Synthesis strategy of a terpyridine bridging ligand was explained.
- Bridging ligand
- Terpyridine
- bis(2-pyridylmethyl)amine
- bridging ligand
4'-(p-(N,N′-bis(2-pyridylmethyl)amine)-N"-methylphenyl)-2,2':6',2"-terpyridine (bpat)
Ramin Zibaseresht 1,2*
1. Introduction
We have begun a research programme aimed at the synthesis of heterodinuclear complexes. In these studies, we are focusing on the preparation and use of ditopic ligands where the two metal ion binding sites are differentiated by the number of donor atoms in each site, the configuration of the binding site, or the types of donor atom that are present in the sites. This binding site differentiation offers the prospect of using the different coordination properties of the binding sites to control the regiochemistry of complexation, ensuring that the correct metal ion is incorporated at the correct binding site in the ligand.
1 Faculty of Sciences, Maritime University of Imam Khomeini, Noshahr, Iran. Email: rzi12@uclive.ac.nz
2. Application
2 Biomaterials and Medicinal Chemistry Research Centre, Aja University of Medical Sciences, Tehran, Iran. Email: rzi12@uclive.ac.nz
In order to prepare heterodinuclear metal complexes, or a variety of inorganic polymers, the ligand acts as linker between the metal atoms. Those who are particularly interested in minimising the number of stereoisomers that might be produced on synthesis of the heterodinuclear systems, this type of linker can be one of their choices.
3. Ligand Synthesis
About author:
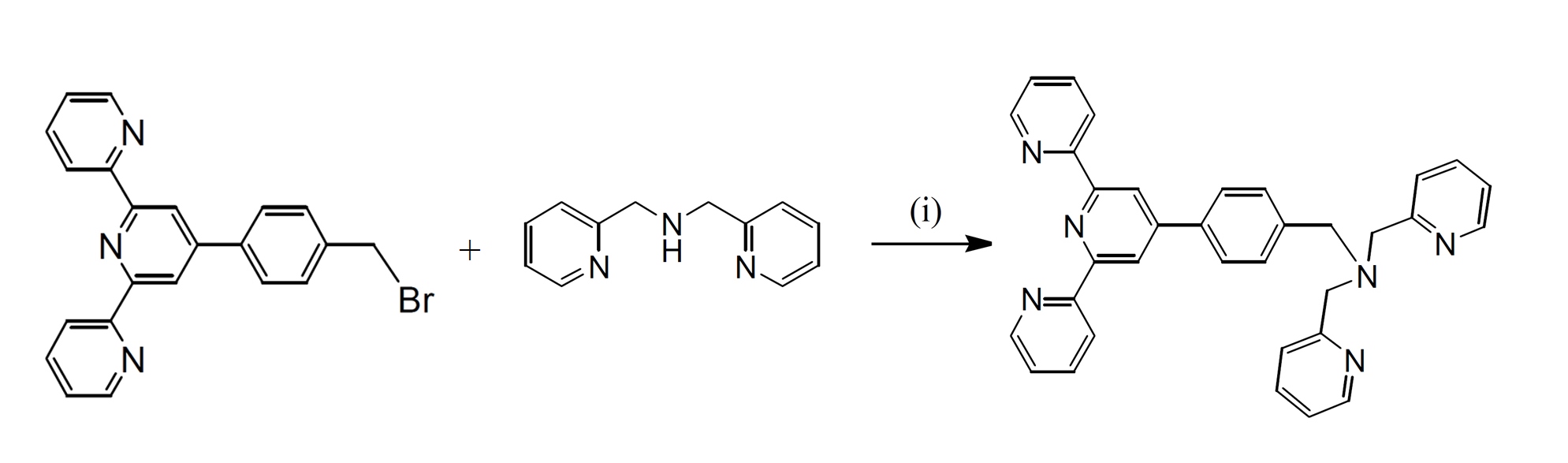
Ditopic ligands 4'-(p-(1,5-bis(phthalimido)-3-azapentan-3-yl)methylphenyl)-2,2':6',2"-terpyridine (bpat), based on tridentate planar terpyridyl systems has been synthesised in relatively high yield. The ligand was prepared using the method by nucleophilic substitution reaction of 4’-(p-bromomethylphenyl)-2,2’:6’,2’’-terpyridine with the appropriate secondary or tertiary amines in single- or multi-step reactions.
Ramin Zibaseresht is Associate Professor in Chemistry at Maritime University of Imam Khomeini in Noshahr and Adjunct Associate Professor at Aja University of Medical Sciences in Tehran. He received his B.Sc. in Chemistry from Shiraz University, his M.Sc. in Inorganic Chemistry from Pune University, and his PhD in Inorganic Chemistry from the University of Canterbury. He is currently the Head of Biomaterials and Medicinal Chemistry Research Centre in Tehran.
Introduction:
We have begun a research programme aimed at the synthesis of heterodinuclear complexes. In these studies, we are focusing on the preparation and use of ditopic ligands where the two metal ion binding sites are differentiated by the number of donor atoms in each site, the configuration of the binding site, or the types of donor atom that are present in the sites. This binding site differentiation offers the prospect of using the different coordination properties of the binding sites to control the regiochemistry of complexation, ensuring that the correct metal ion is incorporated at the correct binding site in the ligand.
