Emotion is a state and not a trait.
- animal emotions
- animal welfare
- behavior
- sensors
1. Introduction
There are numerous benefits to studying animal emotions. Because we ourselves are animals, studying animal emotions can give us greater insight into our own psyche and how our emotions manifest not just physiologically but also behaviorally and cognitively. Additionally, studying the emotions of farm animals helps us to learn how to be better providers for our livestock, work animals, and pets. To be able to assess the welfare of farm animals thoroughly, better understanding of the affective experiences and emotions of the animals are absolutely needed. This understanding has tangible, practical benefits. For example, Weaver et al. [1] [1] found that cows produced more and higher-quality milk when exposed to serotonin, a neurotransmitter linked to feelings of happiness and wellbeing. Happiness refers to a long-term positive state, while happy (happiness, happier) can also refer to the basic, discrete emotion “happy” [2]. In other words, happier animals have the potential to be more productive animals.
More broadly, learning about animal emotions has the long-term potential to give us better ecological insight than we have at present. Emotional cues from animals may give us an idea of the health of an ecosystem before major problems emerge. This may prove critical to conservation efforts in the wake of extreme climate change. In the agricultural sphere, our system has become highly industrialized over the last several decades. Smaller farms mostly disappeared and instead agriculture has grown massive in scale. These massive farms have heightened the challenges of identifying, monitoring, and caring for large groups of animals. This scale has also made keeping the animals satisfied mentally, and overall productivity of the animals more difficult.
While farmers may be open to utilizing technology to perform managerial and monitoring tasks, the possibilities of this field concerning emotions and mental states and its impact on animal welfare are not yet fully explored. There is tremendous potential in technological sensors for monitoring the emotional conditions of animals, allowing farmers to study behavioral changes, detect diseases [3[3][4],4], and easily make adjustments in care to promote the welfare of their animals and increase the yield on their products. To provide a high quality of life to animals, and to remove stress induced factors on the health and welfare of animals, monitoring and measuring of farm animal emotions becomes crucial.
The literature cited in this review article were collected using the Web of Science, Scopus, and Google scholar tools. To showcase the latest developments and recent findings of this research in this area and to narrow down the search, the authors restricted the search to papers published only in the past five years. Keywords used were, farm animal emotions; pig emotions; dairy cow emotions; sheep emotions; horse and chicken emotions; emotional contagion; animal empathy; animal emotions; sensors for emotions; sensor fusion and emotional contagion; measurement of emotions using sensors; sensors and farm animals; pain measurement in farm animals. Individual searches and Boolean were conducted as part of this study. Only farm animals were chosen from the pool of literature. The number of papers cited in this review is 129, with 20 that were published before 2015. The papers published before 2015 were included as the information on the sensing technologies for measuring emotions in farm animals were scant, and to signify the content on the need for sensors in the emotion measurement of farm animals.
2. Animal Emotions
One barrier to studying animal emotions is that the concept of emotion resists definition and quantification. There is no scientifically agreed-upon definition of what constitutes an emotion, and the term is often used interchangeably with others like disposition, mood, temperament, and mental state [5]. At its broadest, an emotion can be defined as a psychological phenomenon that helps in behavioral management and control, but this definition is too broad to be of immediate use [6]. More practically, the most commonly accepted definition is that emotions are biological states induced by neuropsychological stimulation brought on by physiology, behavior, and cognition [7]. Emotion is a state and not a trait. One useful framework for considering animal emotions is through the lens of affect or affective state. This is defined as the experiences and emotions that drive an organism to function. Affect drives animals towards reward and drives them away from punishment. In other words, affect connects the emotional inner life with the physical outer world [5].
Generally, emotions are considered to consist of four different components: Subjective, Behavioral, Cognitive, and Physiological [8]. Each component in turn has a valence, or direction—whether the experience is positive or negative. Furthermore, they may also vary in the degree of arousal and duration. This conceptual framework of emotion is illustrated in Figure 1. However, while we use this framework to discuss the matter here, the precise labels used to differentiate different components of emotions vary greatly among different research methodologies [5].
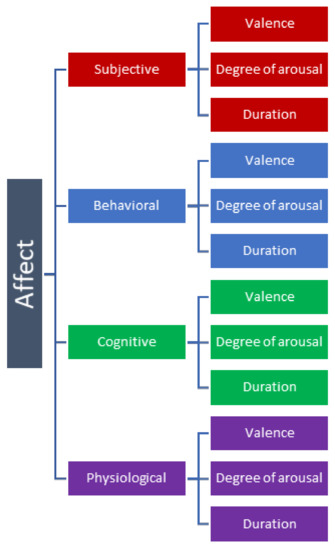
Figure 1.
The framework of affective state.
The Subjective component of emotion refers to the actual feeling experienced in real time by the given organism. Mammals (particularly primates) and birds experience something resembling emotions as we as humans understand them. Behavior in these animals is governed by automatic responses. The implication of this neurological hierarchy is that the subjective component of emotion can affect the behavior and emotional state of animals, but only those that have reached a certain level of cerebral organization.
The Behavioral component of emotion refers to how subjective experiences translate into tangible action. Some researchers argue that feelings cause behavioral changes, while others posit that it is the behaviors themselves that trigger feelings [9]. This component is complicated by the fact that behavioral responses may themselves feedback into the brain, causing further adjustment to the current emotional state. This is known as interoception, and it is not fully understood what effect, if any, this phenomenon has on animal emotions [10].
The Cognitive component of emotion refers to the way in which an organism thinks or makes decisions based on emotions. This component is also debated significantly in the field of animal science. Some researchers feel that cognitive processes and affective (emotional) processes are interdependent, while others believe the two systems are independent of each other [11]. It may be possible that affective processes may predate intellect, evolving from primitive subcortical structures [12].
Measurable aspects of the emotions including behavior, body language, sounds, facial expressions and physiological components are critical to the subject at hand in this review. The physiological component refers to the way that organisms experience bodily reactions in response to emotion. The role of the immune and neuroendocrine systems in emotions is well established in both humans and animals [13]. This paper deals with both the measurement of physiological and behavioral components (sound, body language including facial expression) of the farm animal emotions with a plea to combine these metrics.
2.1. Emotions and Animal Welfare
Animal welfare concepts call for all the activities involved in taking care of an animal physically and providing for its emotional wellbeing. Basic health and functioning; natural living; and affective states form the aspects/pillars of three circles model of animal welfare [14]. For example, a hungry animal may produce vocalizations or show a pronounced difference in posture, indicating a negative emotional state.
We know more about measuring animal welfare concerning the aspects of basic health and functioning and natural living but only a little about the measurement of affective state, particularly positive experiences such as pleasure and satisfaction. Maintaining positive affective states can lead to a greatly improved level of happiness and health for domestic and livestock animals [15].
Most farmers have significant emotional and financial incentive to take good care of their charges. Not only is this humane for the animal, but it also has distinct financial benefit since, as stated earlier, happier animals may be more productive in general. Emotion measurement is one concrete step farmers can take towards caring for their animals better [16].
