Direct compression is a simple, quick and cost-effective drug manufacturing approaches of tableting.
- spray drying
- direct compression
- tablets
- co-processing
1. Introduction
Tablets constitute the most common dosage form due to economical manufacturing, accurate dosing and patient convenience. Tablets can be manufactured by direct compression or via dry, wet or melt granulation of drug(s)/excipient(s) mixture. Direct compression is a simple, quick and cost-effective method of tableting, and is therefore receiving enduring interest. However, it requires that the processed materials have excellent flowability and respond well under compression, a prerequisite which many commercialized excipients and active ingredients do not meet. As such, formulators usually resort to granulation techniques to enhance flow and compression properties of the drug/excipient mixture.
In order to facilitate direct compression, specialized DC excipients and grades of some high-dose drugs have been developed and marketed. These grades are prepared by various techniques to obtain particles with suitable micromeritic and physicomechanical properties. Spray drying is such a strategy that has been extensively exploited in recent decades. It is a continuous one-step process producing powders with uniform particle size and approximately spherical particle shape, rendering them good flowability. Also, the isodiametric shape of spray-dried particles helps particle rearrangement in the die during tableting leading to enhanced compaction behaviour [1]. The process parameters can be controlled, and the formulation can be optimized by employing process analytical technology (PAT) and quality-by-design (QbD) principles [2][3][4][5][2,3,4,5]. With appropriate process and formulation design, it is possible to produce a ready-to-compress (RTC) mixture or an almost final product that needs only one last mixing step with binder/disintegrant/lubricant before tableting (Figure 1).
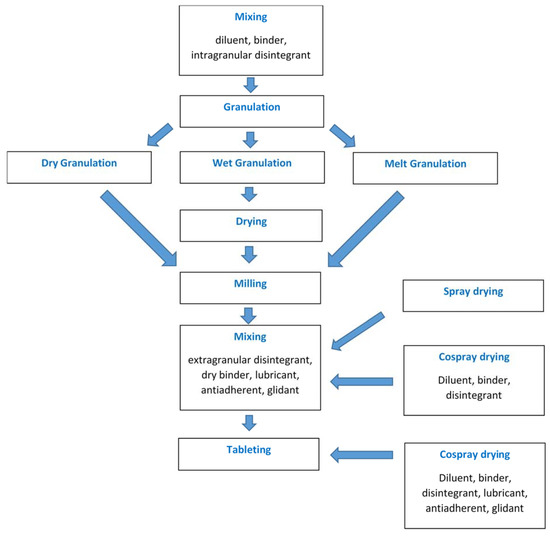
Figure 1. Unit operations employed in the production of tablets via granulation or spray/co-spray drying.
Furthermore, spray drying is a unique co-processing technique allowing the production of composite particles consisting of combinations of different ingredients dissolved or suspended together in a liquid medium. The produced co-spray dried composite particles usually have improved physical form and advanced attributes such as better flowability and compactability or reduced hygroscopicity compared to the individual components or their physical mixtures [7][6][6,7]. Additional functionalities such as sustained release [8][9][8,9], delayed release [10][11] [10,11] and taste masking [11][12][13] [11,12,13] can be achieved by selecting suitable co-excipients and drug state in the feed liquid as solution or suspension.
2. Direct Compression of Spray-Dried APIs
Spray drying has been particularly important for enhancing the compression of poorly compressible high-dose drugs. Although the majority of the studies report combinations of drugs with auxiliaries, there are some studies reporting spray drying of a solution of drug without auxiliaries, aiming at compression improvement via changes in the solid state, particle size or shape. The main investigations of drugs spray-dried alone or co-spray dried with excipients are summarized in Table 1.
Di Martino et al. [14] [34] spray dried acetazolamide from ammonia solutions. The obtained powder was composed of a mixture of polymorphs I and II. On the other hand, compression of the respective pure polymorphs, obtained by crystallization from solution, was not possible, giving capped tablets even at high compression pressures. This was explained by the remarkable improvement of particle rearrangement in the initial stage of compression of the spray-dried polymorphs due to the spherical shape and minor wrinkledness of the particles.
Paluch et al. [15] [35] obtained nanocrystalline microparticles of the poorly compactable chlorothiazide by spray drying a drug solution in water/acetone mixture. Tablets compacted from the obtained nanocrystalline microparticles (Figure 4) had remarkably higher tensile strengths than those from micronized chlorothiazide raw material. In another study from the same group, the compression behavior of several solid forms of chlorothiazide sodium and potassium salts were evaluated. The results proved the superior tabletability of the spray-dried microparticles of both salts that were composed of primary nanoparticles, which offered a larger interparticle contact area during the compaction stage [16][36].
3. Direct Compression of Co-Spray Dried APIs with Excipients
The majority of spray drying applications of drugs involve co-processing with excipients. The presence of the excipients favorably modifies the solid-state crystalline properties and may exercise some binding effect as well. The most commonly investigated excipients are carbohydrates and polymers.