Application:
In order to prepare heterodinuclear metal complexes, or a variety of inorganic polymers, the ligand acts as linker between the metal atoms. Those who are particularly interested in minimising the number of stereoisomers that might be produced on synthesis of the heterodinuclear systems, this type of linker can be one of their choices.
Ligand Synthesis:
Ditopic ligands 4'-(p-(1,5-bis(phthalimido)-3-azapentan-3-yl)methylphenyl)-2,2':6',2"-terpyridine (bpat), based on tridentate planar terpyridyl systems has been synthesised in relatively high yield. The ligand was prepared using the method by nucleophilic substitution reaction of 4’-(p-bromomethylphenyl)-2,2’:6’,2’’-terpyridine with the appropriate secondary or tertiary amines in single- or multi-step reactions.
Scheme 1. Syntheses of ligand bpat. (i) dipicolylamine (
Scheme 1. Syntheses of ligand bpat. (i) dipicolylamine (
N
,
N
’-bis(2-pyridylmethyl)amine), K2CO3, dry CH3CN, 3 days, 60o C
Ligand bpat was synthesised using direct functionalisation of dipicolylamine (
N
,
N
’-bis(2-pyridylmethyl)amine) with the bromo compound. The reactions were carried out in dry CH3CN and in the presence of K2CO3 to afford the desired products in excellent yields (95-98%). The reaction mixtures were monitored by 1H NMR spectroscopy and thin layer chromatography (TLC) in order to determine the optimum conditions. It was noted that prolonged reactions (up to 3 days) and also using 1.2 equivalents of the amines resulted in analytically pure products, with the best yields. The product was characterised using 1H and 13C NMR spectroscopy, COSY, NOESY, HSQC, and electrospray ionisation mass spectrometry.
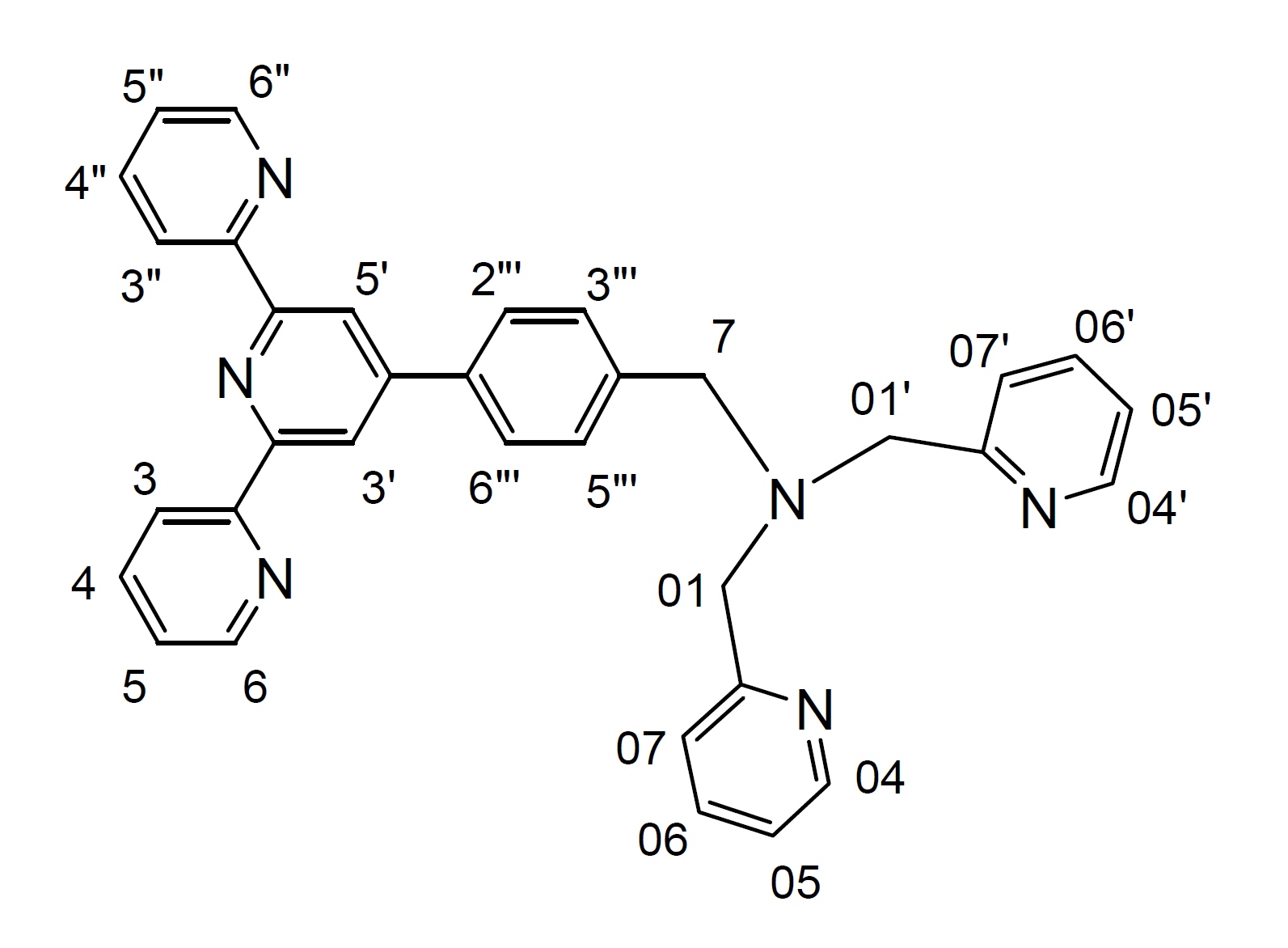
A mixture of (N,N'-bis(2-pyridylmethyl)amine), (0.24 g, 1.2 mmol) and 4'-(p-bromomethylphenyl)-2,2':6',2"-terpyridine (btp) (0.4 g, 1.0 mmol), and anhydrous K2CO3 (1.4 g, 10 mol) in dry CH3CN (50 mL) was stirred at 55-60° C under argon for 3 days. The progress of the reaction was monitored by TLC. The reaction mixture was then allowed to cool at room temperature; filtered and the solvent was removed from the filtrate under reduced pressure to give a pale yellow oil. Purification by column chromatography (Al2O3, 1-2% MeOH in CH2Cl2) afforded the desired