An important consideration when designing a system for animal monitoring is to ensure that the system itself is not detrimental to animal welfare. Since no two individuals are alike, identifying individual farm animals is extremely important to this work. In the past, invasive methods such as branding, in-vivo sensors, and attaching transmitters with hooks or other invasive methods have been used. All of these may have negative impacts on the welfare of the individual in the form of infections, parasites, and emotional distress. They may also prove ineffective (e.g., transmitters can get lost). One of the great benefits of the emerging technology for animal monitoring is the potential for non-invasive identification. Today, software technology akin to facial recognition in humans has been developed for use in animal management and research. In the 1990s, visual and pattern recognition were combined with digital photography to create Visual Animal Biometrics (VAB) technology [17]. This technology gave us the ability to identify individual animals by their unique physical attributes, including even retinal patters, as no two animals have the exact same markings and colorations. This greatly enhances our ability to obtain accurate counts of populations, follow the animals’ movements, and provide for better welfare of both livestock and wild populations [18]. This recognition can be done remotely, without ever disturbing the animals, whether they are land-based, free-roaming, or aquatic [19]. So, while older methods of data collection sacrificed a small amount of good animal welfare for the benefit of the population at large, no such compromises need be made in future.
2.2. Common Emotional States in Animals and Their Presentation
Animals can experience and express a wide range of both positive and negative emotions [15]. Here, we briefly review the most common emotional states that researchers have studied in animals (Table 1).
Table 1. Summary of emotions expressed by farm animals and sensing parameters related to recognize each emotion.
| Farm Animal Species | Indicators Inferring Emotions and the Emotions/Affective States |
|---|---|
| Sheep | Horizontal ear posture—Neutral state Ears backward—Fear Ears up—Anger Asymmetric ears—Surprise |
| Sheep | Ear flat—Pain not present Ear flipped—Pain fully present/Negative state Shallow U-shaped nose—Pain not present/Neutral or positive state Extended V shaped nose—Pain fully present/Negative state Eye fully open, Pain fully present/Negative state Eye partly closed, Pain not present, Neutral or positive state |
| Lamb | Cheek flattening—Less bulging of nose and cheek area—Pain Ear Posture—Ears tense and point backwards or downwards (no visible inner ear)—Pain Ears relaxed and horizontal or inner ear visible—Not in pain or neutral state Flat and tight lip like horizontal line—‘Smile’ emotion and not in pain Tight nose with decreased nostril size—Pain V shape nose—in pain—U shape nose—Not in pain Squeezing or closing of eye (Orbital tightening)—In Pain |
| Goat | Ears lowered and turn down—Positive emotions Ear tips pointing backwards, and auricles turned down—Negative emotions |
| Horse | Lower oxytocin level—Neutral and positive emotional states Rise in cortisol levels and rise in heart rate parameters—Stress |
| Horse | More eye white region—Negative emotion experience Decrease in eye wrinkle expression—Positive emotion condition Increase in eye wrinkle expression—Increase in negative emotion |
| Horse | Increase in spontaneous blink rate of eye—Stress Increase in dopamine levels—Positive emotion due to reward Increase in salivary cortisol and change in heart rate variability—Stress |
| Horse | Increase in heart rate, eye white increase, nostril dilator, upper eyelid raiser, inner brow raiser, tongue show with increase in ear flicker and blink frequency—All related to increase in stress |
| Cow | Upright ear posture longer—Excitement Forward facing ear posture—Frustration |
| Cow | Half-closed eyes and ears backwards or hung-down—Relaxed State Eye white clearly visible and ears directed forward—Excited State |
| Cow | Decrease in nasal temperature and change in peripheral temperature—Positive experience or increase in arousal |
| Cow | Cow vocalizations—Open mouth calls & a greater number of vocal units per sequence—alert and stress escalation Close mouth calls—Positive emotional state |
| Cow | Visible eye white and maximum eye temperature—Stress |
| Dairy Calves | Lower heart rate—positive emotion Higher heart rate—negative emotion Increase in salivary cortisol—Both positive and negative emotion Higher secretory immunoglobulin A (SIgA), Serum IL-2 and IL-3 levels—Positive emotional states Higher serum of tumor necrosis factor alpha (TNFα)—Negative emotion |
| Hens | Increase in cortisol in serum—Negative emotions and stress Increase in corticosterone levels in feathers—Positive emotions |
| Hens | Increase in corticosterone levels in feathers—Positive excited states |
| Chickens | Tachycardia and bodily fever—Fear Increased locomotion and pacing behaviour—Anxiety or Negative emotion Lower corticosterone—Positive emotion |
| Chickens | Repetitive, high energy calls (sounds)—Distress or negative emotions |
| Pigs | High frequency ear movement—Stress or negative emotion High duration lateral tail movement—Positive emotions or play behavior |
| Pigs | Tail raised and forming a loop—Positive emotion Ears forward—Alert and neutral emotion Ears backward—Negative emotion Hanging ears flipping in the direction of eyes—Normal state (Neutral emotion Standing upright ears—Normal neutral state |
| Pigs | Smaller snout ration and ears forward oriented—Aggression or negative emotion state Ears backward and less open eyes—Retreat from aggression or transition to neutral state |
| Pigs | Tail hang loose—Negative or neutral emotion state |
| Pigs | Curled up tails and ears directed forward—Positive emotion state Tucked under tails—Negative emotion |
2.2.1. Pain
Pain is a dominant, aversive emotion in response to illness or physical injury in animals and it is distinct from human concepts of emotional pain (for example, grief) [20]. Pain in animals may elicit abnormal reactions, changes in motor skills and coordination, and unusual social behavior. Pain is commonly associated with production related diseases namely mastitis and lameness in dairy cattle and tail docking or castration in pigs.
2.2.2. Fear and Aggression
Though animals may not have exact emotions that qualify as “anger,” they tend to be aggressive under certain conditions when they are pushed or provoked [21]. Traditionally, researchers have studied basic emotions such as aggression and fear in farm animals during the past two decades. Fear related studies focused on situations such as the animal facing a threatening situation, or presence of a predator or a novel object, etc. The aggression related experiments typically included conspecific interactions.
2.2.3. Distress
Distress can include a variety of responses of the animal to a changing environment. In animals, it may present as changes in feeding habits, a compromised immune system, or elevated levels of the hormone cortisol [22]. Heat stress is a specific category of distress that occurs when the animal is unable to maintain the appropriate body temperature due to high ambient heat. Heat stress can affect fertility in animals [23]. Heat stress is also frequently accompanied by other health issues like dehydration. Frustration is another form of distress that occurs in animals when their access to a resource they need is cut off. This resource can be nutritional, like food or water, or it can include resources such as access to mates or mating habitats.
2.3. Physiological Indicators of Emotions in Animals
Using physiological cues or biomarkers to monitor animal emotions is just as important as monitoring visible behavior. Although, physiological cues are generally considered less informative on the valence facet of emotion [5] [5], the benefit of using these types of cues is that they can be tracked chemically through biosensors, allowing for a more objective, quantitative analysis of the animal’s emotion, including inferences about the arousal facet.
