptors to be similar [33][42].
3. CD44-HA Interactions
There is quite an extensive number of surface receptors that recognize either the soluble or molecular form of HA. These include proteins, such as CD44, RHAMM, TSG-6/TNFIP6, Brevican, SHAP, LYVE1, TLRs, and others [17][26]. The RHAMM and TLR2,4 receptors preferentially bind smaller HA molecules (below 7 kDa), whereas CD44 requires at least six monosaccharides for the process to occur [reviewed in [34][43]. CD44 contains at least three HA binding sites on the hair loop (encoded by exon 2 and 5). CD44 molecules have been reported to be present in three functional stages: Constitutive binding of HA (most of the tumor cells), non-binding or non-binding until activated by physiological stimuli [8][35][7,44]. The affinity of HA to CD44 is cell-type dependent, regulated by glycosylation in the receptor’s extracellular domain and the phosphorylation of the serine residue in the cytoplasmic tail [36][45]. All forms of CD44 (standard or variants) can bind HA. In the case of immune cells, CD44 was described as the only receptor that has been demonstrated to bind HA [31][40].
Upon HA binding to the intercellular domain, CD44 undergoes conformational changes resulting in the recruitment of adaptor proteins (ERM, Src, etc.) to its intracellular domain, which, in turn, leads to the activation of a number of signaling pathways involved in cell proliferation, adhesion, migration, invasion and metabolic shift [37][38][8,46]. Table 1 summarizes all the currently known signal transduction mechanisms and their outcomes associated with CD44 molecule in cancer cells. Moreover, CD44v isoforms can also serve as co-receptors with the ability to sequester growth factors (i.e., HGF, FGF, EGF) at the cell surface and presenting them to tyrosine kinase receptor (RTK) complexes (i.e., c-Met, EGFR), which in turn, upon activation, can initiate an appropriate signaling pathway. Figure 4 depicts possible intercellular signaling mediated by the CD44 receptor in cancer cells.
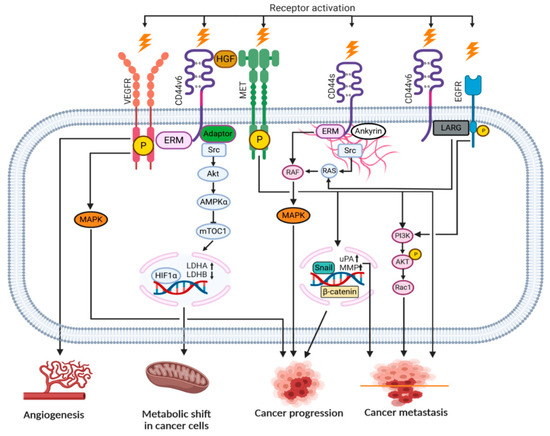
Figure 4. Signaling pathways engaging the CD44 receptor in cancer cells (according to [8][7] with own modifications).
Table 1. Association of the CD44 molecule with signaling pathways in cancer cells.
| Signaling Pathway | Outcome | Reference |
|---|---|---|
| CD44s | ERM/MAPK | Cell division, proliferation |
| PI3K/AKT/NFκB | Inflammatory cytokine release | |
| Migration of ICD fragment into the nucleus | Metastasis, survival | |
| RHAMM/cSrc/RAS | Migration | |
| CD44v | ERM/FAK/Paxillin | Angiogenesis |
| Src/AKT/HIF1α | Metabolic shift (glycolysis increase) | |
| Met/PI3K/AKT | Cell invasion | |
| Met/Snail/MMP | Cell division, proliferation |
CD44–HA interactions were defined as important for many types of cells. For example, macrophages exhibited preferential migration towards HA-enriched stromal structures in breast cancer [38][46]. It was also shown that small HA induced immunophenotypic maturation of human monocyte-derived dendritic cells and an increase in their pro-inflammatory cytokine (IL-1, TNF, and IL-12) production [39][47]. An elevated expression of CD44 has been reported on activated T cells [18][27]. The CD44-dependent adhesion mechanism is likely to be the most important for mobilizing the effector T cells at sites of infection and inflammation, because the expression of CD44 is upregulated whereas L-selectin (CD62L), the lymphocyte-expressed selectin, is downregulated in these cases [40][48]. Activation of CD44 influenced tumor cell metabolism by inducing Hypoxia-inducible factor 1α (HIF1α) binding to nuclear DNA to increase glycolysis, in turn, rendering a metabolic shift in cancer cells [41][49].
