Microencapsulation is a well-known technology for the lipid delivery system. It prevents the oxidation of fatty acids and maintains the quality of lipid after extraction from oil seed and processing. In flaxseed oil, the amount of ω-3 and ω-6 polyunsaturated fatty acids are 39.90–60.42% and 12.25–17.44%, respectively.
- flaxseed oil
- microencapsulation
- Emulsification
- Spray-drying
- Freeze-drying
1. Introduction
Microencapsulation has been explored in order to satisfy the increasing expectation of developing food ingredients with complex properties and functional values. It is an emerging technology which has been receiving interest in food and biopharmaceutical industries. It is used to protect encapsulated bioactive compounds and control their release. In the microencapsulation technology, small droplets of liquid or solid particles are coated within a thin film, known as a wall material or matrix [1][2][22,23]. For the microencapsulation of food-grade bioactive compounds, different techniques have been adopted and they can be classified into three distinctive categories. Those include (a) chemical methods: Entrapping the bioactive compound within the polymerized matrix, (b) physical methods: Spray-drying, spray coating, freeze-drying, and supercritical encapsulation processes, and (c) physico-chemical methods: Complex coacervation, entrapment within the nanostructured lipid matrix, ionotropic gelation, and molecular inclusion [3][24].
2. Microencapsulation of Flaxseed Oil
For the microencapsulation of flaxseed oil, two major steps are: Preparation of flaxseed oil emulsion with an aqueous solution of the matrix and subsequently, spray-drying or freeze-drying.
2.1. Emulsification
The emulsion preparation plays a key role in the encapsulation efficiency. An emulsion is a mixture of two or more immiscible liquids [4][25]. The emulsion stability is controlled by many factors. Flocculation, a reversible aggregation of droplets and coalescence, an irreversible fusion of droplets are two main types of emulsion instabilities. The emulsifier can make a bridge between polar and non-polar components, and provides stability in the emulsion [5][26]. To prepare the emulsion, two different types of technologies can be adopted. Those are (a) high energy consuming technologies using mechanical devices to mix up the water and oil phase, such as (i) ultrasound generator and (ii) high pressure homogenizers, as well as (b) low energy consuming technologies, such as (i) phase inversion temperature, (ii) membrane emulsification, and (iii) spontaneous emulsification of two immiscible liquids without any significant external thermal or mechanical energy [6][27]. In Table 1, a summary of different emulsification technologies is provided. The emulsion stability, droplets size, and their distribution are considerably affected by the adopted technologies [7][28]. It has been reported that the fine emulsion increases the organoleptic properties of microcapsules [8][29].
Table 1. Summary of different emulsification technologies.
| Emulsification Techniques | Description | Reference | ||
|---|---|---|---|---|
| High-Energy Consuming Methods | Ultrasound generator | Due to the ultrasound (physical shear force), fine droplets are created. At a certain range of sound, a source pressure amplitude cavitation takes place and emulsification of the immiscible liquids occurs. | [9] | [30] |
| High pressure homogenizer | In a homogenizer, with the help of a pump the liquid is pressed with high pressure to a narrow channel, which offers shear force on immersible liquids. It creates cavitation and leads to emulsion with a small droplet size. | [10] | [31] | |
| Low-Energy Techniques | Phase inversion temperature | Due to the change in factors, such as temperature or pH, the activity of the emulsifier in terms of its hydrophilic—lipophilic balance is affected. It helps create the emulsion. | [11] | [32] |
| Membrane emulsification | Membrane emulsification is performed with the porous membrane. Hydrophilic liquid (oil) in a dispersed phase is pressed through the membrane pores to a continuous phase, generally hydrophilic liquid and the emulsion is formed in a continuous phase. | [12] | [33] | |
| Spontaneous emulsification | In spontaneous emulsification, the immiscible liquids, such as oil and water along with the emulsifier create the emulsion without an external energy source. | [13][14] | [34,35] | |
2.2. Spray-Drying
Spray-drying is a commonly used technology to prepare the encapsulation of vegetable oil [15][36]. In the spray-drying process, due to high heat, the water content is evaporated. Subsequently, the phase of the matrix is altered and solidification of the matrix takes place. Oil droplets are encapsulated within the molten matrix in a non-homogeneous way and the size of the microcapsule ranges between 10 to 400 µm depending on the initial parameters [16][37]. Compared to other microencapsulation technologies, spray-drying is simple, may operate with a continuous mode, and has a low production cost. On the other hand, the disadvantages of spray-drying are (a) the availability of water-soluble matrixes is limited and (b) loss of heat energy [17][38]. In Figure 1Figure 3, the process flow diagram of the spray-drying technology is represented.