In humans, for example, states of anxiety and or tension are related to elevated or augmented blood lactate levels [48][24]. Blood lactate concentrations in livestock indicate the severity of stressors and underlying disease conditions such as respiratory diseases or neonatal diarrhea, or displacement of abomasum [49][25]. In beef cattle, such states have been studied using cortisol measurements in the hair matrix [50,51][26][27]. Cortisol concentration in saliva has been used as a biomarker for changing stress levels in pigs [52][28]. Chewable silicone stick-based (popsicle or lollipop) sensing devices have the potential to measure salivary concentrations in pigs and cattle. Salivary oxytocin in pigs, cattle, and goats have been shown to influence positive human-animal interactions and can be an effective biomarker of positive emotions [53,54][29][30].
Measuring sample matrices and biochemical signatures in urine, nasal, and saliva secretions of farm animals indicating emotions is not well established. Serotonin (5-hydroxytryptamine, 5-HT) is a prototypical neuromodulator and significantly impacts animal cognition and behavior, and this neuromodulator is fundamentally involved in the adaptation of animals [55][31]. Because dopamine cannot be directly measured, researchers opted to measure catecholamines in livestock as a stress indicator. Increased level of catecholamines in beef cattle is considered as an objective measurement of pre-slaughter stress in cattle [56][32]. However, it should be noted that there are no commercially available on-the-spot measurement devices or sensors for any all these biomarkers.
2.4. Reading Animal Emotions
One of the greatest challenges of monitoring animal emotions is that many of the methods that might prove useful for humans, such as surveys or interviews, are useless for creatures that cannot read, write, or speak. In addition to language, humans also have a strong body language. The relative ease with which it is possible to identify human emotions has led to several technological systems for sensing human emotions [57][33].
Most animals, with the arguable exception of monkeys and apes, lack these mechanisms and must rely on other means to convey their emotions. Some animals use vocalizations such as growls, murmurs, barks, roosting calls, or purrs. Other animals use tails and body posture, like wagging a tail when happy or swishing a tail to convey anger. These types of signals can communicate, or even spread, the animal’s emotions within species or to humans [58,59][34][35].
However, such signals are not without fault. Even among humans, they are often misinterpreted, leading to embarrassing, and sometimes unfortunate results. A major complication in reading these signals is that the majority of animal behavior and physiology studies are not done on free range or wild animals. Rather, they are usually undertaken with domesticated or captive animals.
In general, emotional states fall into two main categories as described by Jaak Panksepp [60][36]; primary and secondary emotions, and each can be further categorized into positive and negative states. While primary emotions are generally easier to interpret, since they are based upon instinctual responses and thus may be similar across individuals of a species, secondary emotions are more nuanced. Interpreting farm animal emotions thus requires a solid knowledge of the species in question, as well as familiarity with the individual.
2.5. Relationship between Emotions, Facial Patterns, and Sounds
Two behavioral indicators of emotion relevant for sensor technology in farm animals are facial expressions and sounds. The ability to connect the face and sounds of an animal to an emotional state is critical for many practical applications, due to the fact that most livestock animals are mammals capable of changing their facial expression to a certain degree. An early study of the relationship between the expression of the face and emotions was published in 1964 [61] [37]. However, it and many of the studies to follow were focused primarily on human emotions rather than animals.
Today, the scope of the research has expanded, and facial expressions are widely considered to be a great means of assessing the internal state of an animal. Pain expression is difficult in animals, and research is only now emerging on the use of facial changes in response to pain or stress [62][38]. Horses in particular have also been shown to have positive facial expressions [63][39]. One challenge of these types of studies is the difference in indicators of fear and stress within a species, overlapping of emotions, and false indicators based on other, unknown stimuli.
Farm animals also convey emotional states through sound. Sounds have been demonstrated to be indicators of emotions in several animals including horses [64][40], pigs [65][41], poultry and cattle [66][42]. Many animal vocalizations, particularly those indicating a negative emotion, are involuntary. This suggests that sounds may often indicate primary emotional responses as a first reaction.
3. Technologies for Measuring Animal Emotions
At present, direct measurement of emotion (as in the subjective component) is not possible, even for humans. Indirect measurements of emotion are time-sensitive and are difficult to take manually. However, modern technology is making observation and analysis of animal behavior and physiology faster and more effective. In this section, we discuss different technologies for monitoring farm animal emotions, including sensors, facial expression, sound analysis, and multimodal integrated technology approaches.
3.1. Sensors
Visual sensors (cameras) and biosensors constitute a significant part of the solution to automate the monitoring process of farm animals [67][43]. Sensors and biosensors in this context refer to devices that collect data about a specific physical, chemical, biological or biochemical parameter that can then be measured and analyzed [19].
Sensors can be affixed to a part of the barn, placed in a grazing field, or placed on or implanted within the farm animals themselves. They can be classified as wearables or non-wearable remote types and are invasive or non-invasive depending on the location. Noninvasive sensors are those located external to the animal, immobile, and nonattached. Alternatively, they can be attached to the animal’s body to collect information [68][44].
Invasive sensors are those that are implanted into the animal. While invasive sensors can provide more accurate, individualized data, they may induce stress that skews the data or harms the animal’s welfare, so these sensors must be used carefully or avoided. Biosensors can be invasive or wearable and non-invasive and detect the presence of specific biological compounds, such as a hormone or enzyme [67][43]. Each category of sensors has its benefits and drawbacks, and each can be used to attempt to quantify the emotional experience of the animal. While wearable sensors are frequently more accurate in terms of the parameter they measure, they also require large numbers of individual sensors to get a sufficient dataset to assess the emotional state of all individual animals. On the other hand, a small number of immobile sensors can be used for a large number of animals, as long as they are placed in locations where the animals will frequently be present.
There are several categories of sensors commercially available and are under development, and each measures a distinct parameter and has its own benefits and drawbacks (Table 2).
Table 2.
Pros and cons of different sensor systems related to emotions measurement.
| System | Pros | Cons |
|---|---|---|
| Global Positioning System | Long-lasting system, noninvasive | Expensive at startup, battery life, issues with accuracy, noise |
| Thermal Infrared Imaging | Accurate indicator of temperature, noninvasive | Subject to interference from external heat sources |
| Electrocardiograph | Likely reliable indicator of positive affect through heart rate measurement | Deployability issues due to motion artefacts; Not practical for real-time or on-site monitoring |
| Electroencephalography | Accurate measure of brain activity irrespective of subject movement | Dissociation between EEG states and emotional valences; Real-time non-invasive sensors are not yet available |
| Electromyogram | Useful for many diagnostics | Subject to interference; Only measures surface muscles |
| Respirometer | Especially useful for diagnostics and for animals with distinct breath patterns | Difficult to implement and influenced by many factors including motion |
| Olfactory and chemical sensors | Strongly linked to emotion | Do not use data from the animal directly; Indirect measurement as validated benchmarks is unavailable |
3.2. Global Positioning System
The global positioning system (GPS) is satellite-based standard sensing technology used for tracking farm animals’ location. Despite its longevity, initial cost of installation and implementation of this technology is quite high [69][45]. GPS sensors continuously monitor and maps the places the animals wander. These data can then be used to draw conclusions about the collective habits of animals in a group, or of individual animals. GPS is the primary tool for behavioral insights in grazing animals. GPS is also useful for monitoring wild animals, making it a frequent choice for measuring emotions in farm animals [70][46]. RFID and UWB are terms more often used in farm animals kept indoors than GPS.
