Metabolic reprogramming (MR) is an upregulation of biosynthetic and bioenergetic pathways to satisfy increased energy and metabolic building block demands of tumors. This includes glycolytic activity, which deprives the tumor microenvironment (TME) of nutrients while increasing extracellular lactic acid.
- bladder
- urothelial cancer
- metabolic reprogramming
- immunotherapy
1. Introduction
Worldwide, there were approximately 550,000 new cases and 200,000 deaths from bladder cancer (BC) in 2018 [1]. Up to 90% of BC cases originate from the luminal urothelial lining of the bladder and produce urothelial carcinomas (UC). Non-muscle invasive UC (NMIUC) accounts for 75% of BC patients and is commonly treated by transurethral tumor resection (TUR) with and without adjuvant intravesical instillations [2]. In contrast, muscle-invasive UC (MIUC) is treated with either cisplatin-based neoadjuvant chemotherapy (NAC) followed by surgical removal of the bladder (cystectomy) or external beam radiotherapy with or without chemotherapy. Despite extensive treatment, half of MIUC patients will progress to metastatic urothelial carcinoma (mUC) [3]. First-line treatment option in mUC is gemcitabine + cisplatin ("“gem/cis"”) [3][4][3,4]. However, 30% of mUC patients are cisplatin-ineligible due to poor performance status and other comorbidities [5][6][5,6]. Cisplatin-ineligible patients, mostly due to renal compromise, are treated with gemcitabine + carboplatin ("“gem/carbo"”), which is less effective than cisplatin combinations [7]. Regardless of the platinum-based chemotherapy used, most mUC patients will ultimately progress [8]. In recent years, immune checkpoint therapy (ICT) has emerged as a new option for platinum-relapsed or cisplatin-ineligible patients [3]. ICT targets cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed death (ligand) 1 (PD-1/PDL1), used by tumor cells to inhibit anticancer immune responses [9]. ICT has shown superior efficacy over 2nd line chemotherapy in platinum-relapsed mUC patients [10][11][12][10,11,12]. Currently, several PD-1/PD-L1 inhibitors have been FDA, and EMA approved for the treatment of mUC in the first line (no prior platinum-based chemotherapy) and/or second-line (after the failure of platinum-based chemotherapy) [10][11][12][13][14][10,11,12,13,14]. Approved agents used PD-L1 inhibitors: atezolizumab, durvalumab, and avelumab, and PD-1 inhibitors: nivolumab and pembrolizumab [10][11][12][10,11,12]. Treatment of platinum-relapsed mUC patients with pembrolizumab, nivolumab, or atezolizumab was associated with an ORR of ~20% [10][11][12][10,11,12]. Robust biomarkers that can predict clinical response to ICT are lacking due to the complexity of tumor-immune interactions that contribute to ICT resistance [15]. However, it was found recently that a mechanism associated with resistance to ICT is metabolic competition between immune cells and cancer cells in the tumor microenvironment (TME) [16][17][16,17].
2. Glucose Metabolism in Urothelial Carcinoma
Glucose metabolism produces energy in the form of ATP and precursor metabolites used for biosynthesis. Glucose metabolism starts with a process called glycolysis that consists of stepwise conversions of glucose that ultimately generates pyruvate. The rate of glycolysis is regulated by hexokinase (HK), glucose-6-phosphate dehydrogenase (G6PD), phosphofructokinase (PFK), and pyruvate kinase (PK) (Figure 1). Pyruvate participates in the tricarboxylic acid (TCA) cycle, also known as the citric acid cycle (CAC), or Krebs cycle, where it is ultimately oxidized into water and carbon dioxide. This oxygen-dependent process is called oxidative phosphorylation and ultimately produces 32–38 ATP molecules from one glucose molecule.
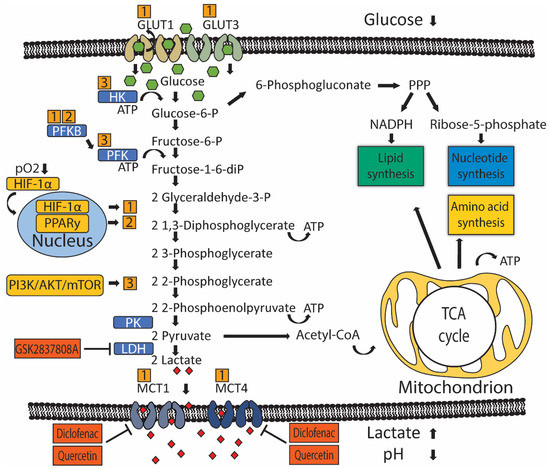
Figure 1.
