The fluid catalytic cracking (FCC) process is an alternative olefin production technology, with lower CO2 emission and higher energy-saving.
- Light olefin production
- FCC process
- Catalyst Types
- Operating conditions
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Olefins, also known as alkenes, are critical components in the chemical industry. Light olefins are used to produce many different derivatives used in our daily life, such as packaging, solvents, synthetic fibers, construction, and coatings. Olefins are aliphatic hydrocarbons with one or more C=C double bonds, with the general molecular formula of CnH2n. Alkenes are called unsaturated because the number of hydrogen atoms in alkenes is less than the maximum possible number per carbon atoms [1-4][1][2][3][4]. Ethene (or ethylene) with the molecular formula of C2H4 is the simplest alkene molecule and is followed by propylene (C3H6), butene (C4H8), pentene (C5H10), and other homologues.
Olefin production depends on the crude oil fractions or the products of natural gas processing. It is reported that annually 400 million tons of olefins are produced through different routes such as steam cracking (SC), fluid catalytic cracking (FCC), and dehydrogenation using one billion tonnes of hydrocarbon as feedstock. A wide range of products, such as gasoline, kerosene, jet fuel, and diesel, can be produced by cracking the large molecules. Light gases and olefins, liquefied petroleum gas (LPG), and butanes can also be generated via cracking. Heat is required for breaking of the C–C bonds in the cracking process. Thermal cracking processes comprise steam cracking, coking, and visbreaking, and the catalytic processes comprise hydrocracking and fluid catalytic cracking. In the hydrocracking process, due to the heat generation during the hydrogenation of cracked fragments, the net basis hydrocracking is exothermic [1].
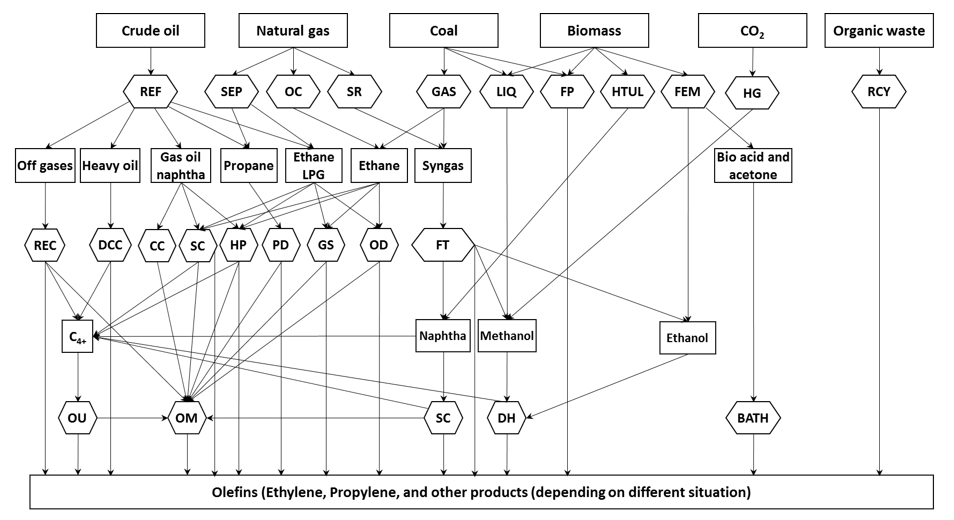
Almost 40% of the global feedstocks are used in SC processes, and about 60% in FCC units, where 59% of olefins are produced by FCC process, and about 39% by steam cracking of ethane, LPG, and liquid feeds [5]. Different technologies have been used to produce light olefins using different feedstocks such as methane and light alkanes, and naphtha (Figure 1). Steam cracking is the well-known conventional leading technology for olefin production, where the hydrocarbons that mainly originated from fossil resources (such as ethane, naphtha, etc.) are cracked in tubular reactors suspended in a gas-fired furnace [4,6-8][4][6][7][8]. However, the SC process is one of the most energy-intensive processes in the petrochemical industry, but it is clear that this process, with low CO2 emission, is still the best–performing technology. It seems to be very difficult to replace this well-established technology without significant breakthroughs in process intensification. Due to the high energy consumption, emission of pollutants during olefin production, and more burdensome environmental regulations, many efforts are being made to develop technologies with low CO2 emissions.