However, GPS is not without limitations. Battery life, accuracy, and loss of data due to noise or external factors are all issues that may arise with a GPS tracking system. Despite these limitations, GPS is still widely used. Interestingly, Fogarty et al. [4] [4] found that GPS was the most frequently used type of sensor to study sheep but was not used in studies on sheep welfare. This suggests that location data are at present not the primary parameter in the measurement of emotions of livestock.
3.3. Thermal Infrared Imaging Sensors
Thermal imaging captures images of animals using infrared light as opposed to the visible spectrum [46][47]. This results in an accurate indicator of temperature throughout the animal because infrared radiation is directly linked to heat. In order for this system to function, it must be able to continuously and precisely monitor body temperature. But once that is successful, it is a valuable tool. Additional benefits of infrared radiation include the fact that the sensors are no more invasive or destructive than a regular camera [70][46].
Thermal infrared imaging has been successfully used to detect pregnancy, measure heat stress, monitor foot lesions in cattle, and detect diseases like bovine respiratory disease complex and foot and mouth disease [71][48]. Interestingly, it is likely that thermal imaging may even be used to measure emotion. For example, changes in nasal temperature in cows have been associated with positive emotional states [48][24], eye temperature has been used to evaluate stress in meat goats [72][49], in combination with behavioral data temperature of the inner corner of the eyes that seems to be related to stress and negative emotions in sheep [73][50].
3.4. Electrocardiography
Electrocardiography (ECG) is a system that measures the electrical potential difference between two electrodes that are placed at the opposing ends of the cardiac flow, effectively measuring the electrical activity of the circulatory system. A third neutral electrode is set to remove the noise or the readings from other animal systems to give accurate results [74][51]. The recurring cardiac flow pattern is measured to monitor the functioning of the heart. Emotional reactivity, such as avoidance of other cows, can be reliably measured from the baseline values of the changing heart rate [75] [52]. ECG systems greatly simplify the task of monitoring livestock and detecting problems with the heart and respiratory system. Based on the results of this monitoring, preventive measures or actions can be taken to handle the problem if needed. One major disadvantage of ECG monitoring is that it is generally not possible to continuously monitor animals with ECG. Often, the system is only employed when there is already probable cause to suspect a health issue. Currently, research is underway to design and develop wearable non-invasive ECG sensor systems for humans and these sensors will only need a few iterations before being able to adopt for farm animal applications.
3.5. Heart Rate Variability
Heart rate variability (HRV) is typically defined as variation in the beat-to-beat fluctuations of the cardiac cycle length under normal sinus rhythm [76][53]. Unlike ECG, there are portable systems available for storing heart rate variability data [70][46]. Two electrode rods are placed for optimal readings with a specific transmitter for horses and cattle. Different sized electrodes are available for smaller animals like calves and sheep [67][43]. There are also different systems for recording HRV in farm animals that require restriction in the movement to avoid motion artifacts in collecting data. These systems have been tested on poultry, pigs, and goats [77][54]. Differences between inter-beat intervals of heart rate along with vocalization sensing data have been shown to objectively assess emotional valence in pigs [78][55].
Heart rate variability has been extensively used in studies to research sympathovagal balance as it relates to stress, emotional states, and temperament of farm animals. For instance, postpartum fever in dairy cows is directly proportional to increased heart rate [79][56]; pigs’ stress response to heat episodes has been shown to be evaluated by heart rate variability [80][57]. Besides sympathovagal balance, the inter-beat interval (IBI) has been used in diagnosis of certain cardiac conditions as well as monitoring stress and anxiety in farm animals. The IBIs are coded to avoid data corruption from other readings in the area [80][57].
3.6. Electroencephalography
Electroencephalography (EEG) is a critical technique for pain research and nociception [81][58]. Much like ECG, EEG uses electrodes to monitor electrical activity within the body, but EEG targets the brain instead of the heart. Animals must be anesthetized before being subjected to EEG, but once the electrodes are in place EEG can provide an accurate reading of the brain activity irrespective of the movement of the subject. Currently, EEG has been particularly useful in measuring stress in animals [82][59] as well as responses to noxious stimulation [83][60]. Emotions in humans and non-human animals can be recognized through correlation from brain activity with the help of EEG signals [84,85][61][62] since EEG is also useful for emotion measurement, considering that it can be used on animals right up to the point of slaughter.
One weakness of EEG is the dissociation between mental states and EEG readings. This is to say that not every emotional state produces a distinct reading, so analysis requires objective knowledge of principles of EEG and its correlation to physiological functions and emotions of animals [86][63]. Figures of merit and additional validation and benchmarking need to be established through research to overcome the adoption of EEG as a wearable sensor for measuring the activity of farm animal brains.
3.7. Electromyogram
An electromyogram (EMG) measures the electrical activity of the muscles. It detects the electrical impulses produced by skeletal muscles. EMG has proven a useful technique to study muscle activity during pregnancy in sheep and humans [87][64]. It has also seen use in invasive and noninvasive evaluation of equine performance and muscle condition [88][65]. These data are especially important for labor animals like horses, because understanding the muscle activity of horses facilitates training.
This technique is used sparingly, as it records only superficial muscle activity and is subject to interference from many ambient factors such as temperature. Additional barriers to the extensive use of EMG are the difficulties in establishing solid benchmarks against which to compare experimental subjects [89][66]. In general, EMG is a useful research and diagnostic tool, but not yet applicable for day-to-day monitoring of muscle activity of animals on the farm and thereby it could be used in animal emotion research. The potential link of muscle activity such as tensions in muscles when the animals are in a fearful state has yet to be explored through sensors technology.
3.8. Respiratory Rate Analysis
Respiration pattern such as the velocity and depth indicate changes in emotions [90][67]. Respiratory rate (RR) analysis is a veritable tool in the arsenal of farmers; however, it is a time-consuming process that consists of monitoring flank movements to measure RR. Due to the sheer number of animals usually present on a farm, this is not a practical method for day-to-day monitoring. However, respiratory rate is a reliable measurement for medical diagnosis and research [57][33], for instance an increase in RR is indicative of high stress and potential illness in animals. Moreover, RR is also useful for animals with characteristic respiratory patterns, like dogs [91][68], and could be employed in pigs and dairy cattle as well.
An ideal RR sensor should be differential, pressure-based, transmit continuously, and sustainable. As it works today, RR is not constant and is influenced by factors like heat, high milk yield, and physical activity [92][69]. Using RR systems over the long term may prove to be useful in animal emotion research, but for this more research is needed. Thus, the abundance of interfering ambient factors and conditions make this, at present, an unreliable technique when used in isolation. That said, RR is an excellent complement to other sensor measurement systems.
