The clustering of Kv1.1/Kv1.2 channels at the axon initial segment and juxtaparanodes is based on interactions with cell adhesion molecules and cytoskeletal linkers.
- myelin
- node of Ranvier
- Kv1 channels
1. Introduction
The precise distribution of K
+ channels at the axon initial segment (AIS) and the nodes of Ranvier is critical to ensure the appropriate initiation and faithful propagation of action potentials (APs) along myelinated axons. The AIS and nodes of Ranvier are fascinating structures to examine the mechanisms involved in neuronal polarity and subcellular patterning of axonal functional domains [1,2]. Membrane subdomains are distinctly segregated at the nodes of Ranvier with the node itself highly concentrated in voltage-gated Na
channels at the axon initial segment (AIS) and the nodes of Ranvier is critical to ensure the appropriate initiation and faithful propagation of action potentials (APs) along myelinated axons. The AIS and nodes of Ranvier are fascinating structures to examine the mechanisms involved in neuronal polarity and subcellular patterning of axonal functional domains [1][2]. Membrane subdomains are distinctly segregated at the nodes of Ranvier with the node itself highly concentrated in voltage-gated Na
+
(Nav) channels and the juxtaparanodes enriched in voltage-gated K
+ (Kv) channels. These two domains are separated by the paranodal junctions anchoring the myelin terminal loops on both sides of the nodal gap. The mechanisms underlying the segregation of Nav1 channels at the AIS and nodes of Ranvier have been thoroughly examined in the PNS and CNS [3,4,5,6,7,8]. However, it is still unclear how the Kv1 channels are anchored at the AIS and juxtaparanodes. This review will focus on the dynamic processes that are involved in the subcellular targeting of the Kv1.1/Kv1.2 channels associated in complex with cell adhesion molecules and cytoskeleton linkers, including axonal transport, membrane diffusion and trapping, or internalization. A comprehensive view of the functional role of the diverse axonal K
(Kv) channels. These two domains are separated by the paranodal junctions anchoring the myelin terminal loops on both sides of the nodal gap. The mechanisms underlying the segregation of Nav1 channels at the AIS and nodes of Ranvier have been thoroughly examined in the PNS and CNS [3][4][5][6][7][8]. However, it is still unclear how the Kv1 channels are anchored at the AIS and juxtaparanodes. This review will focus on the dynamic processes that are involved in the subcellular targeting of the Kv1.1/Kv1.2 channels associated in complex with cell adhesion molecules and cytoskeleton linkers, including axonal transport, membrane diffusion and trapping, or internalization. A comprehensive view of the functional role of the diverse axonal K
+
channels begins to emerge, taking into consideration the neuronal cell-type specificity. In pathological conditions such as demyelinating diseases in the PNS or CNS, disturbance of the nodes of Ranvier is associated with alteration of paranodal junctions and exposure of juxtaparanodal Kv1 channels that may contribute to neurological disorders.