In the absence of oxygen, pyruvate is transformed into lactate, producing 2 ATP molecules for every glucose molecule. In cancer cells, metabolic reprogramming (MR) refers to an upregulation of biosynthetic and bioenergetic pathways to produce the necessary materials and energy required for tumor growth. MR includes a shift in glucose metabolism from oxidation to glycolysis despite the presence of oxygen. This is commonly known as aerobic glycolysis [18] [18] or the Warburg effect and favors the usage of glucose'’s carbon atoms for gaining biomass (i.e., metabolic building blocks) over energy (i.e., ATP) production. Because cancer cells also need more energy, yet this arrangement is energy inefficient, there is a compensatory increase in glucose consumption [19]. Glucose uptake is seen in UC by way of positron emission tomography/computed tomography (PET/CT), which uses radioactively labeled glucose-analog fluorodeoxyglucose (18F-FDG) to visualize primary tumors and metastases [3]. Human UC cell lines also show increased uptake of glucose compared to untransformed urothelial cells and produce increased levels of pyruvate and lactate [20]. Evaluation of the patient'’s UC tumor samples indicates glucose quantity was significantly lower compared to normal urothelium [21]. Furthermore, late TCA cycle intermediates were also increased in UC, suggesting flux into the TCA cycle to replenish intermediates extracted from the cycle for biosynthesis—a process called anaplerosis [21]. A significant increase of ribose, the end-product of the pentose phosphate pathway (PPP), was also observed in UC, suggesting upregulation of the PPP [21]. The PPP occurs in the cytosol and consists of an oxidative phase that produces NADPH, which is required for reductive processes such as fatty acid synthesis and scavenging of reactive oxygen species, and a non-oxidative phase that produces pentoses like ribose, which are important precursors for nucleotide synthesis. Therefore, the PPP helps metabolically active or proliferating cells to meet their anabolic demands and combat oxidative stress [22][23][22,23]. Thus, UC alters its metabolism and consumes glucose to produce energy via glycolysis, biomass through PPP and anaplerosis, and to counter oxidative stress through PPP.
3. Regulation of Glucose Transport and Metabolism in UC
Cellular glucose utilization is regulated by oxygen-dependent and oxygen-independent mechanisms that rely on several common glucose transporters and glycolytic enzymes (Figure 1). Oxygen-dependent mechanisms are mediated by transcription factor hypoxia-inducible factor 1-alpha (HIF-1α) [24][25][24,25]. Low oxygen tension stabilizes HIF-1α protein expression, which translocates to the nucleus and binds to target genes, thereby upregulating gene expression [26][27][26,27]. HIF-1α indirectly stimulates glycolysis through inhibition of mitochondrial biogenesis and oxygen consumption through induction of pyruvate dehydrogenase kinase 1 (PDK1), which subsequently inhibits pyruvate dehydrogenase from catalyzing oxidative decarboxylation of pyruvate [28][29][28,29]. The steroid receptor coactivator-3 (SRC-3) is a HIF-1α co-activator required for the expression of several HIF1-1α target genes in T24 UC cells under hypoxia [30]. Another HIF-1α co-activator is histone demethylase JMJD1A, whose H3K9 demethylase activity is required at promotor sites to induce expression of several key glycolytic enzymes [31]. Interestingly, JMJD1A was found upregulated in 46 UC patient samples, compared to 14 normal bladder samples [31]. In summary, UC has higher levels of HIF-1α co-activators, which leads to more glycolysis and reduced oxidative phosphorylation.
Oxygen-independent mechanisms of glucose utilization in UC are primarily mediated through activation of the PI3K/AKT/mTOR pathway [32][33][32,33]. The PI3K/AKT/mTOR pathway consists of activators: phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT), mammalian target of rapamycin (mTOR), and PI3K-inhibitor: phosphatase and tensin homolog (PTEN). Mutations in genes of the PI3K/AKT/mTOR pathway are present in 42/131 (38%) patients with MIUC [34]. Besides activating mutations, other factors may also promote PI3K/AKT/MTOR signaling in UC. For example, microRNA 21 (Mir-21) activates PI3K/AKT/mTOR signaling through inhibition of PTEN expression in UC cell line T24, thereby stimulating glycolysis [32][35][32,35]. Furthermore, long non-coding RNA UCA1 is associated with mTOR-mediated glucose consumption and lactate production in 5637 human bladder carcinoma cells, although no direct interaction between mTOR and UCA1 was demonstrated [33].
Peroxisome proliferator-activated receptor gamma (PPARy) has been implicated as a driver of oxygen-independent activation of glycolysis in breast cancer and hepatocellular carcinoma murine models through transcriptional activation of key glycolytic enzymes [36][37][36,37]. In UC, the increased transcriptional activity of PPARy was associated with increased mRNA expression of glycolytic enzymes and decreased recurrence-free survival in a subset of 140 non-invasive (pTa) bladder tumors [38]. Interestingly, PPARy, like PI3K/AKT/mTOR signaling, is commonly associated with the luminal subtype of MIUC, which has a relatively good prognosis [39][40][41][39,40,41].