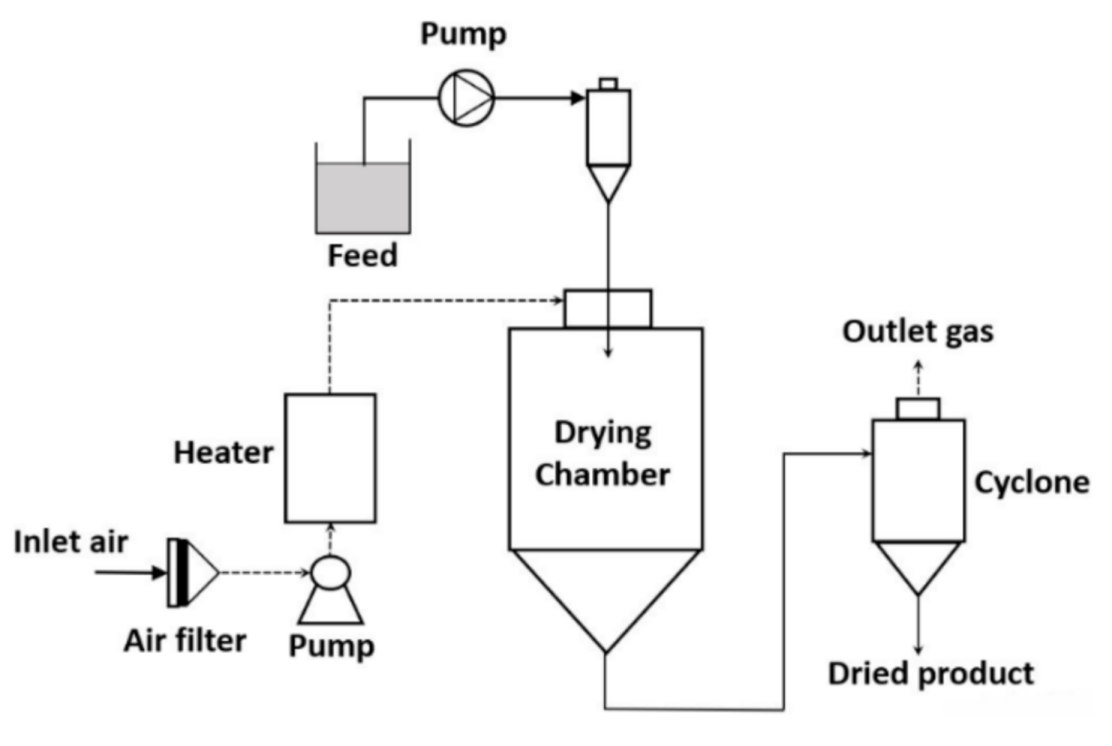
To obtain an optimum encapsulation efficiency with a minimum amount of oil on the surface of the matrix and maximum retention of the active compound, the composition of the emulsion, technology to prepare the emulsion, and parameters of the spray-drying process are taken into consideration [17][20][21][38,41,42]. The composition of the emulsion, size of droplets, and the viscosity of emulsion influence the quality of the spray-dried product. In the emulsion, the ratio of oil and matrix affects the stability of emulsion and encapsulation efficiency. It also affects the physical and biochemical properties of the spray-dried product [22][23][43,44]. The lower oil content and higher matrix to oil ratio lead to a smaller droplet of oil bodies in the emulsion. In this case, the amount of oil on the surface of the matrix is reduced and encapsulation efficiency is increased. In the emulsion, viscosity is directly proportional to the droplet size and inversely proportional to the emulsion stability. These two conditions affect the encapsulation efficiency. The type of atomizer and its operating parameters are important to prepare the microcapsule since they influence the particle size. Among the existing atomizers used for breaking the bulk feed into a smaller droplet, the centrifugal wheel atomizer and spray pressure nozzle are the most used for the encapsulation of oil [19][22][40,43]. In the spray-drying process, the particle size of the product is increased with the increase in the emulsion flow. In the case of the spray pressure nozzle, the particle size of the spray-dried product is increased with the increase in the nozzle orifice diameter and decrease in the atomization pressure. In the case of the centrifugal wheel atomizer, an increase in the wheel diameter and speed provides a smaller size of the particle [22][43]. Furthermore, drying parameters provide the desired quality of the final product. The major drying parameters are the drying air flow, and inlet and outlet temperatures [22][23][43,44]. A high inlet temperature in the spray-drying process may lead to deterioration of the encapsulated active compound and an imbalanced evaporation of water from the matrix, which affects the encapsulation efficiency. On the other hand, a higher water content in the encapsulated product and agglomeration of the microcapsule may take place at a very low inlet temperature in the spray-drying process. Furthermore, the outlet temperature in the spray-drying process influences the stability of the microcapsule and retention of the encapsulated product. However, the outlet temperature in the spray-drying process cannot be regulated in a direct way, it can be monitored in an indirect way by controlling the solid content in the feed, inlet temperature, and feed flow rate. The spray air flow rate affects the quality of the final product. It controls the stickiness of the dried particles, deposited onto the wall of the drying chamber [22][43].