2. Diversity of K
+
Channels at the Axon Initial Segment and Nodes of Ranvier
The initiation and propagation of APs in myelinated axons depends on the high concentration of voltage-gated Na
+
channels at the AIS and the nodes of Ranvier, respectively. A variety of K
+
channels have been identified with precise axonal distribution to modulate neuronal excitability and the shape and frequency of APs. A common feature for myelinated axons both in the CNS and PNS is the presence of voltage-gated K
+ channels including Kv1.1/Kv1.2 channels localized at the juxtaparanodes under the myelin sheath [9] and Kv7.2/Kv7.3 (also named KCNQ2/3) that are present at the node itself [10,11]. Kv1 and Kv7 family members are also found at the AIS regulating excitability at the site where APs are generated [12,13]. Moreover, other subtypes of axonal K
channels including Kv1.1/Kv1.2 channels localized at the juxtaparanodes under the myelin sheath [9] and Kv7.2/Kv7.3 (also named KCNQ2/3) that are present at the node itself [10][11]. Kv1 and Kv7 family members are also found at the AIS regulating excitability at the site where APs are generated [12][13]. Moreover, other subtypes of axonal K
+
channels are found along myelinated axons, with the voltage-gated Kv3.1b segregated at the nodal gap in a subset of CNS large myelinated axons [14]. The nodal K
Ca
3.1 channels activated by an activity-dependent influx of Ca
2+
, have been shown to secure continuous spike propagation in Purkinje cells [15]. The BK/K
Ca
1.1 channels, also activated by changes in membrane potential and intracellular Ca
2+
concentration, localize to the paranodes solely in axons of cerebellar Purkinje cells to support the high-fidelity of AP firing at high frequency [16]. Recently, the two-pore-domain K
+ channels TREK-1 and TRAAK, displaying thermal and mechanical sensitivity, have been identified at the nodes of Ranvier both in the CNS and PNS, and are not found at the AIS [17,18]. The K2P channels generate high-leak K
channels TREK-1 and TRAAK, displaying thermal and mechanical sensitivity, have been identified at the nodes of Ranvier both in the CNS and PNS, and are not found at the AIS [17][18]. The K2P channels generate high-leak K
+
current at the node, hyperpolarizing the membrane resting potential, thereby increasing Na
+
channel availability for AP propagation [17]. As shown using pressure-patch-clamp recording at the nodes of Ranvier of trigeminal nerves, TREK-1 and TRAAK, most likely forming heteromers, drive rapid AP repolarization at the nodes and permit high speed and high-frequency AP conduction along myelinated axons [18]. In contrast, AP repolarization is not modified by TEA, a blocker of Kv1 and Kv7 channels, at the nodes of Ranvier of sensory nerves. Thus, AP repolarization may not mainly depend on voltage-gated K
+
channels at the nodes of Ranvier as it occurs at the neuronal cell bodies.
The role of nodal Kv7.2/7.3 channels mediating a slow K
+ current could be to finely modulate the excitability at the nodal region [19,20]. Moreover, voltage-gated K
current could be to finely modulate the excitability at the nodal region [19][20]. Moreover, voltage-gated K
+ channels may finely tune excitability and secure axonal conduction at transition zones, including at branch points or in the region distal to the last myelinated segment near the nerve terminal [21,22]. The safety factor is altered at these sites because of impedance mismatch. Strikingly, recent reports indicate that myelination can be discontinuous in the cortical pyramidal neurons mostly in the superficial layers [23]. Non-uniform myelination is also found in parvalbumin GABAergic neurons in the cortex and hippocampus, which show irregular myelinated segments along with their branched axonal trees [24,25]. Transition zone excitability may have profound implications for signal integration in axonal trees. The precise distribution and function of the diverse K
channels may finely tune excitability and secure axonal conduction at transition zones, including at branch points or in the region distal to the last myelinated segment near the nerve terminal [21][22]. The safety factor is altered at these sites because of impedance mismatch. Strikingly, recent reports indicate that myelination can be discontinuous in the cortical pyramidal neurons mostly in the superficial layers [23]. Non-uniform myelination is also found in parvalbumin GABAergic neurons in the cortex and hippocampus, which show irregular myelinated segments along with their branched axonal trees [24][25]. Transition zone excitability may have profound implications for signal integration in axonal trees. The precise distribution and function of the diverse K
+
channels at these transition zones deserve further investigation.
