The ligand, 4'-(p-(1,5-bis(amino)-3-azapentane)methylphenyl)-2,2':6',2"-terpyridine (dint) contains a terpyridine fragment and a tri-amine fragment, which may allow synthetic differentiation between the tridentate binding sites at both sides and may offer the benefits of polydentate ligands and minimisation of isomeric possibilities upon coordination to metal ions.
- bridging ligand
- terpyridine
- polyamine
- Bridging ligand
- Terpyridine
- Polyamine
4'-(p-(1,5-bis(amino)-3-azapentane)methylphenyl)-2,2':6',2"-terpyridine (dint)
1. Introduction
Ramin Zibaseresht1,2*
1 Faculty of Sciences, Maritime University of Imam Khomeini, Noshahr, Iran. Email: rzi12@uclive.ac.nz
2 Biomaterials and Medicinal Chemistry Research Centre, Aja University of Medical Sciences, Tehran, Iran. Email: rzi12@uclive.ac.nz
Introduction:
We have begun a research programme aimed at the synthesis of heterodinuclear complexes. In these studies, we are focusing on the preparation and use of ditopic ligands where the two metal ion binding sites are differentiated by the number of donor atoms in each site, the configuration of the binding site, or the types of donor atom that are present in the sites. This binding site differentiation offers the prospect of using the different coordination properties of the binding sites to control the regiochemistry of complexation, ensuring that the correct metal ion is incorporated at the correct binding site in the ligand [1].
2. Application
Application:
In order to prepare heterodinuclear metal complexes, or a variety of inorganic polymers, the ligand acts as linker between the metal atoms. Those who are particularly interested in minimising the number of stereoisomers that might be produced on synthesis of the heterodinuclear systems, this type of linker can be one of their choices.
3. Ligand Synthesis
Ligand Synthesis:
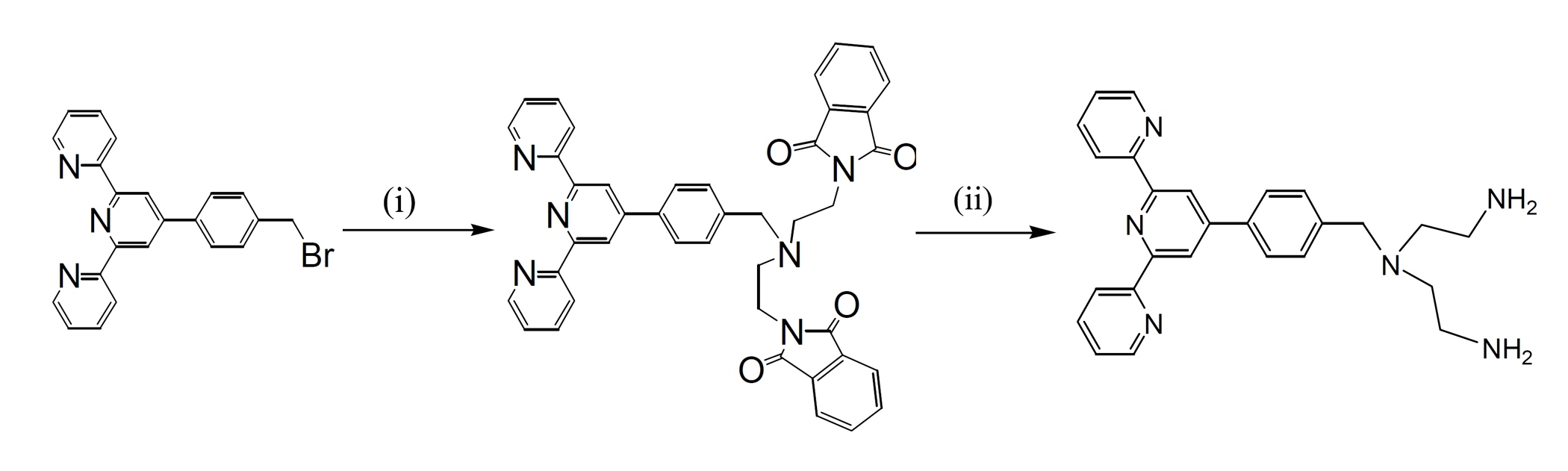
Ditopic ligands 4'-(p-(1,5-bis(phthalimido)-3-azapentan-3-yl)methylphenyl)-2,2':6',2"-terpyridine (bpat), based on tridentate planar terpyridyl systems has been synthesised in relatively high yield. The ligand was prepared using the method by nucleophilic substitution reaction of 4’-(p-bromomethylphenyl)-2,2’:6’,2’’-terpyridine with the appropriate secondary or tertiary amines in single- or multi-step reactions.
Scheme 1. Syntheses of ligand dint. (i) 1,5-bis(phthalimido)-3-azapentane, K2CO3, dry CH3CN, 3 days, 60o C; (ii) ethylenediamine, MeOH, overnight, r.t.