Depending on the feedstock's composition in steam crackers, about 1–1.6 tonne of CO2 is produced per tonne of ethylene, and in general, more than 300 million tonnes of CO2 per annum is emitted through the steam cracking process [9] [9]. The CO2 emission could be caused by the chemical CO2 produced in the reaction, as well as the energy requirement of the process (i.e., fuel combustion) [9]. It has been reported that steam cracking plants are responsible for around 30% of all pollution from chemical plants, which is mainly due to the NOx emissions and unburned hydrocarbons in the flame needed for heating of the cracking furnace [10]. Concerning the expected increase in the production rates by increasing the global population and raising the living standards, development and improvements in the olefin production technologies could significantly affect the common challenge for reducing the adverse effect of climate change [9].
Several studies have been done for a considerable increase in ethylene production, and use of biomass and waste stream for olefin production has become more promising and attracted more attention. The processes of catalytic dehydrogenation of light alkanes are another promising technology with high selectivity for olefin production. These processes have low capital investment, and they are independent of the oil price; production of hydrogen as a value-added byproduct is also another advantage of these processes. Due to the endothermic characteristics of these reactions, high temperatures are required to obtain appropriate productivity, and these processes are known as energy-consuming processes. Another problem of the high-temperature reaction is the acceleration of side reactions and reducing the selectivity. Therefore, the catalyst deactivation and energy efficiency need to be considered and improved in this process [11].
Contrasted with direct dehydrogenation oxidative dehydrogenation process is an exothermic reaction. In this process, due to the presence of oxygen, side-reactions result in the formation of oxygen-containing byproducts, decreasing the selectivity. The separation of light olefins from the product mixture increases the process cost; simultaneously, this process generates a large amount of waste gases that reduce its ecological index [11,12][11][12].
Abundantly available methane can also be used as feedstock for olefin production. Several methods for this process, including the oxidative coupling of methane (OCM) [13-15][13][14][15], Fischer-Tropsch synthesis (FTS) [16-18][16][17][18], and methanol to olefins (MTO) [19-21][19][20][21], are potential alternatives for SC process. However, MTO and FTS technologies are well-established processes that already have some operational plants globally, but due to the required step of syngas production in both methods, these methods are not very efficient. In addition, in FTS, the light olefins are not the only products, and a considerable amount of fuel-range hydrocarbons are also produced during the reaction. However, increasing the selectivity of target products is possible by improvements of the catalyst design. The OCM process, with a highly exothermic feature, needs more improvement for catalyst design and development of a reactor suitable for this highly exothermic reaction [6]; this process, with the lowest emission of CO2 energy (CO2 emission resulting from the energy requirement of the process i.e. fuel combustion), suffers from the relatively low ethylene selectivity and high chemical CO2 emission which is produced in the reaction. The direct catalytic conversion of CO2 to value-added chemicals such as light olefins has recently attracted high attention due to its potentially serious effects on climate and environment; therefore, the development of stable and efficient catalysts for light olefins production with excellent olefin selectivity is highly desirable [22-27][22][23][24][25][26][27].
Figure 1. Different technologies for olefins production. BATH: Bio-acid acetone to hydrocarbons; CC: Catalytic Cracking or Catalytic Pyrolysis; DCC: Deep Catalytic Cracking; DH: De-hydration process (e.g., methanol to olefins, methanol to propylene, and ethanol dehydration); FM: Fermentation; FP: Flash Pyrolysis, sometimes in the presence of methane; FT: Fischer-Tropsch synthesis; GAS: Gasification; GS: Gas stream reactor technologies, e.g., shockwave reactors; HG: Hydrogenation; HP: Hydro-Pyrolysis; HTUL: Hydro-Thermal Upgrading Liquefaction which produces naphtha from biomass feedstock; LIQ: liquefaction; OC: Oxidative Coupling of methane via ethane; OD: Oxidative Dehydrogenation of ethane; OM: Olefin Metathesis; OU: Olefins Upgrading (conversion of C4-C10) to light olefins; PD: Propane Dehydrogenation; RCY: Re-cycling pyrolysis using organic waste, such as discarded plastics, used rubber; REC: Recovery of refinery off-gases, which contains ethylene, propylene, propane, etc.; REF: Refinery processes. Distillation of crude oil produces naphtha and heavy oil. Catalytic cracking produces off-gases. Cryogenic separation and absorption produce ethane and LPG; SC: Steam cracking (conventional); SEP: Gas separation process produces methane, ethane, and propane; SR: Steam Reforming of natural gas to produce methanol. Reproduced from [28].