3.9. Olfactory and Chemical Sensors
Olfactory and chemical sensors have tremendous potential in assessing animal emotion because animals use their sense of smell for a variety of essential processes: searching for food, sensing danger, and even determining when and with whom to mate [93][70]. Sense of smell is also well-connected with emotional and social responses in humans and animals [94][71]. Many farm animals have superior olfactory senses. Pigs, for example, are known to have an excellent sense of smell, but grazing animals like sheep or goats also have an exceptional sense of smell, which they use to avoid toxic plants and weeds [40,95][72][73]. Chemical and olfactory sensors monitor animals indirectly by detecting chemicals external to the animal. Chemical sensors may also be used to monitor chemicals present in bodily excretions like saliva. Olfactory sensors can also provide an early intervention for certain animal disease, like flystrike in sheep [96][74]. In this way, olfactory sensors could provide information on the overall state of the farm as opposed to consistently monitoring individual animals. Emotional states are also reflected in the odours of animals. For example: fearful pigs emits ‘alarming substances and volatile metabolites’ (allelochemics) that can be smelled by other animals [97][75]. Odour cues and olfactory awareness expressed by animals can be measured using sensing platforms to understand the correlation between the emotional states and the expression of various allelochemics. Adoption of sensors based analytical tools may be a game-changer in using odour as a biomarker for determining farm animal emotions through decoding the social volatilome.
3.10. Sound Analysis Sensing Platform
Sound analysis is a well-researched, sensor-based method for measurement of emotions [98][76]. Precision livestock farming with sound analysis is relatively easy to implement. Sound analysis sensing platform is comparatively more manageable to set up than other sensors, since the sensor itself consists of a simple audio recorder. The sensor is fixed to one location and records ambient sound. Therefore, this method can use a single sensor to monitor many animals [99][77].
The field of bioacoustics, or the extracting of valuable biological information from sounds makes this effort possible. Sound analysis has been successfully undertaken with pigs [100][78], poultry [66][42], and cattle [22]. The animals are first placed in situations that trigger certain vocal responses. Neuroscientists have shown the interconnectedness of neurons and the physiology and expression of emotions [101,102][79][80]. The neural and physiological responses expressed in the form of vocalizations in the farm animals are then measured. It is assumed that fearful or stressful situations may evoke negative emotions, which allows this benchmark measurement to be used to identify this emotion through comparison later. It is easiest to use a sound analysis system in animals without a large range of vocal sounds.
In pigs, stress such as throat, heat, and cold stress was found to be easily measured, as there is not much vocal modulation [100,103][78][81]. In addition, piglets seem to indicate through vocalizations when they are in pain or hungry [100][78]. Sensor-based vocalization data has to overcome the interference from ambience, and hence the filters for signal processing play a vital role in creating insights. Another example of where sound analysis is used is in the health management of broilers chickens. When suffering from respiratory diseases, the broilers tends to make an abnormal sound like coughing. Sound analysis was found to be efficient in identifying stress, diseases, and behavioral changes in these animals. Additionally, this is a technique that can also be implemented in a closed commercial building such as barns or pens, rather than an open space farm [104][82].
References
- Weaver, S.R.; Prichard, A.P.; Endres, E.L.; Newhouse, S.A.; Peters, T.L.; Crump, P.M.; Akins, M.S.; Crenshaw, T.D.; Bruck-maier, R.M.; Hernandez, L.L. Elevation of circulating serotonin improves calcium dynamics in the peripartum dairy cow. J. Endocrinol. 2016, 230, 105–123, doi:10.1530/JOE-16-0038.
- Webb, L.E.; Veenhoven, R.; Harfeld, J.L.; Jensen, M.B. What is animal happiness? Ann. N. Y. Acad. Sci. 2019, 1438, 62–76, doi:10.1111/nyas.13983.
- Jukan, A.; Masip-Bruin, X.; Amla, N. Smart computing and sensing technologies for animal welfare: A systematic review. Comput. Soc. 2020, 50, 1–27, doi:10.1145/3041960.
- Fogarty, E.S.; Swain, D.L.; Cronin, G.; Trotter, M. Autonomous on-animal sensors in sheep research: A systematic review. Comput. Electron. Agric. 2018, 150, 245–256, doi:10.1016/j.compag.2018.04.017.
- Kremer, L.; Holkenborg, S.E.J.K.; Reimert, I.; Bolhuis, J.E.; Webb, L.E. The nuts and bolts of animal emotion. Neurosci. Biobe-hav. Rev. 2020, 113, 273–286, doi:10.1016/j.neubiorev.2020.01.028.
- Bekoff, M. Animal emotions: Exploring passionate natures. Bioscience 2020, 50, 861–870, doi:10.1641/0006-3568(2000)050[0861:AEEPN]2.0.CO;2.
- Mauss, I.B.; Robinson. M.D. Measures of emotion: A review. Cogn. Emot. 2009, 23, 209–237, doi:10.1080/02699930802204677.
- Adolphs, R. How should neuroscience study emotions? By distinguishing emotion states, concepts, and experiences. Soc. Cogn. Affect. Neurosci. 2017, 12, 24–31, doi:10.1093/scan/nsw153.
- Anderson, D.J.; Adolphs, R. A framework for studying emotions across species. Cell 2014, 157, 187–200, doi:10.1016/j.cell.2014.03.003.
- Adolphs, R.; Mlodinow, L.; Barrett, L.F. What is an emotion? Curr. Biol. 2019, 29, R1–R5, doi:10.1016/j.cub.2019.09.008.
- Storbeck, J.; Clore, G.L. On the interdependence of cognition and emotion. Cogn. Emot. 2007, 21 1212–1237, doi:10.1080/02699930701438020.
- Robinson, M.D.; Watkins, E.R.; Harmon-Jones, E. (Eds.) Cognition and emotion: An introduction. In Handbook of Cognition and Emotion; The Guilford Press: New York, NY, USA, 2013; pp. 3–16, ISBN 9781462509997.
- Steptoe, A.; Wardle, J.; Marmot, M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc. Natl. Acad. Sci. USA 2005, 102, 6508–6512, doi:10.1073/pnas.0409174102.
- Fraser, D. Understanding animal welfare. Acta Vet. Scand. 2008, 50, 1–7, doi:10.1186/1751-0147-50-S1-S1.
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Jangbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2017, 92, 375–397, doi:10.1016/j.physbeh.2007.02.003.
- Spinka, M. Social dimension of emotions and its implication for animal welfare. Appl. Anim. Behav. Sci. 2012, 138, 170–181, doi:10.1016/j.applanim.2012.02.005.
- Burghardt, T.; Campbell, N. December. Individual animal identification using visual biometrics on deformable coat patterns. ICVS 2007, doi:10.2390/biecoll-icvs2007-121.
- Nawroth, C.; Langbein, J.; Coulon, M.; Gabor, V.; Oesterwind, S.; Benz-Schwarzburg, J.; von Borell, E. Farm animal cogni-tion—linking behavior, welfare and ethics. Front. Vet. Sci. 2019, 6, 24, doi:10.3389/fvets.2019.00024.
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens Bio Sens. Res. 2020, 29, 1–8, doi:10.1016/j.sbsr.2020.100367.
- Olsson, I.A.; Nicol, C.J.; Niemi, S.M.; Sandøe, P. From Unpleasant to Unbearable—Why and How to Implement an Upper Limit to Pain and Other Forms of Suffering in Research with Animals. Inst. Lab. Anim. Res. J. 2020, doi:10.1093/ilar/ilz018.