The role of voltage-gated Kv1.1/Kv1.2 channels has been finely analyzed at the AIS where they are involved in the control of neuronal excitability, spike shape, and frequency. The low-threshold fast-activated Kv1 channels play a highly localized role in shaping the axonal AP at the AIS of cortical pyramidal neurons [26] or in dampening near-threshold excitability in fast-spiking cortical GABAergic interneurons [27], likely depending on Kv1 subunit composition in the different neuronal cell types. The slow inactivation of Kv1.2 enriched at the AIS of spinal motoneurons promotes the nonlinear spiking associated with the rhythmic locomotor activity [28]. However, their role at the nodes of Ranvier is a matter of speculation. The fast Kv1.1/1.2 channels are sequestered along myelinated axons on both sides of the node of Ranvier at the juxtaparanodes under the myelin sheath and do not normally influence AP repolarization. Kv1 channels are separated from the nodal gap by septate-like paranodal junctions anchoring the terminal myelin loops to the axolemma. The transmembrane septate-like junctions and the short paranodal width (3–5 nm instead of 10–20 nm at internode) are thought to reduce the current flows between nodal and internodal extracellular spaces. An intriguing observation is that the juxtaparanodal Kv1 channels are forming rosette particles aligned with Connexin29 channels in apposed myelin membrane as observed in freeze-fracture replica immunogold labeling of mouse sciatic nerves [29]. This would support a leak K
The role of voltage-gated Kv1.1/Kv1.2 channels has been finely analyzed at the AIS where they are involved in the control of neuronal excitability, spike shape, and frequency. The low-threshold fast-activated Kv1 channels play a highly localized role in shaping the axonal AP at the AIS of cortical pyramidal neurons [26] or in dampening near-threshold excitability in fast-spiking cortical GABAergic interneurons [27], likely depending on Kv1 subunit composition in the different neuronal cell types. The slow inactivation of Kv1.2 enriched at the AIS of spinal motoneurons promotes the nonlinear spiking associated with the rhythmic locomotor activity [28]. However, their role at the nodes of Ranvier is a matter of speculation. The fast Kv1.1/1.2 channels are sequestered along myelinated axons on both sides of the node of Ranvier at the juxtaparanodes under the myelin sheath and do not normally influence AP repolarization. Kv1 channels are separated from the nodal gap by septate-like paranodal junctions anchoring the terminal myelin loops to the axolemma. The transmembrane septate-like junctions and the short paranodal width (3–5 nm instead of 10–20 nm at internode) are thought to reduce the current flows between nodal and internodal extracellular spaces. An intriguing observation is that the juxtaparanodal Kv1 channels are forming rosette particles aligned with Connexin29 channels in apposed myelin membrane as observed in freeze-fracture replica immunogold labeling of mouse sciatic nerves[29]. This would support a leak K
+
conductance directly from juxtaparanodal axoplasm into the myelin cytoplasm that would not be dependent on voltage-gating. In the CNS, the inward-rectifying Kir4.1 channels are expressed by oligodendrocytes on the inner tongue of the myelin sheath facing the internodal axonal membrane. These channels ensure that the K
+ released during neuronal firing is buffered by oligodendrocytes [30,31].
released during neuronal firing is buffered by oligodendrocytes [30][31].
Kv channels are the most diverse family of voltage-gated ion channels in vertebrates of which a significant proportion plays a critical role in controlling neuronal excitability and is of major relevance to neurological diseases. In particular, mutations of genes encoding Kv1 and Kv7 subfamily channels have been associated with a range of epilepsies and encephalopathies. Their pathogenic role is complex and partly defined by the axonal distribution of those channels [11,32,33]. This review will particularly focus on the role of Kv1.1/1.2 channels in physiological conditions and de- or dys-myelinating autoimmune diseases. The Shaker-related Kv1 subfamily comprises at least eight members (Kv1.1–Kv1.8). The α-subunits from the same subfamily assemble to form functional homo- or hetero-tetrameric channels (
Kv channels are the most diverse family of voltage-gated ion channels in vertebrates of which a significant proportion plays a critical role in controlling neuronal excitability and is of major relevance to neurological diseases. In particular, mutations of genes encoding Kv1 and Kv7 subfamily channels have been associated with a range of epilepsies and encephalopathies. Their pathogenic role is complex and partly defined by the axonal distribution of those channels [11][32][33]. This review will particularly focus on the role of Kv1.1/1.2 channels in physiological conditions and de- or dys-myelinating autoimmune diseases. The Shaker-related Kv1 subfamily comprises at least eight members (Kv1.1–Kv1.8). The α-subunits from the same subfamily assemble to form functional homo- or hetero-tetrameric channels (
A). The juxtaparanodes of myelinated axons contain generally heteromeric Kv1.1 and Kv1.2 channels associated with Kvß2 auxiliary subunits. The physiological role of these channels may be critical during development as their mature localization pattern is not achieved at the onset of myelination. Kv1.1/ Kv1.2 are first present at the node and paranodes in immature nerves when the axo-glial septate-like junctions are not fully established (
Figure 1B) [34,35,36]. This transient localization may prevent aberrant excitations during the transition between continuous to saltatory conduction. Importantly, the juxtaparanodal Kv1 channels may also play a critical function in pathological situations when the paranodal junctions are altered and the myelin sheaths retracted.