2. Fluid catalytic cracking
Catalytic cracking is an olefin production technology, with lower CO2 emission and higher energy saving. The FCC units were initially developed to convert low-value feedstock into gasoline, but by increasing the demand for some of its byproducts, such as propylene, some modifications have been made to the process and the unit to achieve this aim. Therefore, FCC unit, process, and catalysts were upgraded and redesigned to produce propylene as a co-product instead of the byproduct [29]. The fluid catalytic cracking leads to a much lower operating temperature than conventional steam cracking [8]. Additionally, the control and propylene/ethylene ratios in the olefins produced via steam cracking are limited, while the propylene demand is growing quicker than ethylene. Employing a catalyst in a cracking reaction could also provide the possibility of tuning the product selectivity, such as increasing the propylene selectivity instead of ethylene, and it helps to increase the propylene/ethylene ratios [8,30][8][30]. The light olefins production research through the catalytic cracking of hydrocarbons such as naphtha started in the late 1960s [31]. The FCC process used zeolite catalysts implemented by US refineries in 1977 and helped to save about 30 million tonnes of crude oil in the US alone [28]. The FCC process was initially designed to produce gasoline via upgrading low-value feedstocks, such as vacuum gas oil (VGO), and atmospheric residue (AR). The lighter feedstocks such as naphtha need a relatively higher cracking temperature than heavier feedstocks [8]. Owing to the flexibility of the FCC process, the operating conditions, and type of catalysts can be changed according to the type and quality of the feedstock [29]. A schematic of an FCC reactor is shown in Figure 2 [32].
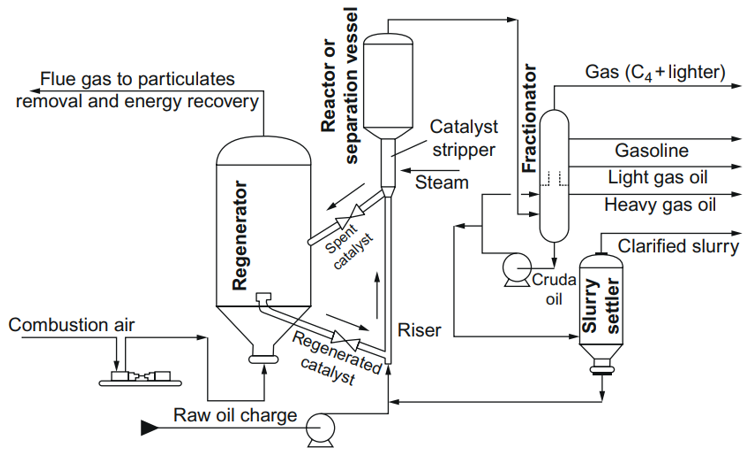
Figure 2. Detailed schematic of a fluid catalytic cracking reactor [32].
In the FCC process, the hot catalyst material is aerated with air or pre-heated feedstock at the bottom of the riser reactor, and the catalyst is moving through pipes. Therefore, a vaporized feedstock and fluidized catalyst simultaneously flow into a reactor chamber where the catalyst forms beds in the reaction chamber, and the cracking reactions occur. The temperature at the bottom of the riser is about 550 °C, whereas the catalyst to oil ratio (CTO) is larger than 1 (typically 5.5). The cracking reaction and formation of gases resulted in the expansion of the reactant mixture, and the mixture of the feedstock and the catalyst is quickly (at the speed of 40 m/s) transported up the riser reactor. The contact time in a riser is in the order of seconds. The temperature at the top of the reactor decreased to about 500 °C due to the endothermic nature of the catalytic cracking process. Cracked vapors go through the cyclone placed at the top of the reaction chamber and the spent catalysts are separated from the cracked vapors using the centrifugal force. Then, the cleaned cracked vapors fractionate into the products such as gasoline and cracked heavy and light gas oils. During the cracking process, the catalysts are contaminated with coke, and the coke deposited on the catalysts is removed by burning in the regenerator. The regenerated catalysts are added to the fresh catalyst to be used again in the FCC process. The temperature in the regenerator can reach 760 °C, and the heat of the catalysts at the entrance of the reaction chamber is high enough for the evaporation of the fresh feed before entering the reactor [32,33][32][33].