- Lischinsky, J.E.; Lin, D. Neural mechanisms of aggression across species. Nat. Neurosci. 2020, 23, 1317–1328, doi:10.1038/s41593-020-00715-2.
- Canozzi, M.E.A.; Mederos, A.; Manteca, X.; Turner, S.; McManus, C.; Zago, D.; Barcellos, J.O.J. A meta-analysis of cortisol concentration, vocalization, and average daily gain associated with castration in beef cattle. Res. Vet. Sci. 2017, 114, 430–443, doi:10.1016/j.rvsc.2017.07.014.
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–38, doi:10.1093/af/vfy027.
- Grosz, H.J.; Farmer, B.B. Blood lactate in the development of anxiety symptoms: A critical examination of Pitts and McClure’s hypothesis and experimental study. Arch. Gen. Psychiatry 1969, 21, 611–619, doi:10.1001/archpsyc.1969.01740230099014.
- Lindholm, C.; Altimiras, J. Point-of-care devices for physiological measurements in field conditions. A smorgasbord of in-struments and validation procedures. Comp. Biochem. Phys. A 2016, 202, 99–111, doi:10.1016/j.cbpa.2016.04.009.
- Tarantola, M.; Biasato, I.; Biasibetti, E.; Biagini, D.; Capra, P.; Guarda, F.; Leporati, M.; Malfatto, V.; Cavallarin, L.; Minis-calco, B.; et al. Beef cattle welfare assessment: Use of resource and animal-based indicators, blood parameters and hair 20β-dihydrocortisol. Ital. J. Anim. Sci. 2020, 19, 341–350, doi:10.1080/1828051X.2020.1743783.
- Otten, W.; Heimbürge, S.; Kanitz, E.; Tuchscherer, A. It’s getting hairy–External contamination may affect the validity of hair cortisol as an indicator of stress in pigs and cattle. Gen. Comp. Endocrinol. 2002, 113531, doi:10.1016/j.ygcen.2020.113531.
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171, doi:10.1186/s12917-016-0791-8.
- Lürzel, S.; Bückendorf, L.; Waiblinger, S.; Rault, J.L. Salivary oxytocin in pigs, cattle, and goats during positive human-animal interactions. Psychoneuroendocrinology 2020, 115, 104636, doi:10.1016/j.psyneuen.2020.104636.
- López-Arjona, M.; Mateo, S.V.; Manteca, X.; Escribano, D.; Cerón, J.J.; Martínez-Subiela, S. Oxytocin in saliva of pigs: An assay for its measurement and changes after farrowing. Domest. Anim. Endocrinol. 2020, 70, 106384, doi:10.1016/j.domaniend.2019.106384.
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deur-waerdère, P. Serotonin in animal cognition and behavior. Int. J. Mol. Sci. 2020, 21, 1649, doi:10.3390/ijms21051649.
- Loudon, K.M.; Tarr, G.; Pethick, D.W.; Lean, I.J.; Polkinghorne, R.; Mason, M.; Dunshea, F.R.; Gardner, G.E.; McGilchrist, P. The use of biochemical measurements to identify pre-slaughter stress in pasture finished beef cattle. Animals 2019, 9, 503, doi:10.3390/ani9080503.
- Dzedzickis, A.; Kaklauskas, A.; Bucinskas, V. Human emotion recognition: Review of sensors and methods. Sensors 2020, 20, 592, doi:10.3390/s20030592.
- Briefer, E.F. Vocal contagion of emotions in non-human animals. Proc. R. Soc. B 2018, 285, 20172783, doi:10.1098/rspb.2017.2783.
- Adriaense, J.E.C.; Koski, S.E.; Huber, L.; Lamm, C. Challenges in the comparative study of empathy and related phenomena in animals. Neurosci. Biobehav. Rev. 2020, 112, 62–82, doi:10.1016/j.neubiorev.2020.01.021.
- Panksepp, J. Affective Neuroscience: The Foundations of Human and Animal Emotions; Oxford University Press: New York, NY, USA, 2004; ISBN 9780198025672.
- Tomkins, S.S.; McCarter, R. What and where are the primary affects? Some evidence for a theory. Percept. Mot. Skills 1964, 18, 119–158, doi:10.2466/pms.1964.18.1.119.
- Di Giminiani, P.; Brierley, V.L.; Scollo, A.; Gottardo, F.; Malcolm, E.M.; Edwards, S.A.; Leach, M.C. The assessment of facial expressions in piglets undergoing tail docking and castration: Toward the development of the piglet grimace scale. Front. Vet. Sci. 2016, 3, 1–10, doi:10.3389/fvets.2016.00100.
- Lansade, L.; Nowak, R.; Lainé, A.L.; Leterrier, C.; Bonneau, C.; Parias, C.; Bertin, A. Facial expression and oxytocin as possi-ble markers of positive emotions in horses. Sci. Rep. 2018, 8, 1–11, doi:10.1038/s41598-018-32993-z.
- Stomp, M.; Leroux, M.; Cellier, M.; Henry, S.; Lemasson, A.; Hausberger, M. An unexpected acoustic indicator of positive emotions in horses. PLoS ONE 2018, 13, e0197898, doi:10.1371/journal.pone.0197898.
- Friel, M.; Kunc, H.P.; Griffin, K.; Asher, L.; Collins, L.M. Positive and negative contexts predict duration of pig vocalizations. Sci. Rep. 2019, 9, 1–7, doi:10.1038/s41598-019-38514-w.
- Du, X.; Lao, F.; Teng, G. A sound source localization analytical method for monitoring the abnormal night vocalizations of poultry. Sensors 2018, 18, 2906, doi:10.3390/s18092906.
- Neethirajan, S. Recent advances in wearable sensors for animal health management. Sens. Bio Sens. Res. 2017, 12, 15–29, doi:10.1016/j.sbsr.2016.11.004.
- Massawe, E.A.; Michael, K.; Kaijage, S.; Seshaiyer, P. Design and Analysis of smart sensing system for animal emotions recognition. Int. J. Comput. Appl. 2017, 169, 975–8887, doi:10.5120/IJCA2017914797.
- Bailey, D.W.; Trotter, M.G.; Knight, C.W.; Thomas, M.G. Use of GPS tracking collars and accelerometers for rangeland live-stock production research. Trans. Anim. Sci. 2018, 2, 81–88, doi:10.1093/tas/txx006.
- Neethirajan, S. Transforming the adaptation physiology of farm animals through Sensors. Animals 2020, 10, 1512, doi:10.3390/ani10091512.
- Paoli, M.A.; Lahrmann, H.P.; Jensen, T.; D’Eath, R.B. Behavioural differences between weaner pigs with intact and docked tails. Anim. Welf. 2016, 25, 287–296.
- Jeelani, R.; Jeelani, R. Thermal imagery for monitoring livestock. Int. J. Life Sci. Appl. Sci. 2019, 1, 58–69.
- Bartolomé, E.; Azcona, F.; Cañete-Aranda, M.; Perdomo-González, D.I.; Ribes-Pons, J.; Terán, E.M. Testing eye temperature assessed with infrared thermography to evaluate stress in meat goats raised in a semi-intensive farming system: A pilot study. Arch. Anim. Breed. 2019, 62, 199–204, doi:10.5194/aab-62-199-2019.
- Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Dalla Costa, E. Thermography as a non-invasive measure of stress and fear of humans in sheep. Animals 2018, 8, 146, doi:10.3390/ani8090146.
- Balters, S.; Steinert, M. Capturing emotion reactivity through physiology measurement as a foundation for affective engi-neering in engineering design science and engineering practices. J. Intell. Manuf. 2012, 28, 1585–1607, doi:10.1007/s10845-015-1145-2.
- Frondelius, L.; Järvenranta, K.; Koponen, T.; Mononen, J. The effects of body posture and temperament on heart rate varia-bility in dairy cows. Physiol. Behav. 2015, 139, 437–441, doi:10.1016/j.physbeh.2014.12.002.
- Hayano, J. Introduction to heart rate variability. In Clinical Assessment of the Autonomic Nervous System; Iwase, S., Hayano, J., Orimo, S., Eds.; Springer: Tokyo, Japan, 2017; pp 109–127, ISBN 978-4-431-56010-4.
- Düpjan, S.; Krause, A.; Moscovice, L.R.; Nawroth, C. Emotional contagion and its implications for animal welfare. CAB Rev. 2020, 15, 1–6, doi:10.1079/PAVSNNR202015046.
- Goursot, C.; Düpjan, S.; Tuchscherer, A.; Puppe, B.; Leliveld, L.M. Visual laterality in pigs: Monocular viewing influences emotional reactions in pigs. Animal Behav. 2019, 154, 183–192.
- Aoki, T.; Itoh, M.; Chiba, A.; Kuwahara, M.; Nogami, H.; Ishizaki, H.; Yayou, K.I. Heart rate variability in dairy cows with postpartum fever during night phase. PLoS ONE 2020, 15, e0242856, doi:10.1371/journal.pone.0242856.
- Byrd, C.J.; Johnson, J.S.; Radcliffe, J.S.; Craig, B.A.; Eicher, S.D.; Lay, D.C. Nonlinear analysis of heart rate variability for evaluating the growing pig stress response to an acute heat episode. Animal 2020, 14, 379–387, doi:10.1017/S1751731119001630.
- Lenoir, D.; Willaert, W.; Coppieters, I.; Malfliet, A.; Ickmans, K.; Nijs, J.; Vonck, K.; Meeus, M.; Cagnie, B. Electroenceph-alography during Nociceptive stimulation in chronic pain patients: A systematic review. Pain Med. 2020, doi:10.1093/pm/pnaa131.
- de Camp, N.V.; Ladwig-Wiegard, M.; Geitner, C.I.; Bergeler, J.; Thöne-Reineke, C. EEG based assessment of stress in horses: A pilot study. Peer J. 2020, 8, e8629, doi:10.7717/peerj.8629.
- Kells, N.J.; Beausoleil, N.J.; Sutherland, M.A.; Johnson, C.B. Post-natal development of EEG responses to noxious stimulation in pigs (Sus scrofa) aged 1–15 days. Anim. Welf. 2019, 28, 317–329, doi:10.7120/09627286.28.3.317.
- Egger, M.; Ley, M.; Hanke, S. Emotion recognition from physiological signal analysis: A review. Electron. Notes Theor. Comput. Sci. 2019, 343, 35–55, doi:10.1016/j.entcs.2019.04.009.
- Leshin, J.C.; Lindquist, K.A. Neuroimaging of Emotion. In Oxford Handbook of Emotion Dysregulation; Oxford Handbooks: Oxford, UK, 2020.
- Klemm, W.R. Are there EEG correlates of mental states in animals? Neuropsychobiology 1992, 26, 151–165, doi:10.1159/000118911.
- Leskošek, B.; Pajntar, M. Electromyography of the pregnant uterus in humans and sheep. In Proceedings of the 2nd European Medical & Biological Engineering Conference, Portorož, Slovenia, 29 November–3 December 2020; Volume 4, pp. 544–545.
- Williams, J.M. Electromyography in the horse: A useful technology. J. Equine Vet. Sci. 2018, 60, 43–58, doi:10.1016/j.jevs.2017.02.005.
- Valentin, S.; Zsoldos, R.R. Surface electromyography in animal biomechanics: A systematic review. J. Electromyogr. Kines. 2016, 28, 167–183, doi:10.1016/j.jelekin.2015.12.005.
- Zhang, Q.; Chen, X.; Zhan, Q.; Yang, T.; Xia, S. Respiration-based emotion recognition with deep learning. Comput. Ind. 2017, 92, 84–90, doi:10.1016/j.compind.2017.04.005.
- Lopedote, M.; Valentini, S.; Musella, V.; Vilar, J.M.; Spinella, G. Pulse rate, respiratory rate and rectal temperature in working dogs before and after three different field trials. Animals 2020, 10, 733, doi:10.3390/ani10040733.
- Pinto, S.; Hoffmann, G.; Ammon, C.; Heuwieser, W.; Levit, H.; Halachmi, I.; Amon, T. Effect of two cooling frequencies on respiration rate in lactating dairy cows under hot and humid climate conditions. Ann. Anim. Sci. 2019, 19, 821–834, doi:10.2478/aoas-2019-0026.
- Clouard, C.M.; Bolhuis, J.E. Olfactory behaviour in farm animals. In Olfaction in Animal Behaviour and Welfare; Nielsen, B.L., Ed.; CABI Publishing: Jouy en Josas, France, 2017; pp. 161–175, ISBN 9781786391599.
- Sullivan, R.M.; Wilson, D.A.; Ravel, N.; Mouly, A.M. Olfactory memory networks: From emotional learning to social behav-iors. Front. Behav. Neurosci. 2015, 9, 36, doi:10.3389/fnbeh.2015.00036.
- Marino, L.; Merskin, D. Intelligence, complexity, and individuality in sheep. Anim. Sentien. 2019, 4, 1–26.
- Brunjes, P.C.; Feldman, S.; Osterberg, S.K. The pig olfactory brain: A primer. Chem. Senses 2016, 41, 415–425, doi:10.1093/chemse/bjw016.
- Cramp, A.P.; Sohn, J.H.; James, P.J. Detection of cutaneous myiasis in sheep using an ‘electronic nose’. Vet. Parasitol. 2009, 166, 293–298, doi:10.1016/j.vetpar.2009.08.025.
- Bombail, V. The Role of Olfaction in Relation to Stress and Fear. In Olfaction in Animal Behaviour and Welfare; CABI Publishing: Jouy en Josas, France, 2017.
- Adelman, J.S.; Estes, Z.; Cossu, M. Emotional sound symbolism: Languages rapidly signal valence via phonemes. Cognition 2018, 175, 122–130, doi:10.1016/j.cognition.2018.02.007.
- Bishop, J.C.; Falzon, G.; Trotter, M.; Kwan, P.; Meek, P.D. Livestock vocalisation classification in farm soundscapes. Comp. Electron. Agric. 2019, 162, 531–542.
- da Silva, J.P.; de Alencar Nääs, I.; Abe, J.M.; da Silva Cordeiro, A.F. Classification of piglet (Sus Scrofa) stress conditions using vocalization pattern and applying paraconsistent logic Eτ. Comput. Electron. Agric. 2019, 166, 105020, doi:10.1016/j.compag.2019.105020.
