Trichuriasis is the clinical disease of animals infected with the parasite of the genus Trichuris. This review attempts to present information on Trichuris spp. infestation in neo-tropical rodents that are utilized for meat consumption by humans
- agouti
- lappe
1. Introduction
The neo-tropics is a geographical region located in the western hemisphere between the Tropic of Cancer and the Tropic of Capricorn. Geographical territories present within this zone include the southern parts of North America, all of Central America, the northern parts of South America, and all of the Caribbean[1]. Animals that are present in this region can be categorized into three groups: imported domesticated animals[2], domesticated animals originating from the neo-tropics[3], and non-domesticated neo-tropical animals[4]. For the purpose of this review, neo-tropical rodents that are included belong to the domesticated and non-domesticated groups. Domesticated neo-tropical rodents, such as the guinea pig, are utilized in South America for their meat and are reared in captivity to provide meat protein for rural villages. The guinea pig is able to utilize household waste and provide income and food for these communities[5][6]. Neo-tropical rodents on the verge of domestication are the agouti, lappe, and capybara. These animals have been reared in captivity in South America and the Caribbean for their meat [1]. These animals have been able to breed in captivity: the agouti produces four offspring per year [7] the lappe produces two offspring per year[8], and the capybara can produce eight offspring per year[9][10]. These animals are ideal in that they can utilize local feed resources and are adapted to local conditions of high heat and humidity. The meats produced by these rodents are highly nutritious, with high protein values and low fat and cholesterol concentration[11][12][13][14].
Trichuris
Trichuris
Figure 1) [16]. Animals become infected by the ingestion of infective eggs[16]. However, there has been limited information on the effects of
Trichuris
Trichuris that parasitizes these rodents, the effect of this parasite on these animals, and the zoonotic potential of this pathogen.
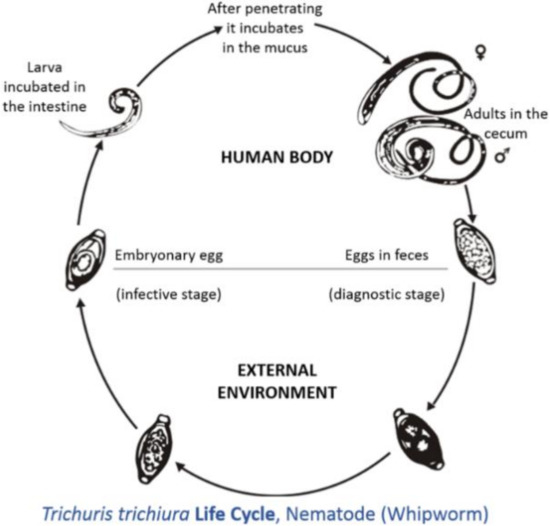
Figure 1.
Tichuris trichiura (taken from[17]).
2. Trichuris spp. of Veterinary and Public Health Importance
2.1. Trichuriasis of Man
Trichuriasis is one of the major infectious diseases of children in developing countries[18].
Trichuris trichiura is a major, soil-transmitted helminth targeted by the World Health Organization in their mass drug administration program for pre-school and primary school children in endemic developing countries[18]. There have been several cases of trichuriasis reported in humans. In some cases, it has been due to three
Trichuris
T. trichiura
T. vulpis
T. suis
T. vulpis, and the diagnosis was made based on the morphology of the eggs and vulva from an adult female[19]. Molecular techniques were used on
Trichuris
T. suis
T. trichiura in human populations from Thailand[20].
T. suis
Trichuris eggs 40 days post-infection[21]. Experimentally treated patients showed no symptoms of gastrointestinal distress[21]. In contrast to the previous studies, Kradin et al.[22] showed that iatrogenic infection with
T. suis
Trichuris trichiura has human and non-human primates as its natural hosts[23]. Mixed infections with various
Trichuris
T. vulpis
T. trichiura [24][25]. The identification of the species of
Trichuris spp. was based on the morphology of eggs[24] and polymerase chain reactions of the helminth eggs [25].