The high capacities of a cracking unit and its positive effect on the overall refinery economics have made it an essential object for innovation. In order to improve olefin production, researchers have made more effort to redesigning the FCC units [34]. Catalytic pyrolysis process (CPP) and deep catalytic cracking process (DCC) are technologies developed based on Riser FCC by Sinopec, Research Institute of Petroleum Processing [35]. The first commercial DCC unit has been demonstrated in 1990 and commercialized since 1994, and now, 10 commercial units in operation in China (eight units), Thailand (one unit), and Saudi Arabia (one unit) [36,37][36][37]. Later, the CPP process was designed and developed by modification of DCC process to facilitate production of ethylene and propylene [37] [37]. Depending on the type of feedstock, operating conditions, and nature of the catalyst, a low amount of olefins are produced (~1–2 wt.% ethylene and ~3–6 wt.% propylene) in the conventional FCC process, which can be enhanced by choosing a proper catalyst and optimizing the operating conditions. The Indian Oil Corporation’s Research and Development Center developed the Indmax fluid catalytic cracking (I-FCC) process for the production of light olefins from heavy feedstocks. This process is able to produce more than 20 wt.% propylene using a wide range of feedstocks including the residues [38,39][38][39]. Another process, high-severity down-flow FCC (HS-FCC), developed by an alliance of Saudi Aramco, King Fahd University of Petroleum and Minerals (KFUPM), and JX Nippon Oil & Energy (JX), can produce up to 25 wt.% propylene through the cracking of heavy hydrocarbons at a temperature of 550 °C to 650 °C [40,41][40][41].
References
- Hsu, C.S.; Robinson, P.R. Petroleum Science and Technology; Springer: Cham, Switzerland, 2019.
- Speight, J.G. Organic chemistry. In Environmental Organic Chemistry for Engineers; Elsevier BV: Cambridge, United States, 2017; pp. 43–86.
- Stauffer, E.; Dolan, J.A.; Newman, R. Chapter 3 - Review of Basic Organic Chemistry. In Fire Debris Analysis, Stauffer, E., Dolan, J.A., Newman, R., Eds. Academic Press: Burlington, United States, 2008; pp. 49–83.
- Fakhroleslam, M.; Sadrameli, S.M. Thermal cracking of hydrocarbons for the production of light olefins; A review on optimal process design, operation, and control. Eng. Chem. Res. 2020, 59, 12288–12303, doi:10.1021/acs.iecr.0c00923.
- Zacharopoulou, V.; Lemonidou, A.A. Olefins from biomass intermediates: A review. Catalysts 2017, 8, 2, doi:10.3390/catal8010002.
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New trends in olefin production. Engineering 2017, 3, 171–178, doi:10.1016/j.eng.2017.02.006.
- Sadeghbeigi, R. Fluid Catalytic Cracking Handbook: An Expert Guide to the Practical Operation, Design, and Optimization of FCC Units; Elsevier Science: Waltham, United States, 2020.
- Akah, A.; Williams, J.; Ghrami, M. An overview of light olefins production via steam enhanced catalytic cracking. Surv. Asia 2019, 23, 265–276, doi:10.1007/s10563-019-09280-6.
- Amghizar, I.; Dedeyne, J.N.; Brown, D.J.; Marin, G.B.; Van Geem, K.M. Sustainable innovations in steam cracking: CO2 neutral olefin production. Chem. Eng. 2020, 5, 239–257, doi:10.1039/c9re00398c.
- Subramanian, R.; Schmidt, L.D. Renewable olefins from biodiesel by autothermal reforming. Chem. Int. Ed. 2005, 44, 302–305, doi:10.1002/anie.200460790.
- Shelepova, E.V.; Vedyagin, A.A. Intensification of the dehydrogenation process of different hydrocarbons in a catalytic membrane reactor. Eng. Process. Process Intensif. 2020, 155, 108072, doi:10.1016/j.cep.2020.108072.
- Wu, T.; Yu, Q.; Roghair, I.; Wang, K.; Annaland, M.V.S. Chemical looping oxidative dehydrogenation of propane: A comparative study of Ga-based, Mo-based, V-based oxygen carriers. Eng. Process. Process Intensif. 2020, 157, 108137, doi:10.1016/j.cep.2020.108137.
- Lima, D.S.; Perez-Lopez, O.W. Oxidative coupling of methane to light olefins using waste eggshell as catalyst. Chem. Commun. 2020, 116, 107928, doi:10.1016/j.inoche.2020.107928.
- Luo, L.; You, R.; Liu, Y.; Yang, J.; Zhu, Y.; Wen, W.; Pan, Y.; Qi, F.; Huang, W. Gas-phase reaction network of Li/MgO-catalyzed oxidative coupling of methane and oxidative dehydrogenation of ethane. ACS Catal. 2019, 9, 2514–2520, doi:10.1021/acscatal.8b04728.