The ditopic ligand 4'-(p-(1,5-bis(amino)-3-azapentan-3-yl)methylphenyl)-2,2':6',2"-terpyridine (dint), based on tridentate planar terpyridyl systems (Scheme 1) has been synthesised in relatively high yields. The ligand shown in Scheme 1 were all prepared using nucleophilic substitution reaction of 4’-(p-bromomethylphenyl)-2,2’:6’,2”-terpyridine, with the appropriate amine in multi-step reaction.
The reactions were carried out in dry CH3CN and in the presence of K2CO3 to afford the desired products in excellent yields (95-98%). The reaction mixtures were monitored by 1H NMR spectroscopy and thin layer chromatography (TLC) in order to determine the optimum conditions. It was noted that prolonged reactions (up to 3 days) and also using 1.2 equivalents of the amines resulted in analytically pure products, with the best yields. The ligand bptt was then converted to ligand dint by deprotection using ethylenediamine, in MeOH, in nearly quantitative yield. All products were characterised using 1H and 13C NMR spectroscopy, COSY, NOESY, HSQC, and electrospray ionisation mass spectrometry.
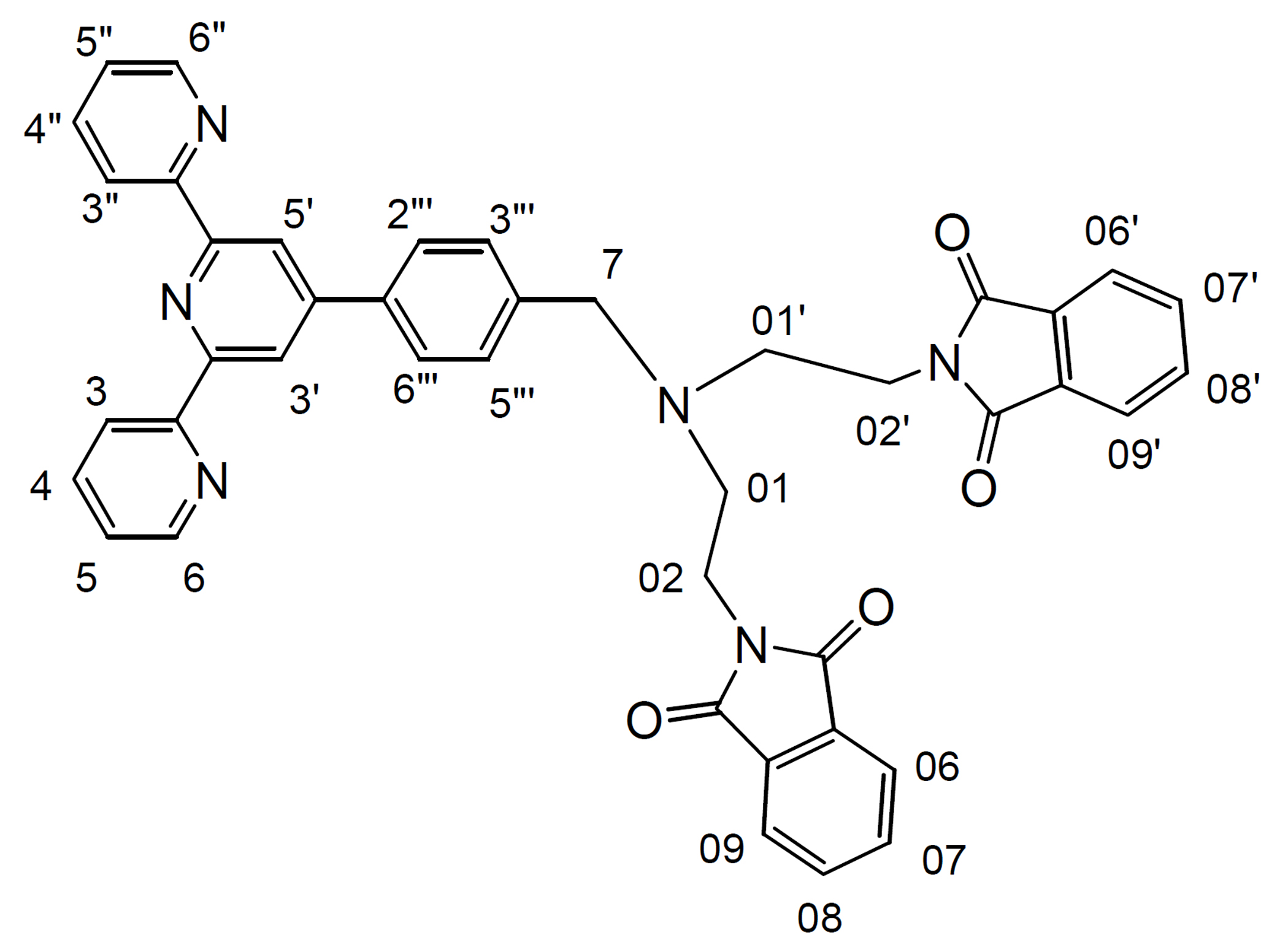
(1) 4'-(p-(1,5-bis(phthalimido)-3-azapentane)methylphenyl)-2,2':6',2"-terpyridine (bptt)
A mixture of 1,5-bis(phthalimido)-3-azapentane, (0.43 g, 1.2 mmol) and 4'-(p-bromomethylphenyl)-2,2':6',2"-terpyridine (btp) (0.4 g, 1.0 mmol), and anhydrous K2CO3 (1.4 g, 10 mmol) in dry CH3CN (50 mL) was stirred at 55-60° C under argon for 3 days. The progress of the reaction was monitored by TLC. The reaction mixture was then