Trichuris trichiura
T. vulpis
Trichuris spp. to humans, but further work needs to be done to validate this finding[25].
T. vulpis have been reported in children and adults [19][26][27]. However, all cases of trichuriasis in humans caused by
T. vulpis have had some association with dogs, and the diagnosis was made based on morphology of eggs present in the feces. Clinical signs reported in humans are abdominal discomfort, epigastric pain, nausea, vomiting, diarrhea, and poor appetite[24]. Patients with
T. trichiura[19] have been treated with mebendazole and albendazole with improvements of clinical signs [19][24][26][27]. However, in vivo studies on albendazole and mebendazole have shown little efficacy against
T. trichiura[28]. At 14 days post-treatment, there was no difference in the disease prevalence seen between treatments of patients with 400 grams of albendazole [28]. Therefore, alternative anthelmintic treatment against
T. trichiura
Trichuris
2.2. Morphological and Molecular Identifications of Trichuris spp.
2.2.1. Morphological Identification of
2.2.1. Morphological Identification of
Trichuris spp. in Pigs, Dogs, Cats, Humans, and Non-Human Primates
Morphological analysis of
spp. in Pigs, Dogs, Cats, Humans, and Non-Human Primates
Trichuris
spp. has been used for identification within various host species.Trichuris trichiura
infection has been investigated in humans, non-human primates, and pigs, but based on morphological analysis, theT. trichiura found in humans and non-human primates were indistinguishable[29]. In pigs,
found in humans and non-human primates were indistinguishable [29]. In pigs,T. suis
was differentiated fromT. trichiura
, based on the lack of peri-cloacal papillae in adult specimens. In female specimens, there were no morphological differentiation betweenT. suis
andT. trichiura[29]. Ruminants evaluated in India using morphological analysis identified
[29]. Ruminants evaluated in India using morphological analysis identifiedT. ovis as the major parasite [30]. [
Further research was done in domestic cats in St. Kitts. Based on the size of the
as the major parasite [30].Trichuris
spp. identified, authors believed that it wasT. campanula
, but based on the vulva structure the authors confirmed it wasT. serrata
. In conclusion, the authors, identified the parasite asT. serrata, but recommended that molecular studies must be done in order to reliably identify this parasite[31]. In dogs, male and female adult
, but recommended that molecular studies must be done in order to reliably identify this parasite [31]. In dogs, male and female adultT. vulpis could be identified based on nine parameters (including body length, length of cuticular processes, and width of body at tail part)[32]. Male
could be identified based on nine parameters (including body length, length of cuticular processes, and width of body at tail part) [32]. MaleT. vulpis can be distinguished from other species by spicule sheath ornamentation (the dimensions of the spicule) [32].