- Wang, P.; Zhang, X.; Zhao, G.; Liu, Y.; Lu, Y. Oxidative coupling of methane: MOx-modified (M = Ti, Mg, Ga, Zr) Mn2O3-Na2 WO4 /SiO2 catalysts and effect of MOx Chin. J. Catal. 2018, 39, 1395–1402, doi:10.1016/s1872-2067(18)63076-1.
- Pedersen, E.Ø.; Svenum, I.-H.; Blekkan, A. Mn promoted Co catalysts for Fischer-Tropsch production of light olefins—An experimental and theoretical study. Catal. 2018, 361, 23–32, doi:10.1016/j.jcat.2018.02.011.
- Tian, Z.; Wang, C.; Yue, J.; Zhang, X.; Ma, L. Effect of a potassium promoter on the Fischer–Tropsch synthesis of light olefins over iron carbide catalysts encapsulated in graphene-like carbon. Sci. Technol. 2019, 9, 2728–2741, doi:10.1039/c9cy00403c.
- Di, Z.; Zhao, T.; Feng, X.; Luo, M. A newly designed core-shell-like zeolite capsule catalyst for synthesis of light olefins from syngas via Fischer–Tropsch synthesis reaction. Lett. 2019, 149, 441–448, doi:10.1007/s10562-018-2624-9.
- Yang, M.; Fan, D.; Wei, Y.; Tian, P.; Liu, Z. Recent progress in methanol‐to‐olefins (MTO) catalysts. Mater. 2019, 31, e1902181, doi:10.1002/adma.201902181.
- Hua, J.; Dong, X.; Wang, J.; Chen, C.; Shi, Z.; Liu, Z.; Han, Y. Methanol-to-olefin conversion over small-pore DDR zeolites: Tuning the propylene selectivity via the olefin-based catalytic cycle. ACS Catal. 2020, 10, 3009–3017, doi:10.1021/acscatal.9b05521.
- Kang, J.H.; Alshafei, F.H.; Zones, S.I.; Davis, M.E. Cage-defining ring: A molecular sieve structural indicator for light olefin product distribution from the methanol-to-olefins reaction. ACS Catal. 2019, 9, 6012–6019, doi:10.1021/acscatal.9b00746.
- Ding, J.; Huang, L.; Gong, W.; Fan, M.; Zhong, Q.; Russell, A.G.; Gu, H.; Zhang, H.; Zhang, Y.; Ye, R.-P. CO2 hydrogenation to light olefins with high-performance Fe30Co0.15Zr0.45K0.10O1.63. J. Catal. 2019, 377, 224–232, doi:10.1016/j.jcat.2019.07.036.
- Goud, D.; Gupta, R.; Maligal-Ganesh, R.; Peter, S.C. Review of catalyst design and mechanistic studies for the production of olefins from anthropogenic CO2. ACS Catal. 2020, 10, 14258–14282, doi:10.1021/acscatal.0c03799.
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Commun. 2019, 10, 5698, doi:10.1038/s41467-019-13638-9.
- Numpilai, T.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Optimization of synthesis condition for CO2 hydrogenation to light olefins over In2O3 admixed with SAPO-34. Energy Convers. Manag. 2019, 180, 511–523, doi:10.1016/j.enconman.2018.11.011.
- Wang, X.; Wu, D.; Zhang, J.; Gao, X.; Ma, Q.; Fan, S.; Zhao, T.-S. Highly selective conversion of CO2 to light olefins via Fischer-Tropsch synthesis over stable layered K–Fe–Ti catalysts. Catal. A Gen. 2019, 573, 32–40, doi:10.1016/j.apcata.2019.01.005.
- Wu, T.; Lin, J.; Cheng, Y.; Tian, J.; Wang, S.; Xie, S.; Pei, Y.; Yan, S.; Qiao, M.; Xu, H.; et al. Porous Graphene-Confined Fe–K as highly efficient catalyst for CO2 direct hydrogenation to light olefins. ACS Appl. Mater. Interfaces 2018, 10, 23439–23443, doi:10.1021/acsami.8b05411.
- Ren, T.; Patel, M.K.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451, doi:10.1016/j.energy.2005.04.001.
- Akah, A.; Al-Ghrami, M. Maximizing propylene production via FCC technology. Petrochem. Res. 2015, 5, 377–392, doi:10.1007/s13203-015-0104-3.
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Catal. A Gen. 2011, 398, 1–17, doi:10.1016/j.apcata.2011.03.009.