The influence of different carbohydrates on spray drying processability and tableting-related properties (flowability, compactability and hygroscopicity) of spray-dried high-dose drugs has been investigated in a series of published papers [17][18][19][20][37,38,39,40]. Spray drying of binary and ternary paracetamol-excipient mixtures demonstrated the efficiency of three carbohydrates to improve the physical properties and compactability of this high-elastic relaxation drug. Mannitol and maltodextrin enhanced compactability, while erythritol increased powder density and flowability [17][37]. A D-optimal mixture design was employed to evaluate the effects of the three carbohydrates in co spray-dried mixtures. The composition containing 20.9% w/w erythritol, 13.9% w/w maltodextrin and 11.6% w/w mannitol was found optimal regarding powder flow, mechanical strength and disintegration of paracetamol tablets [18][38]. In a further study by the same research group, optimization of processing parameters was sought out to obtain RTC mixtures. A mixture of paracetamol with carbohydrates (erythritol, maltodextrin, mannitol), disintegrant (crospovidone), glidant (colloidal silica) and surfactant (polysorbate 80) was co-spray dried. Inlet and outlet air temperatures were found to affect the median particle size, moisture content and flowability. However, regression models for the effect of processing parameters on tablet hardness, friability and disintegration time were not statistically acceptable [19][39]. The combination of erythritol, maltodextrin, mannitol, crospovidone, colloidal silica and polysorbate 80 considerably improved the compactability of paracetamol, ibuprofen and cimetidine via co-spray drying in a lab-scale apparatus. Furthermore, high contents of paracetamol (70% w/w) and ibuprofen (75% w/w) were successfully loaded using a production scale spray-drier [20][40].
McDonagh et al. [21] [41] investigated the role of the soluble fraction of paracetamol and α-lactose monohydrate on the tableting of their crystallo-co-spray dried agglomerates. Different inlet feed solvent compositions were employed to modify the soluble fraction of lactose in the inlet feed. It was found that an increase in the soluble fraction of lactose resulted in greater mixing in the final spray-dried product and greater improvement in compressibility and tabletability [21][41].
Polymers with good capability to act concomitantly as binders and crystallinity modifiers are also interesting additives for co-spray drying. They offer the advantage of being used at lower percentage than low-molecular-weight carbohydrates (Table 1) and thus to avoid enlargement of tablet volume of high-dose drugs. Joshi et al. [22] [42] prepared amorphous celecoxib and celecoxib-PVP-meglumine ternary mixture by spray drying and compared their compaction behavior with crystalline celecoxib. They found that tablets formed from ternary mixture gave higher tensile strength in comparison with amorphous or crystalline celecoxib. Also, pressure-induced devitrification that inhibited direct compaction of the amorphous celecoxib occurred to a lesser extent in the ternary mixture, thus making it suitable for direct compaction.
Al-Zoubi et al. [23] [43] co-spray dried naproxen and naproxen sodium with HPMC seeking out enhancement of compression behavior. Co-processing resulted in reduced crystallinity of naproxen and higher dihydrate content of naproxen sodium. When mixed with suitable processing aids, the co-spray dried powders formed tablets with superior mechanical properties compared to unprocessed or spray-dried alone drug powders.
Chinta et al. [24] [44] investigated co-spray drying aqueous acidic solutions of propranolol HCl with chitosan and lactose. Three chitosan grades (low-, medium-, and high-molecular-weight) were evaluated. The spray-dried agglomerates prepared with lactose and chitosan showed excellent flow and were suitable for tableting.
Rathod et al. [25] [45] co-processed cefuroxime axetil with chitosan chlorhydrate and mannitol by co-spray drying 5% slurry of drug and carriers at 1:1 drug:excipient ratio in isopropanol. They employed a full factorial design to optimize mannitol:chitosan chlorhydrate ratio and inlet air temperature as independent variables. Analysis of the results using the Kawakita and Heckel models revealed that the batch produced from the optimized conditions had better compressibility than the physical mixture.
Vanhoorne et al. [26] [46] co-spray dried paracetamol with mannitol and PVP. Compaction properties of the co-spray dried powders were compared to physical mixtures for compositions containing 75% paracetamol, 20–25% spray-dried mannitol, and 0–5% PVP. Mixtures of co-spray dried paracetamol with 5% PVP and 20% mannitol produced tablets with higher tensile strength than the corresponding physical mixtures at all applied compressions pressures (34–229 MPa). The improvement of mechanical strength by co-spray drying was attributed to the coating of paracetamol crystals with δ mannitol and PVP.