Recently, the morphometric approach analyzing the adult worms and eggs of
can be distinguished from other species by spicule sheath ornamentation (the dimensions of the spicule) [32].Trichuris spp. of non-human primates were analyzed[33][34]. Morphometric data on the adult worms showed that features present in the females made them indistinguishable for species characteristics, but adult male worms may be used to differentiate
spp. of non-human primates were analyzed [33,34]. Morphometric data on the adult worms showed that features present in the females made them indistinguishable for species characteristics, but adult male worms may be used to differentiateTrichuris populations[33]. Geometric morphometric analysis is a new diagnostic tool that can be used to differential
populations [33]. Geometric morphometric analysis is a new diagnostic tool that can be used to differentialTrichuris spp. present in non-human primates. However, further data must be collected to determine the sensitivity and specificity of this diagnostic tool[34]. Combination of various techniques, such as the use of molecular and morphological analysis, should be performed for confirmation of various
spp. present in non-human primates. However, further data must be collected to determine the sensitivity and specificity of this diagnostic tool [34]. Combination of various techniques, such as the use of molecular and morphological analysis, should be performed for confirmation of variousTrichuris
spp. [33].2.2.2. Molecular Identification of Trichuris spp. in Domestic and Non-Domestic Ruminants
Trichuris
Trichuris
Trichuris
T. discolor
T. ovis
T. globulosa
T. skrjabini—have been identified as inhabiting the caecum and colon of ruminants[35][36][37][38][39][40][41][42][43][44][45][46]. One of the major discoveries was the identification of
T. globulosa
T. ovis as the same species by isoenzymes [35], using second, internally transcribed spacer ribosomal DNA (ITS2 rDNA)[38] and ITS1-5.8S-1TS2 [37]. Further molecular analysis was done comparing
T. ovis
T. discolor, where the entire mitochondrial DNA (mtDNA) was analyzed[42], and with the use of internally transcribed spacers 1, 2, and 16S, partial DNA sequencing (ITS1, 2, 16rDNA) was completed[44]. Based on mtDNA and rDNA,
T. ovis
T. discolor
Trichuris skrjabini
T. skrjabini
Trichuris
Trichuris discolor
Capreolus capreolus
Cervus nippon
Cervus elephus
Dama dama
Ovis orientalis musimon)[43][44][45]. In wild ruminants,
T. discolor was identified with use of ITS1-5.8S-1TS2[43][44][45], but in cattle different populations of
T. discolor
T. discolor
T. discolor
2.2.3. Molecular Identification of Trichuris spp. in Cats, Dogs, Pigs, Humans, and Non-Human Primates
Trichuris spp. has also been identified molecularly in pets, such as dogs and cats. In cats it is associated with typhlitis, which also occurs in other animals[46]. Identification of
T. serrata
Trichuris vulpis (dogs) was accomplished through the use of 18S rDNA (cats) and enzyme-linked immunosorbent assay (ELISA) and ITS1-5.8S-1TS2 (dogs)[47][48]. Comparative genetic studies were done of the
T. vulpis
T. suis
T. vulpis
T. suis
T. suis
Sus scrofa scrofa
Sus scrofa domestica) showed no sequential genetic differences[49].
T. suis found in pigs using isoenzymes[50], ITS 1 and ITS2 regions of rDNA [51], large mitochondrial subunits and ITS2[52], and nuclear ribosomes (18S, ITS2)[18]. Due to the zoonotic potential of
T. suis
T. trichiura previous molecular studies have been done in both human and non-human primates[53][54][55].
Trichuris
Colobus guereza kikuyensis
Nomascus gabriellae
T. suis
T. trichiura
Colobus guereza kikuyensis
Nomascus gabriellae[53]. Nissen et al. [54] conducted a similar study to Cutillas et al.[53], but
T. suis
T. trichiura
T. suis
T. trichiura
T. suis
Trichuris
The research done by Cutillas et al. [53]and Nissen et al.[54] highlights the fact that humans and non-human primates may be infected with several species of
Trichuris
T. trichiura.
Trichuris
Macaca fuscata
Trichuris
Figure 2). Ravasi et al.[57] investigated the genotype of human and non-human primates in Central Africa. Sequencing of the rDNA (ITS1-5.8S-1TS2) revealed two
Trichuris genotypes that infect both humans and non-primates[57]. Ghai et al.[58] found similar results to Ravasi et al.[57], but three
Trichuris
Colobus guereza
Cercopithecus mitis
Lophocebus albigena
Cercopithecus lhoesti
Papio anubis
Procolobus rufomitratus
Cercopithecus ascanius
Pan troglodytes
Trichuris
Colobus guereza
Procolobus rufomitratus)[58]. Furthermore, this new species of
Trichuris
Presbytis francoisi
Colobus guereza kikuyensis using mtDNA, rDNA, and morphometry[59][60].