- Yoshimura, Y.; Kijima, N.; Hayakawa, T.; Murata, K.; Suzuki, K.; Mizukami, F.; Matano, K.; Konishi, T.; Oikawa, T.; Saito, M.; et al. Catalytic cracking of naphtha to light olefins. Surv. Jpn. 2001, 4, 157–167, doi:10.1023/a:1011463606189.
- Speight, J.G. Chapter 11—Fouling during catalytic cracking. In Fouling in Refineries; Speight, J.G., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2015; pp. 271–302.
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: recent developments on the grand old lady of zeolite catalysis. Soc. Rev. 2015, 44, 7342–7370, doi:10.1039/c5cs00376h.
- Maadhah, A.G.; Fujiyama, Y.; Redhwi, H.; Abul-Hamayel, M.; Aitani, A.; Saeed, M.; Dean, C. A new catalytic cracking process to maximize refinery propylene. J. Sci. Eng. 2008, 33, 17–28.
- Zhu, G.; Xie, C.; Li, Z.; Wang, X. Catalytic processes for light olefin production. In Springer Handbook of Nanotechnology; Springer: Cham, Switzerland, 2017; pp. 1063–1079.
- Yujian, L.; Jun, L.; Huiping, T.; Yun, X.; Liuzhou, Z. Advances in DCC process and catalyst for propylene production from heavy oils. China Pet. Process. Petrochem. Technol. 2011, 13, 1–5.
- Genquan, Z.; Chaogang, X. Research and commercial application of CPP technology for producing light olefins from heavy oil. China Pet. Process. Petrochem. Technol. 2013, 15, 7–12.
- Soni, D.S.; Shorey, S.; Rao, M.R. Maximizing propylene through catalytic cracking: Indmax fluid catalytic cracking (I-FCC) process. In Proceedings of the 2008 AIChE Spring National Meeting, New Orleans, LA, USA, 6–10 April 2008.
- Banjare, M.; Pal, M.P.; Nanacharaiah, C.; Desai, V.S.; Guria, S.; Vardhan, H.; Mondal, B.C. Use of phased array ultrasonic testing for sizing of hydrogen blisters in LPG wash water vessel in INDMAX unit. J. Mech. Ind. Eng. 2012, 1, 203–207, doi:10.47893/ijmie.2012.1040.
- Fujiyama, Y.; Al-Tayyar, M.H.; Dean, C.F.; Aitani, A.; Redhwi, H.H. Chapter 1 Development of high-severity FCC process: an overview. In Studies in Surface Science and Catalysis, Ocelli, M.L., Ed. Elsevier, Amsterdam, Netherlands, 2007; Volume 166, pp. 1–12.
- Parthasarathi, R.S.; Alabduljabbar, S.S. HS-FCC high-severity fluidized catalytic cracking: A newcomer to the FCC family. Petrochem. Res. 2014, 4, 441–444, doi:10.1007/s13203-014-0087-5.
References
- Hsu, C.S.; Robinson, P.R. Petroleum Science and Technology; Springer: Cham, Switzerland, 2019.
- Speight, J.G. Organic chemistry. In Environmental Organic Chemistry for Engineers; Elsevier BV: Cambridge, United States, 2017; pp. 43–86.
- Stauffer, E.; Dolan, J.A.; Newman, R. Chapter 3 - Review of Basic Organic Chemistry. In Fire Debris Analysis, Stauffer, E., Dolan, J.A., Newman, R., Eds. Academic Press: Burlington, United States, 2008; pp. 49–83.
- Fakhroleslam, M.; Sadrameli, S.M. Thermal cracking of hydrocarbons for the production of light olefins; A review on optimal process design, operation, and control. Eng. Chem. Res. 2020, 59, 12288–12303, doi:10.1021/acs.iecr.0c00923.
- Zacharopoulou, V.; Lemonidou, A.A. Olefins from biomass intermediates: A review. Catalysts 2017, 8, 2, doi:10.3390/catal8010002.
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New trends in olefin production. Engineering 2017, 3, 171–178, doi:10.1016/j.eng.2017.02.006.
- Sadeghbeigi, R. Fluid Catalytic Cracking Handbook: An Expert Guide to the Practical Operation, Design, and Optimization of FCC Units; Elsevier Science: Waltham, United States, 2020.
- Akah, A.; Williams, J.; Ghrami, M. An overview of light olefins production via steam enhanced catalytic cracking. Surv. Asia 2019, 23, 265–276, doi:10.1007/s10563-019-09280-6.