B) [34][35][36]. This transient localization may prevent aberrant excitations during the transition between continuous to saltatory conduction. Importantly, the juxtaparanodal Kv1 channels may also play a critical function in pathological situations when the paranodal junctions are altered and the myelin sheaths retracted.
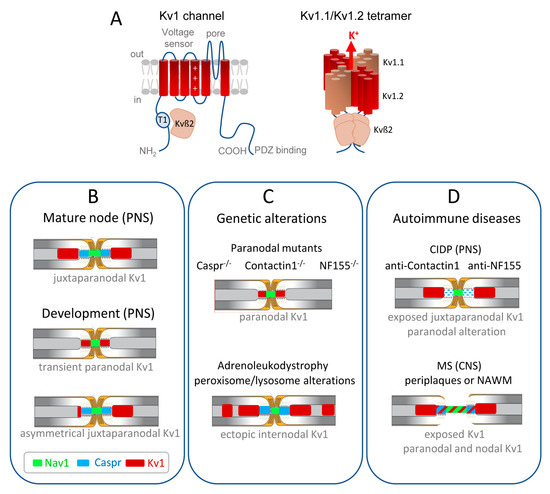
Figure 1.
Distribution of the Kv1.1/1.2 channels in myelinated axons during development and misdistribution associated with genetic or autoimmune diseases. (
A
) (
Left
) Transmembrane topology of one Kv1 channel α-subunit, with the voltage-sensing module comprising transmembrane segments S1–S4 with the positive charges shown in S4, and the pore module comprising S5–S6. The T1 tetramerization domain is located in the N-terminal tail and the C-terminus contains a binding site for PDZ-proteins. The Kv1 α-subunit associates with an auxiliary Kvß2 subunit that is essential for regulation via its T1 linker. (
Right
) Juxtaparanodal Kv1 channels are composed of heterotetramers of Kv1.1 and Kv1.2 α-subunits forming the pore of the channel co-assembled with four Kvß2 subunits. (
B
) The Kv1 channels are transiently present at the paranodes in immature nerves when the axo-glial septate-like junctions are not fully established. In the PNS, the juxtaparanodes are first assembled asymmetrically before being settled on both sides of the paranodes. (
C
) Kv1 channels are mislocalized at the paranodes when the septate-like junctions are disrupted, as shown in mice deficient for the cell adhesion molecules, Caspr, Contactin, or Neurofascin155. Ectopic internodal clusters of Kv1 channels are observed associated with the alteration of myelin ganglioside in a mice model of X-adrenoleukodystrophy. (
D
) In autoimmune demyelinating neuropathy (CIDP), a subset of patients produce anti-Contactin or anti-Neurofascin155 IgG4 that induce the selective loss of paranodal transverse bands, but the distribution of Kv1 is unknown. In multiple sclerosis (MS) patients, early paranodal alterations occur as observed at the border of MS lesions in periplaques and normal-appearing white matter (NAWM), with the Kv1 channels abutting or even overlapping the nodal region.
References
- Rasband, M.N. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 2010, 11, 552–562.
- Normand, E.A.; Rasband, M.N. Subcellular patterning: Axonal domains with specialized structure and function. Dev. Cell 2015, 32, 459–468.
- Salzer, J.L. Switching myelination on and off. J. Cell Biol. 2008, 181, 575–577.