allowed to cool at room temperature; filtered and the solvent was removed from the filtrate under reduced pressure to give a pale yellow oil. To the crude product was added water (100 mL); then was extracted with CH3Cl (3 × 100 mL). After drying over MgSO4, it was filtered and the solvent was removed from the filtrate to afford the analytically pure desired product (0.67 g, 98%) as a non-solid material. 1H NMR (500 MHz; solvent CDCl3): δ 8.77 (2H, d, H6, H6"), 8.68 (2H, d, H3, H3") ,8.60 (2H, s, H3', H5'), 7.90-7.86 (4H, m, H4, H4"), 7.74-7.69 (8H, m, H06-09, H06'-09'), 7.39 (2H, d, H2"', H6"'), 7.36 (2H, m, H5, H5"), 7.17 (2H, d, H3"', H5"'), 3.79 (4H, m, H02, H02'), 3.69 (2H, s, H7), 2.84 (4H, m, H01, H01'). 13C NMR (75 MHz; solvent CDCl3): δ 168.20 (4C, C04), 156.27, 155.72, 149.76, 149.06 (2C, C6, C6"), 140.12, 136.81 (2C, C4, C4"), 136.59, 133.71 (4C, aromatic phthalimide), 132.31 (4C), 129.57 (2C, C3"', C5"'), 126.76 (2C, C2"', C6"'), 123.77 (2C, C5, C5"), 123.00 (4C, aromatic phthalimide), 121.27 (2C, C3, C3"), 118.64 (2C, C3', C5'), 57.76 (C7), 51.91 (2C, C01, C01'), 35.71 (2C, C02, C02'). IR (KBr): /cm-1 = 3051 w, 3016 w, 2943 w, 2822 m, 1773 s, 1711 ssh, 1603 m, 1583 s, 1568 m, 1542 m, 1514 w, 1468 m, 1431 m, 1396 ssh, 1327 m, 1265 w, 1190 w, 1170 w, 1159 w, 1086 s, 1038 m, 1018 w, 991 w, 966 w, 895 w, 872 w, 831 w, 791 s, 746 m, 719 ssh, 687 w, 660 m, 621 m, 530 m, 478 w, 469 w, 415 w. ESI-MS: m/z 684.9885 ([M+H]+, 100%).
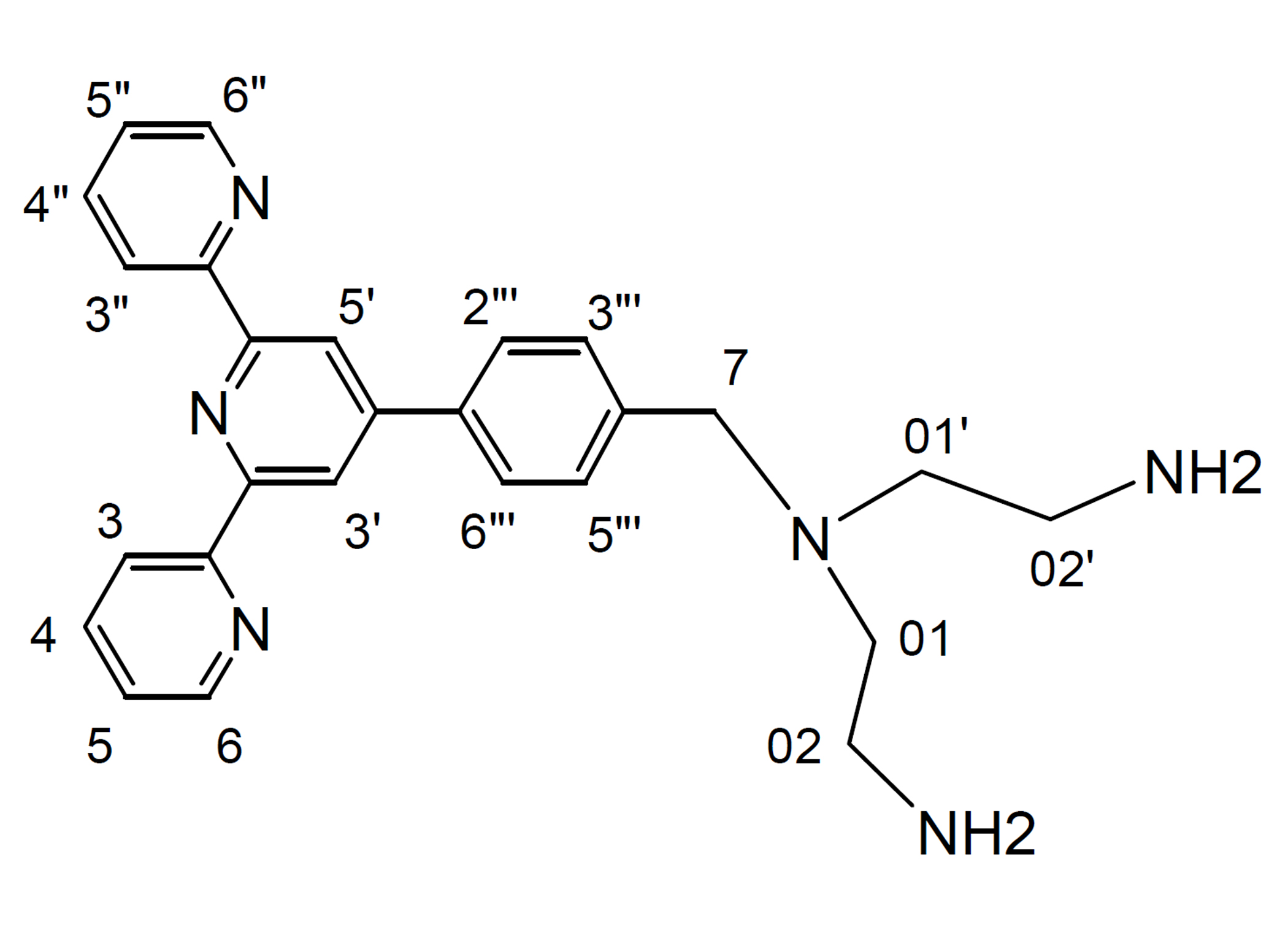
(2) 4'-(p-(1,5-bis(amino)-3-azapentane)methylphenyl)-2,2':6',2"-terpyridine (dint)