- Amghizar, I.; Dedeyne, J.N.; Brown, D.J.; Marin, G.B.; Van Geem, K.M. Sustainable innovations in steam cracking: CO2 neutral olefin production. Chem. Eng. 2020, 5, 239–257, doi:10.1039/c9re00398c.
- Subramanian, R.; Schmidt, L.D. Renewable olefins from biodiesel by autothermal reforming. Chem. Int. Ed. 2005, 44, 302–305, doi:10.1002/anie.200460790.
- Shelepova, E.V.; Vedyagin, A.A. Intensification of the dehydrogenation process of different hydrocarbons in a catalytic membrane reactor. Eng. Process. Process Intensif. 2020, 155, 108072, doi:10.1016/j.cep.2020.108072.
- Wu, T.; Yu, Q.; Roghair, I.; Wang, K.; Annaland, M.V.S. Chemical looping oxidative dehydrogenation of propane: A comparative study of Ga-based, Mo-based, V-based oxygen carriers. Eng. Process. Process Intensif. 2020, 157, 108137, doi:10.1016/j.cep.2020.108137.
- Lima, D.S.; Perez-Lopez, O.W. Oxidative coupling of methane to light olefins using waste eggshell as catalyst. Chem. Commun. 2020, 116, 107928, doi:10.1016/j.inoche.2020.107928.
- Luo, L.; You, R.; Liu, Y.; Yang, J.; Zhu, Y.; Wen, W.; Pan, Y.; Qi, F.; Huang, W. Gas-phase reaction network of Li/MgO-catalyzed oxidative coupling of methane and oxidative dehydrogenation of ethane. ACS Catal. 2019, 9, 2514–2520, doi:10.1021/acscatal.8b04728.
- Wang, P.; Zhang, X.; Zhao, G.; Liu, Y.; Lu, Y. Oxidative coupling of methane: MOx-modified (M = Ti, Mg, Ga, Zr) Mn2O3-Na2 WO4 /SiO2 catalysts and effect of MOx Chin. J. Catal. 2018, 39, 1395–1402, doi:10.1016/s1872-2067(18)63076-1.
- Pedersen, E.Ø.; Svenum, I.-H.; Blekkan, A. Mn promoted Co catalysts for Fischer-Tropsch production of light olefins—An experimental and theoretical study. Catal. 2018, 361, 23–32, doi:10.1016/j.jcat.2018.02.011.
- Tian, Z.; Wang, C.; Yue, J.; Zhang, X.; Ma, L. Effect of a potassium promoter on the Fischer–Tropsch synthesis of light olefins over iron carbide catalysts encapsulated in graphene-like carbon. Sci. Technol. 2019, 9, 2728–2741, doi:10.1039/c9cy00403c.
- Di, Z.; Zhao, T.; Feng, X.; Luo, M. A newly designed core-shell-like zeolite capsule catalyst for synthesis of light olefins from syngas via Fischer–Tropsch synthesis reaction. Lett. 2019, 149, 441–448, doi:10.1007/s10562-018-2624-9.
- Yang, M.; Fan, D.; Wei, Y.; Tian, P.; Liu, Z. Recent progress in methanol‐to‐olefins (MTO) catalysts. Mater. 2019, 31, e1902181, doi:10.1002/adma.201902181.
- Hua, J.; Dong, X.; Wang, J.; Chen, C.; Shi, Z.; Liu, Z.; Han, Y. Methanol-to-olefin conversion over small-pore DDR zeolites: Tuning the propylene selectivity via the olefin-based catalytic cycle. ACS Catal. 2020, 10, 3009–3017, doi:10.1021/acscatal.9b05521.
- Kang, J.H.; Alshafei, F.H.; Zones, S.I.; Davis, M.E. Cage-defining ring: A molecular sieve structural indicator for light olefin product distribution from the methanol-to-olefins reaction. ACS Catal. 2019, 9, 6012–6019, doi:10.1021/acscatal.9b00746.
- Ding, J.; Huang, L.; Gong, W.; Fan, M.; Zhong, Q.; Russell, A.G.; Gu, H.; Zhang, H.; Zhang, Y.; Ye, R.-P. CO2 hydrogenation to light olefins with high-performance Fe30Co0.15Zr0.45K0.10O1.63. J. Catal. 2019, 377, 224–232, doi:10.1016/j.jcat.2019.07.036.
- Goud, D.; Gupta, R.; Maligal-Ganesh, R.; Peter, S.C. Review of catalyst design and mechanistic studies for the production of olefins from anthropogenic CO2. ACS Catal. 2020, 10, 14258–14282, doi:10.1021/acscatal.0c03799.