product (0.52 g, 96%) as a pale yellow oil. 1H NMR (500 MHz; solvent CDCl3): δ 8.729 (2H, s, H3', H5'), 8.725 (2H, d, H6, H6"), 8.67 (2H, d, H3, H3"), 8.54 (2H, d, H04, H04'), 7.87 (2H, m, H4, H4"), 7.86 (2H, d, H2"', H6"'), 7.69 (2H, m, H06, H06'), 7.61 (2H, d, H07, H07'), 7.56 (2H, d, H3"', H5"'), 7.35 (2H, m, H5, H5"), 7.17 (2H, m, H05, H05'), 3.86 (4H, s, H01, H01'), 3.77 (2H, s, H7). 13C NMR (75 MHz; solvent CDCl3): δ 159.61, 156.24, 155.87, 150.03, 149.09 (2C, C6, C6"), 148.98 (2C, C04, C04'), 140.18, 137.23, 136.83 (2C, C4, C4" or C2"', C6"'), 136.46 (2C, C06, C06'), 129.35 (2C, C3"', C5"'), 127.26 (2C, C4, C4" or C2"', C6"'), 123.78 (2C, C5, C5"), 122.85 (2C, C07, C07'), 122.00 (2C, C05, C05'), 121.31 (2C, C3, C3"), 118.74 (2C, C3', C5'), 60.06 (2C, C01, C01'), 58.20 (C7). ESI-MS: m/z 261.1370 ([M+2H]2+, 100%), 521.2880 ([M+H]+, 14%).
A mixture of (N,N'-bis(2-pyridylmethyl)amine), (0.24 g, 1.2 mmol) and 4'-(p-bromomethylphenyl)-2,2':6',2"-terpyridine (btp) (0.4 g, 1.0 mmol), and anhydrous K2CO3 (1.4 g, 10 mol) in dry CH3CN (50 mL) was stirred at 55-60° C under argon for 3 days. The progress of the reaction was monitored by TLC. The reaction mixture was then allowed to cool at room temperature; filtered and the solvent was removed from the filtrate under reduced pressure to give a pale yellow oil. Purification by column chromatography (Al2O3, 1-2% MeOH in CH2Cl2) afforded the desired product (0.52 g, 96%) as a pale yellow oil. 1H NMR (500 MHz; solvent CDCl3): δ 8.729 (2H, s, H3', H5'), 8.725 (2H, d, H6, H6"), 8.67 (2H, d, H3, H3"), 8.54 (2H, d, H04, H04'), 7.87 (2H, m, H4, H4"), 7.86 (2H, d, H2"', H6"'), 7.69 (2H, m, H06, H06'), 7.61 (2H, d, H07, H07'), 7.56 (2H, d, H3"', H5"'), 7.35 (2H, m, H5, H5"), 7.17 (2H, m, H05, H05'), 3.86 (4H, s, H01, H01'), 3.77 (2H, s, H7). 13C NMR (75 MHz; solvent CDCl3): δ 159.61, 156.24, 155.87, 150.03, 149.09 (2C, C6, C6"), 148.98 (2C, C04, C04'), 140.18, 137.23, 136.83 (2C, C4, C4" or C2"', C6"'), 136.46 (2C, C06, C06'), 129.35 (2C, C3"', C5"'), 127.26 (2C, C4, C4" or C2"', C6"'), 123.78 (2C, C5, C5"), 122.85 (2C, C07, C07'), 122.00 (2C, C05, C05'), 121.31 (2C, C3, C3"), 118.74 (2C, C3', C5'), 60.06 (2C, C01, C01'), 58.20 (C7). ESI-MS: m/z 261.1370 ([M+2H]2+, 100%), 521.2880 ([M+H]+, 14%).