Metformin HCl is a problematic drug for DC processing due to poor compactability and high administered dose (500–1000 mg). Barot et al. [27] [47] co-spray dried aqueous solutions of PVP K30 (0–3% w/v) with metformin HCl in an attempt to develop a directly compressible grade. They found that metformin HCl co-spray dried with 2% PVP K30 showed excellent flowability and compressibility as well. Further investigation was sought out by Al-Zoubi et al. [28][48], who compared five hydrophilic polymers (HPMC, PVP, copovidone, sodium alginate and sodium carmellose) regarding their ability to enhance the compression behavior of metformin HCl. Co-spray drying with the polymers resulted in increased amorphous drug content associated with increased deformability and reduced ejectability. A significant correlation was found between compaction work and relative crystallinity (p = 0.042), which confirmed the importance of crystalline to amorphous conversion on the deformability and particle bonding mechanisms. However, compactability and tabletability of metformin HCl were improved only by co-spray drying with the anionic sodium alginate and sodium carmellose polymers that could make ionic interactions with the positively charged ammonium of the drug. [28][48].
The above results demonstrate the ability of spray drying to convert the crystalline state of the drug to amorphous, which improves interactivity, enhances plasticity and hence deformability and contact area. Additionally, they demonstrate the drug’s ability in the spray-dried particulate form to retain chemical bonds with the excipient formed during spray drying and thus enable modification and improvement of the mechanical properties. Furthermore, the spray-dried particles may present higher polymer concentration at the surface due to the rapidness of the drying process, enhancing bond formation during tableting. Therefore, all the aforementioned mechanisms should be considered when selecting polymers for co-spray drying with the view of tableting improvement.
Honick et al. [29] [49] co-spray dried hypromellose acetate succinate (HPMCAS) with itraconazole from organic solutions. They showed that the compacts from solid dispersions (SDDs) had a higher elastic recovery and much greater tendency to laminate, particularly at higher compression speeds, in comparison with physical mixtures. However, the intact compacts from SDDs tended to have higher mechanical strength than those produced from physical mixtures, probably due to the smaller particle size of the SDDs.
Table 1. Illustrative studies attempting direct compression of drugs by spray dying alone or with excipients.
| Drug | Nominal Content Per Tablet (mg) | Additive (s) | Additives Nominal Percentage (%) | Alterations Due to Spray Drying Affecting Functional Properties Related to Direct Compression Improvement |
|---|---|---|---|---|
| Spray-dried APIs | ||||
| Acetazolamide | 250 | N/A | 0 | Formation of a mixture of polymorphs I and II; More isodiametric microparticles; Reduced elastic recovery; Higher tensile strength |
| Chlorothiazide | 250–500 | N/A | 0 | Microparticles composed of primary nanoparticles; Higher tablet tensile strength and higher tablet porosity obtained by spray drying |
| Chlorothiazide sodium | N/A | 0 | Microparticles composed of primary nanoparticles; Higher specific surface area and superior tabletability | |
| Chlorothiazide potassium | N/A | 0 | Microparticles composed of primary nanoparticles; Higher specific surface area and superior tabletability | |
| Co-spray dried API-excipients | ||||
| Paracetamol | 325–650 | Erythritol Maltodextrin Mannitol |
50.8 | Improved flowability and compactability; Prevented capping and lamination |
| Paracetamol | 325–650 | Erythritol Mannitol Maltodextrin Crospovidone Colloidal silicon dioxide Polysorbate 80 |
30–58.1 | Improved flowability and compactability, Production of an RTC mixture |
| Ibuprofen | 200–800 | Erythritol Mannitol Maltodextrin Crospovidone Colloidal silicon dioxide Polysorbate 80 |
25–55 | Improved flowability and compactability, Production of an RTC mixture |
| Cimetidine | 200–400 | Erythritol Mannitol Maltodextrin Crospovidone Colloidal silicon dioxide Polysorbate 80 |
30–55 | Improved flowability and compactability |
| Paracetamol | 325–650 | Lactose | 50 | Improved compressibility and compactability, lower yield pressure |
| Paracetamol | 325–650 | Mannitol | 20–25 | Improved tabletability and decreased friability |
| PVP | 0–5 | |||
| Cefuroxime axetil | 125–500 | Mannitol Chitosan chlorhydrate |
50 | Lower yield pressure (Heckel) |
| Celecoxib | 50–400 | PVP Meglumine |
30 | High degree of amorphization; More isodiametric microparticles; Improved packing (lower Carr’s, Hausner’s indices and angle of repose) and flowability; Lower yield pressure (Heckel); Higher compactability and tabletability |
| Metformin HCl | 500–1000 | PVP | 0–3 | Disruption of crystal lattice; More isodiametric microparticles |
| Metformin HCl | 500–1000 | PVP Copovidone HPMC Sodium alginate Sodium carmellose |
0–5 | Reduced crystallinity; More isodiametric microparticles Reduced elastic recovery; Higher work of compaction; Improved compactability and tabletability by co-spray drying with sodium alginate and sodium carmellose |
| Naproxen | 250–500 | HPMC | 5 | Reduced crystallinity; more isodiametric particles; Higher compactability and tabletability |
| Naproxen sodium | 275–550 | HPMC | 5 | Increased dihydrate content; more isodiametric particles; Higher compactability and tabletability |