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Commun. 2019, 10, 5698, doi:10.1038/s41467-019-13638-9.
- Numpilai, T.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Optimization of synthesis condition for CO2 hydrogenation to light olefins over In2O3 admixed with SAPO-34. Energy Convers. Manag. 2019, 180, 511–523, doi:10.1016/j.enconman.2018.11.011.
- Wang, X.; Wu, D.; Zhang, J.; Gao, X.; Ma, Q.; Fan, S.; Zhao, T.-S. Highly selective conversion of CO2 to light olefins via Fischer-Tropsch synthesis over stable layered K–Fe–Ti catalysts. Catal. A Gen. 2019, 573, 32–40, doi:10.1016/j.apcata.2019.01.005.
- Wu, T.; Lin, J.; Cheng, Y.; Tian, J.; Wang, S.; Xie, S.; Pei, Y.; Yan, S.; Qiao, M.; Xu, H.; et al. Porous Graphene-Confined Fe–K as highly efficient catalyst for CO2 direct hydrogenation to light olefins. ACS Appl. Mater. Interfaces 2018, 10, 23439–23443, doi:10.1021/acsami.8b05411.
- Ren, T.; Patel, M.K.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451, doi:10.1016/j.energy.2005.04.001.
- Akah, A.; Al-Ghrami, M. Maximizing propylene production via FCC technology. Petrochem. Res. 2015, 5, 377–392, doi:10.1007/s13203-015-0104-3.
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Catal. A Gen. 2011, 398, 1–17, doi:10.1016/j.apcata.2011.03.009.
- Yoshimura, Y.; Kijima, N.; Hayakawa, T.; Murata, K.; Suzuki, K.; Mizukami, F.; Matano, K.; Konishi, T.; Oikawa, T.; Saito, M.; et al. Catalytic cracking of naphtha to light olefins. Surv. Jpn. 2001, 4, 157–167, doi:10.1023/a:1011463606189.
- Speight, J.G. Chapter 11—Fouling during catalytic cracking. In Fouling in Refineries; Speight, J.G., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2015; pp. 271–302.
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: recent developments on the grand old lady of zeolite catalysis. Soc. Rev. 2015, 44, 7342–7370, doi:10.1039/c5cs00376h.
- Maadhah, A.G.; Fujiyama, Y.; Redhwi, H.; Abul-Hamayel, M.; Aitani, A.; Saeed, M.; Dean, C. A new catalytic cracking process to maximize refinery propylene. J. Sci. Eng. 2008, 33, 17–28.
- Zhu, G.; Xie, C.; Li, Z.; Wang, X. Catalytic processes for light olefin production. In Springer Handbook of Nanotechnology; Springer: Cham, Switzerland, 2017; pp. 1063–1079.
- Yujian, L.; Jun, L.; Huiping, T.; Yun, X.; Liuzhou, Z. Advances in DCC process and catalyst for propylene production from heavy oils. China Pet. Process. Petrochem. Technol. 2011, 13, 1–5.
- Genquan, Z.; Chaogang, X. Research and commercial application of CPP technology for producing light olefins from heavy oil. China Pet. Process. Petrochem. Technol. 2013, 15, 7–12.
- Soni, D.S.; Shorey, S.; Rao, M.R. Maximizing propylene through catalytic cracking: Indmax fluid catalytic cracking (I-FCC) process. In Proceedings of the 2008 AIChE Spring National Meeting, New Orleans, LA, USA, 6–10 April 2008.
- Banjare, M.; Pal, M.P.; Nanacharaiah, C.; Desai, V.S.; Guria, S.; Vardhan, H.; Mondal, B.C. Use of phased array ultrasonic testing for sizing of hydrogen blisters in LPG wash water vessel in INDMAX unit. J. Mech. Ind. Eng. 2012, 1, 203–207, doi:10.47893/ijmie.2012.1040.
- Fujiyama, Y.; Al-Tayyar, M.H.; Dean, C.F.; Aitani, A.; Redhwi, H.H. Chapter 1 Development of high-severity FCC process: an overview. In Studies in Surface Science and Catalysis, Ocelli, M.L., Ed. Elsevier, Amsterdam, Netherlands, 2007; Volume 166, pp. 1–12.
- Parthasarathi, R.S.; Alabduljabbar, S.S. HS-FCC high-severity fluidized catalytic cracking: A newcomer to the FCC family. Petrochem. Res. 2014, 4, 441–444, doi:10.1007/s13203-014-0087-5.