- Susuki, K.; Chang, K.J.; Zollinger, D.R.; Liu, Y.; Ogawa, Y.; Eshed-Eisenbach, Y.; Dours-Zimmermann, M.T.; Oses-Prieto, J.A.; Burlingame, A.L.; Seidenbecher, C.I.; et al. Three mechanisms assemble central nervous system nodes of ranvier. Neuron 2013, 78, 469–482.
- Eshed-Eisenbach, Y.; Peles, E. The making of a node: A co-production of neurons and glia. Curr. Opin. Neurobiol. 2013, 23, 1049–1056.
- Rasband, M.N.; Peles, E. The nodes of ranvier: Molecular assembly and maintenance. Cold Spring Harb. Perspect. Biol. 2015, 8, a020495.
- Leterrier, C. The axon initial segment, 50years later: A nexus for neuronal organization and function. Curr. Top. Membr. 2016, 77, 185–233.
- Ghosh, A.; Sherman, D.L.; Brophy, P.J. The axonal cytoskeleton and the assembly of nodes of ranvier. Neuroscientist 2018, 24, 104–110.
- Rhodes, K.J.; Strassle, B.W.; Monaghan, M.M.; Bekele-Arcuri, Z.; Matos, M.F.; Trimmer, J.S. Association and colocalization of the kvbeta1 and kvbeta2 beta-subunits with kv1 alpha-subunits in mammalian brain k+ channel complexes. J. Neurosci. 1997, 17, 8246–8258.
- Devaux, J.J.; Kleopa, K.A.; Cooper, E.C.; Scherer, S.S. Kcnq2 is a nodal k+ channel. J. Neurosci. 2004, 24, 1236–1244.
- Trimmer, J.S. Subcellular localization of k+ channels in mammalian brain neurons: Remarkable precision in the midst of extraordinary complexity. Neuron 2015, 85, 238–256.
- Inda, M.C.; DeFelipe, J.; Munoz, A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc. Natl. Acad. Sci. USA 2006, 103, 2920–2925.
- Pan, Z.; Kao, T.; Horvath, Z.; Lemos, J.; Sul, J.Y.; Cranstoun, S.D.; Bennett, V.; Scherer, S.S.; Cooper, E.C. A common ankyrin-g-based mechanism retains kcnq and nav channels at electrically active domains of the axon. J. Neurosci. 2006, 26, 2599–2613.
- Devaux, J.; Alcaraz, G.; Grinspan, J.; Bennett, V.; Joho, R.; Crest, M.; Scherer, S.S. Kv3.1b is a novel component of cns nodes. J. Neurosci. 2003, 23, 4509–4518.
- Grundemann, J.; Clark, B.A. Calcium-activated potassium channels at nodes of ranvier secure axonal spike propagation. Cell Rep. 2015, 12, 1715–1722.
- Hirono, M.; Ogawa, Y.; Misono, K.; Zollinger, D.R.; Trimmer, J.S.; Rasband, M.N.; Misonou, H. Bk channels localize to the paranodal junction and regulate action potentials in myelinated axons of cerebellar purkinje cells. J. Neurosci. 2015, 35, 7082–7094.
- Brohawn, S.G.; Wang, W.W.; Handler, A.; Campbell, E.B.; Schwarz, J.R.; MacKinnon, R. The mechanosensitive ion channel traak is localized to the mammalian node of ranvier. Elife 2019, 8, e50403.
- Kanda, H.; Ling, J.; Tonomura, S.; Noguchi, K.; Matalon, S.; Gu, J.G. Trek-1 and traak are principal k+ channels at the nodes of ranvier for rapid action potential conduction on mammalian myelinated afferent nerves. Neuron 2019, 104, 960–971.
- Battefeld, A.; Tran, B.T.; Gavrilis, J.; Cooper, E.C.; Kole, M.H. Heteromeric kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J. Neurosci. 2014, 34, 3719–3732.
- Schwarz, J.R.; Glassmeier, G.; Cooper, E.C.; Kao, T.C.; Nodera, H.; Tabuena, D.; Kaji, R.; Bostock, H. Kcnq channels mediate i(ks), a slow k(+) current regulating excitability in the rat node of ranvier. J. Physiol.-Lond. 2006, 573, 17–34.