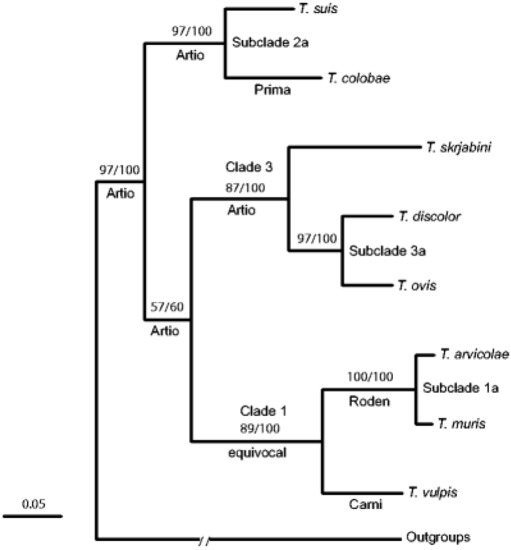
2.2.4. Molecular Identification Trichuris spp. in Rodents
Trichuris
Trichuris
Trichuris muris
Trichuris arvicolae have been found in Arvicolinae rodents using multi-local enzyme electrophoresis[62]and rDNA (ITS1-5.8S-ITS2)[63]. Further investigations were done in the phylogeographic analysis of
T. arvicolae
T. arvicolae
Trichuris
Trichuris novonae
T. pardinasi
Trichuris bainae was identified[66]. Molecular analysis using cox1 and mitochondrial cytochrome b (cob) on the
Trichuris
T. pardinasi
T. bainae
T. navonae)[67]. Further to this,
T. massoiai
Holochilus chacarius
Callejon et al. [41][69] investigated nuclear (18S, triose phosphate isomerase) and mitochondrial (cox1, cob1) genes from
Trichuris
(Colobus guereza kikuyuensis
Papio hamadryas
Homo sapiens
Sus scrofa domesticus
Capra hircus
Canis lupus familiaris
Bos taurus
Mus domesticus
Myodes glareolus
Trichuris
T. arvicolae
T. muris
T.vulpis
T. suis
T. colobae
T. trichiura
T
Papio hamadryas
T. discolor
T. ovis
T. skrjabini[69].
2.3. Immunomodulatory Effect of Trichuris spp.
Trichuris spp. has been used in the treatment of gastrointestinal autoimmune diseases, such as inflammatory bowel disease, Crohn’s disease, and ulcerative colitis [70][71][72].
Trichuris suis (pig whipworm) had been experimentally given to humans with no overt sign of gastrointestinal illness. The eggs produced from the feces remained constant, and only a low percentage of these eggs embryonated in vitro[21]. Some authors also noted that treatment of patients with inflammatory bowel disease, ulcerative colitis, and Crohn’s disease with
Trichuris suis showed improvement in gastrointestinal signs, and in the management of disease the subjects were given ova every three weeks [70][71][72]. Surprisingly, Kradin et al.[22] noted that a patient that underwent treatment for Crohn’s disease using
T. suis
T. suis
T. suis in rats[73]. The investigation of the use of excretory products of
T. suis
T. suis have immunomodulatory effects and can be used as candidates in the treatment of inflammatory bowel disease[73]. The use of ESPs from
T. suis
Trichuris trichiura
Trichuris muris [74][75][76]. Proteins were analyzed from adult worm extract and fragments of
T. trichiura. These extracts and fragments were placed in cell cultures of human peripheral blood monocytes, and elicited the production of IL-10, IL-12, and TNF-α. Some fractions showed the inhibition of IL-5 production. The downregulation of IL-5 is a feature of a Th-2 response[74]. Santos et al. [74] concluded that protein fractions of
T. trichiura
T. muris, and specific immunogenic proteins were identified. The structure of one such protein was Tm16, which was characterized and could be used in the production of a vaccine[75]. Shears et al.[76] noted that ESPs from
T. muris
T. muris
Trichuris