- Chen, X.; Pan, Z.; Wang, P.; Yang, X.; Liu, P.; You, X.; Yuan, J. The integration of facial and vocal cues during emotional change perception: EEG markers. Soc. Cogn. Affect Neurosci. 2016, 11, 1152–1161, doi:10.1093/scan/nsv083.
- Johnstone, T.; Van Reekum, C.M.; Oakes, T.R.; Davidson, R.J. The voice of emotion: An FMRI study of neural responses to angry and happy vocal expressions. Soc. Cogn. Affect Neurosci. 2006, 1, 242–249, doi:10.1093/scan/nsl027.
- Mota-Rojas, D.; Orihuela, A.; Martínez-Burnes, J.; Gómez, J.; Mora-Medina, P.; Alavez, B.; Ramírez, L.; González-Lozano, M. Neurological modulation of facial expressions in pigs and implications for production. J. Anim. Behav. Biometeorol. 2020, 8, 232–243, doi:10.31893/jab.20031.
- Ginovart-Panisello, G.J.; Alsina-Pagès, R.M.; Sanz, I.I.; Monjo, T.P.; Prat, M.C. Acoustic description of the soundscape of a real-life intensive farm and its impact on animal welfare: A preliminary analysis of farm sounds and bird vocalisations. Sen-sors 2020, 20, 4732, doi:10.3390/s20174732.
- Kells, N.J.; Beausoleil, N.J.; Sutherland, M.A.; Johnson, C.B. Post-natal development of EEG responses to noxious stimulation in pigs (Sus scrofa) aged 1–15 days. Anim. Welf. 2019, 28, 317–329, doi:10.7120/09627286.28.3.317.
- Egger, M.; Ley, M.; Hanke, S. Emotion recognition from physiological signal analysis: A review. Electron. Notes Theor. Comput. Sci. 2019, 343, 35–55, doi:10.1016/j.entcs.2019.04.009.
- Leshin, J.C.; Lindquist, K.A. Neuroimaging of Emotion. In Oxford Handbook of Emotion Dysregulation; Oxford Handbooks: Oxford, UK, 2020.
- Klemm, W.R. Are there EEG correlates of mental states in animals? Neuropsychobiology 1992, 26, 151–165, doi:10.1159/000118911.
- Leskošek, B.; Pajntar, M. Electromyography of the pregnant uterus in humans and sheep. In Proceedings of the 2nd European Medical & Biological Engineering Conference, Portorož, Slovenia, 29 November–3 December 2020; Volume 4, pp. 544–545.
- Williams, J.M. Electromyography in the horse: A useful technology. J. Equine Vet. Sci. 2018, 60, 43–58, doi:10.1016/j.jevs.2017.02.005.
- Valentin, S.; Zsoldos, R.R. Surface electromyography in animal biomechanics: A systematic review. J. Electromyogr. Kines. 2016, 28, 167–183, doi:10.1016/j.jelekin.2015.12.005.
- Zhang, Q.; Chen, X.; Zhan, Q.; Yang, T.; Xia, S. Respiration-based emotion recognition with deep learning. Comput. Ind. 2017, 92, 84–90, doi:10.1016/j.compind.2017.04.005.
- Lopedote, M.; Valentini, S.; Musella, V.; Vilar, J.M.; Spinella, G. Pulse rate, respiratory rate and rectal temperature in working dogs before and after three different field trials. Animals 2020, 10, 733, doi:10.3390/ani10040733.
- Pinto, S.; Hoffmann, G.; Ammon, C.; Heuwieser, W.; Levit, H.; Halachmi, I.; Amon, T. Effect of two cooling frequencies on respiration rate in lactating dairy cows under hot and humid climate conditions. Ann. Anim. Sci. 2019, 19, 821–834, doi:10.2478/aoas-2019-0026.
- Clouard, C.M.; Bolhuis, J.E. Olfactory behaviour in farm animals. In Olfaction in Animal Behaviour and Welfare; Nielsen, B.L., Ed.; CABI Publishing: Jouy en Josas, France, 2017; pp. 161–175, ISBN 9781786391599.
- Sullivan, R.M.; Wilson, D.A.; Ravel, N.; Mouly, A.M. Olfactory memory networks: From emotional learning to social behav-iors. Front. Behav. Neurosci. 2015, 9, 36, doi:10.3389/fnbeh.2015.00036.
- Brunjes, P.C.; Feldman, S.; Osterberg, S.K. The pig olfactory brain: A primer. Chem. Senses 2016, 41, 415–425, doi:10.1093/chemse/bjw016.
- Cramp, A.P.; Sohn, J.H.; James, P.J. Detection of cutaneous myiasis in sheep using an ‘electronic nose’. Vet. Parasitol. 2009, 166, 293–298, doi:10.1016/j.vetpar.2009.08.025.
- Bombail, V. The Role of Olfaction in Relation to Stress and Fear. In Olfaction in Animal Behaviour and Welfare; CABI Publishing: Jouy en Josas, France, 2017.
- Adelman, J.S.; Estes, Z.; Cossu, M. Emotional sound symbolism: Languages rapidly signal valence via phonemes. Cognition 2018, 175, 122–130, doi:10.1016/j.cognition.2018.02.007.
- Bishop, J.C.; Falzon, G.; Trotter, M.; Kwan, P.; Meek, P.D. Livestock vocalisation classification in farm soundscapes. Comp. Electron. Agric. 2019, 162, 531–542.
- da Silva, J.P.; de Alencar Nääs, I.; Abe, J.M.; da Silva Cordeiro, A.F. Classification of piglet (Sus Scrofa) stress conditions using vocalization pattern and applying paraconsistent logic Eτ. Comput. Electron. Agric. 2019, 166, 105020, doi:10.1016/j.compag.2019.105020.
- Chen, X.; Pan, Z.; Wang, P.; Yang, X.; Liu, P.; You, X.; Yuan, J. The integration of facial and vocal cues during emotional change perception: EEG markers. Soc. Cogn. Affect Neurosci. 2016, 11, 1152–1161, doi:10.1093/scan/nsv083.
- Johnstone, T.; Van Reekum, C.M.; Oakes, T.R.; Davidson, R.J. The voice of emotion: An FMRI study of neural responses to angry and happy vocal expressions. Soc. Cogn. Affect Neurosci. 2006, 1, 242–249, doi:10.1093/scan/nsl027.
- Mota-Rojas, D.; Orihuela, A.; Martínez-Burnes, J.; Gómez, J.; Mora-Medina, P.; Alavez, B.; Ramírez, L.; González-Lozano, M. Neurological modulation of facial expressions in pigs and implications for production. J. Anim. Behav. Biometeorol. 2020, 8, 232–243, doi:10.31893/jab.20031.
- Ginovart-Panisello, G.J.; Alsina-Pagès, R.M.; Sanz, I.I.; Monjo, T.P.; Prat, M.C. Acoustic description of the soundscape of a real-life intensive farm and its impact on animal welfare: A preliminary analysis of farm sounds and bird vocalisations. Sen-sors 2020, 20, 4732, doi:10.3390/s20174732.