- Zhou, D.; Lambert, S.; Malen, P.L.; Carpenter, S.; Boland, L.M.; Bennett, V. Ankyring is required for clustering of voltage-gated na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998, 143, 1295–1304.
- Zhou, L.; Messing, A.; Chiu, S.Y. Determinants of excitability at transition zones in kv1.1-deficient myelinated nerves. J. Neurosci. 1999, 19, 5768–5781.
- Tomassy, G.S.; Berger, D.R.; Chen, H.H.; Kasthuri, N.; Hayworth, K.J.; Vercelli, A.; Seung, H.S.; Lichtman, J.W.; Arlotta, P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 2014, 344, 319–324.
- Micheva, K.D.; Wolman, D.; Mensh, B.D.; Pax, E.; Buchanan, J.; Smith, S.J.; Bock, D.D. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife 2016, 5, e15784.
- Stedehouder, J.; Couey, J.J.; Brizee, D.; Hosseini, B.; Slotman, J.A.; Dirven, C.M.F.; Shpak, G.; Houtsmuller, A.B.; Kushner, S.A. Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb Cortex 2017, 27, 5001–5013.
- Kole, M.H.; Letzkus, J.J.; Stuart, G.J. Axon initial segment kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 2007, 55, 633–647.
- Goldberg, E.M.; Clark, B.D.; Zagha, E.; Nahmani, M.; Erisir, A.; Rudy, B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking gabaergic interneurons. Neuron 2008, 58, 387–400.
- Bos, R.; Harris-Warrick, R.M.; Brocard, C.; Demianenko, L.E.; Manuel, M.; Zytnicki, D.; Korogod, S.M.; Brocard, F. Kv1.2 channels promote nonlinear spiking motoneurons for powering up locomotion. Cell Rep. 2018, 22, 3315–3327.
- Rash, J.E.; Vanderpool, K.G.; Yasumura, T.; Hickman, J.; Beatty, J.T.; Nagy, J.I. Kv1 channels identified in rodent myelinated axons, linked to cx29 in innermost myelin: Support for electrically active myelin in mammalian saltatory conduction. J. Neurophysiol. 2016, 115, 1836–1859.
- Larson, V.A.; Mironova, Y.; Vanderpool, K.G.; Waisman, A.; Rash, J.E.; Agarwal, A.; Bergles, D.E. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. Elife 2018, 7, e34829.
- Schirmer, L.; Mobius, W.; Zhao, C.; Cruz-Herranz, A.; Ben Haim, L.; Cordano, C.; Shiow, L.R.; Kelley, K.W.; Sadowski, B.; Timmons, G.; et al. Oligodendrocyte-encoded kir4.1 function is required for axonal integrity. Elife 2018, 7, e34829.
- Greene, D.L.; Hoshi, N. Modulation of kv7 channels and excitability in the brain. Cell. Mol. Life Sci. 2017, 74, 495–508.
- Allen, N.M.; Weckhuysen, S.; Gorman, K.; King, M.D.; Lerche, H. Genetic potassium channel-associated epilepsies: Clinical review of the kv family. Eur. J. Paediatr. Neurol. 2020, 24, 105–116.
- Vabnick, I.; Trimmer, J.S.; Schwarz, T.L.; Levinson, S.R.; Risal, D.; Shrager, P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J. Neurosci. 1999, 19, 747–758.
- Poliak, S.; Gollan, L.; Salomon, D.; Berglund, E.O.; Ohara, R.; Ranscht, B.; Peles, E. Localization of caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon. J. Neurosci. 2001, 21, 7568–7575.
- Hivert, B.; Pinatel, D.; Labasque, M.; Tricaud, N.; Goutebroze, L.; Faivre-Sarrailh, C. Assembly of juxtaparanodes in myelinating drg culture: Differential clustering of the kv1/caspr2 complex and scaffolding protein 4.1b. Glia 2016, 64, 840–852.