2.3. Freeze-Drying
Freeze-drying is also known as the lyophilization process. It is used for the dehydration of high temperature sensitive bioactive compounds, including flaxseed oil and aromas. During the freeze-drying process, a reduction of the surrounding pressure, heating of the emulsion, and sublimation of the frozen water in the material take place [18][39]. After crystallization of water in the emulsion, sublimation of water takes place at a minimal temperature. It promotes the transformation of water from a solid phase to vapor, directly [24][45]. In Figure 2Figure 4, the process flow diagram of the freeze-drying technology is represented.
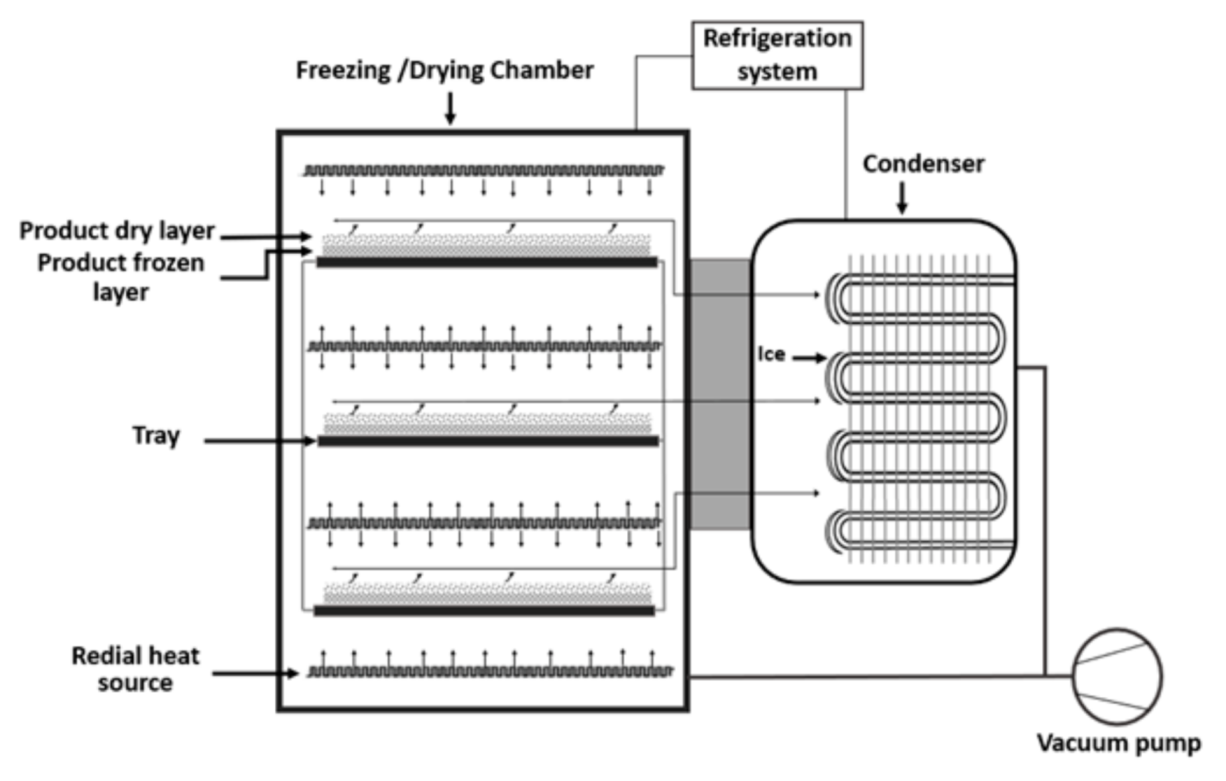
It is considered an expensive drying technology compared to the spray-drying process [26][47]. Furthermore, freeze-drying is a time occupying process, which consumes high energy. Due to the use of low temperature in the freeze-drying process, there is no thermal deterioration in the bioactive compound and less degradation in the heat-sensitive product. The moisture content in the final product is controlled by freeze-drying. Therefore, this technology provides a better quality of the product with a remarkable preservation of sensory properties of food ingredients [18][24][27][39,45,48]. Similar to spray-drying, the properties of the emulsion, characteristics of the matrix, and ratio of the matrix and oil are major factors to ensure the quality of the freeze-dried product. Maltodextrin, gum Arabic, and protein are commonly used as a wall material or matrix for microencapsulation through freeze-drying [19][40]. The freezing rate can affect the morphology of the microcapsule. A faster rate of freezing of the emulsion can lead to aggregation of the freeze-drying products. In the freeze-drying process, the system pressure and temperature influence the properties of the microcapsule. Furthermore, the operational time of drying is important to achieve a stable moisture content in the microcapsule and the stability of the final product [23][24][44,45]. Depending on the process parameters, a particle size of the freeze-dried product remains between 20 to 5000 μm [24][45].
2.4. Matrix (Wall Material)