Compound bptt (0.5 g, 0.73 mmol) in CH2Cl2 (25 mL) was added into a MeOH solution of en (0.5 M, 25 mL), and the mixture was stirred for 48 hr at room temperature, filtered, and the filtrate was concentrated to dryness to give a yellow oil material. The mixture was dissolved into CHCl3 (50 mL). The CHCl3 solution was washed with 3% NH4OH solution (3 × 50 mL), and the organic layer was dried over MgSO4, filtered, the filtrate was concentrated to dryness to give a yellow non-solid material in a quantitative yield. 1H NMR (500 MHz; solvent CDCl3): δ 8.725 (2H, s, H3', H5'), 8.72 (2H, d, H6, H6"), 8.67 (2H, d, H3, H3"), 7.88-7.83 (4H, m, H4, H4", H2"', H6"'), 7.69 (2H, m, H06, H06', 7.44 (2H, d, H3"', H5"'), 7.34 (2H, m, H5, H5"), 3.66 (2H, s, H7), 2.79 (4H, m, H02, H02'), 2.56 (4H, m, H01, H01'), 1.92 (4H, b, NH2). 13C NMR (75 MHz; solvent CDCl3): δ 156.17 (2C), 155.84 (2C), 149.94, 149.06 (2C, C6, C6"), 140.55, 137.19, 136.81 (2C, C4, C4"), 129.35 (2C, C3"', C5"'), 127.23 (2C, C2"', C6"'), 123.76 (2C, C5, C5"), 121.29 (2C, C3, C3"), 118.73 (2C, C3', C5'), 58.92 (C7), 57.08 (2C, C01, C01'), 39.66 (2C, C02, C02'). IR (KBr): /cm-1 = 2802 brs,1653 m, 1585 s, 1568 m, 1541 m, 1516 m, 1470 shs, 1443 w, 1418 w, 1391 m, 1315 w, 1265 w, 1115 brs, 1040 w, 1016 w, 991, w, 895 m, 831 w, 791 shs, 741 m, 719 w, 687 m, 617 m, 579 w, 525 w, 509 w,486 w, 469 w, 459 w, 440 w, 419 m. ESI-MS: m/z 213.1583 ([M+2H]2+, 100%), 425.3123 ([M+H]+, 9%).
Reference:
- Ramin Zibaseresht, 2006, PhD Thesis, University of Canterbury, New Zealand.