Increased glycolytic flux is associated with increased glucose uptake by glucose transporters. Glucose transporter 1 (GLUT1) is the primary glucose transporter overexpressed in cancer [42]. Expression of GLUT3 has also been demonstrated in T24 UC cells [43][44][43,44]. GLUT1 protein overexpression was associated with worse overall and disease-free survival in a pooled analysis of 4079 patients with various tumor types, not including UC [45]. GLUT1 expression evaluated by immunohistochemistry (IHC) in 105 BC samples was associated with an increased grade in both NMIUC and MIUC [46]. Furthermore, GLUT1 overexpression by IHC was an independent predictor of survival following radiotherapy (N = 64) or radical cystectomy (N = 279) for MIUC [47][48][47,48]. GLUT1 expression is generally induced by HIF-1α, indicating oxygen-dependent GLUT1 expression [49]. Likewise, GLUT1 and GLUT3 expression seem also to be controlled by microRNAs that function through altering PI3K/AKT/mTOR signaling in vitro [32]. Mir-218 was found to repress GLUT1 expression and, as a consequence, glucose uptake in T24 cells, while Mir-195-5p did the same for GLUT3 [43][44][43,44]. GLUT1 knockdown elevated intracellular reactive oxygen species (ROS) and increased cisplatin sensitivity in T24 cells [43].
Once glucose is imported into the cell, hexokinase (HK) (Figure 1) is the first rate-limiting enzyme controlling glycolytic flux. HK has four isoforms characterized by different functions and cellular locations. Isoform HK2 is linked to an anabolic function through PPP and has been implicated in UC MR [33][50][51][33,50,51]. T24 cells overexpress HK2 in response to PI3K/AKT/mTOR signaling [32]. Pharmacological inhibition of HK2 in UC cell line UM-UC-3 lowered glucose consumption and lactate production, accentuating a potential role in UC glucose metabolism [52].
UC cells also have upregulated phosphofructokinase (PFK) (Figure 1), which drives increased glycolytic flux. Somatic genetic aberrations that upregulate or amplify PFK family genes are present in ~40% of MIUC patients [50]. In vitro studies with UC cell lines, RT4, and TCCSUP suggested that PFK is primarily important during early phases of cancer progression, as PFK expression was higher in RT4 (representing early-stage, well-differentiated NMIUC) compared to TCCSUP (representing more progressed, anaplastic MIUC) [51]. Moreover, a lower PFK activity was associated with increased pyruvate consumption, implying that more progressed tumors start to directly metabolize pyruvate instead of glucose [51]. PFK is indirectly activated by one of four 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB) enzymes. PFKFB3 is expressed in T24, and knockdown led to decreased lactate production [53]. Another PFKFB family member, PFKFB4, was found expressed in 135 UC radical cystectomy samples [54]. High PFKFB4 expression assessed by IHC was associated with increased tumor stage and grade, and subsequent in vitro experiments demonstrated that PFKFB4 expression was induced during hypoxia in an HIF-1α-dependent manner [54].
The last step of glycolysis converts phosphoenolpyruvate (PEP) and ADP to pyruvate and ATP, and this step is catalyzed by pyruvate kinase (PK) isozymes M1/M2 (PKM1/M2) (Figure 1). UC cell lines have been shown to reexpress PKM2 [55].
In cancer cells, pyruvate is metabolized to lactate-by-lactate dehydrogenase (LDH), which reduces NADH to NAD+ in the same process. Lactate production replenishes cytosolic NAD+, allowing a continuous glycolytic flux [56][57][56,57]. Lactate produced by LDH is exported across the cell membrane by monocarboxylate transporters (MCT) in order to maintain an alkaline intracellular pH, favoring metabolism [58][59][58,59]. Tumor cells depend on MCT4 and, to a lesser extent, on MCT1 for lactate export, and MCT4 is expressed in a HIF-1α dependent manner [59][60][59,60]. MCT4 was overexpressed in approximately 50% of 360 UC patients, as assessed by IHC [61]. Moreover, MCT4 protein overexpression was an independent prognostic factor, predicting poor recurrence-free survival in NMIUC and MIUC patients treated with transurethral resection or radical cystectomy [61]. Likewise, MCT4 mRNA and protein expression predicted poor overall survival in MIUC patients treated with radical cystectomy [62]. Short interference RNA (siRNA) mediated silencing of MCT4 in UC cell lines, reduced proliferation rates, and increased ROS in a glucose-dependent manner [62]. Moreover, stable shRNA knockdown of MCT4 impaired tumor growth in an orthotopic UC xenograft model [62].
In conclusion, evidence shows that UC uses HIF-1α to increase glycolytic flux and to neutralize ROS via the upregulated activity of glucose importers (GLUT1, GLUT3), glycolytic enzymes (PFK), and lactate transporters (MCT4). Meanwhile, PI3K/AKT/mTOR signaling contributes to upregulating glycolytic enzymes (HK). Inhibiting glycolysis and lactate production may target UC either directly by impairing metabolic activity. However, most mechanistic evidence was gathered in small studies investigating parts of UC metabolism in a few human UC cell lines. More comprehensive preclinical investigation of UC metabolism in different stages of the disease is needed to increase the validity of these findings before translation into clinical trials.