For the microencapsulation of flaxseed oil, a selection of the suitable matrix, accepted in the food industry has a great importance. The matrix provides the desired stability of the encapsulated product, increases the encapsulation efficiency, and controls the release of the encapsulated item into the environment. Furthermore, the matrix provides unique physico-chemical and bio-chemical characteristics of the microcapsule [22][28][43,49]. The inexpensive wall material may reduce the cost of the process [28][49]. The water soluble wall material is preferable for the encapsulation of flaxseed oil. In the case of spray-drying, the wall material should be soluble in an aqueous medium to shield the encapsulated material from the external environment [19][40]. The drying characteristics of the matrix influence the moisture content of microcapsules. If the matrix has a chance to dry with high temperature at a minimal time, the moisture content in the microcapsule is reduced. Therefore, the encapsulated item has less chance to be contaminated with water [22][28][43,49]. An aqueous solution of the selected wall material is needed to have low viscosity with a high concentration of the solid. It helps obtain a fine microcapsule (lower particle size) and control the release of the encapsulated product. The Newtonian behavior of the emulsion is desirable for spray-drying, a continuous industrial drying process. In the case of spray-drying, previous knowledges about the glass transition temperature of the oil and matrix are a prerequisite to ensure the stability of the microcapsule, and avoid the stickiness of the obtained powder in the spray-drying chamber [22][43].
2.5. Emulsifier
The emulsifier, also known as “emulgent” and “surface active agents”, has a great influence on the preparation of flaxseed oil emulsion in the aqueous solution of the matrix, prior to the drying process. Emulsifiers are amphiphilic with hydrophilic/polar and hydrophobic/non-polar moieties [29][52]. The emulsifier reduces the interfacial tension between hydrophilic and hydrophobic compounds and makes them miscible [30][53]. Furthermore, emulsifiers have an antimicrobial property [31][54]. During the emulsification process, the hydrophilic group of emulsifier binds with water or the wall material and the hydrophobic group binds with the flaxseed oil. The concentration of the emulsifier and hydrophile–lipophile balance influence the stability of the emulsion, as well as the encapsulation efficiency [2][30][32][23,53,55]. For the preparation of flaxseed oil encapsulation, emulsifier Tween 80 [33][56] and soya lecithin [34][13] were used by several researchers.
