Most investigations of iodine metabolism in humans and animals have focused on its role in thy-roid function. However, considerable evidence indicates that iodine could also be implicated in the physiopathology of other organs.
- molecular iodine
- antioxidant
- differentiator
- immune modulator
- cancer
- mitochondria
- peroxi-some proliferator-activated receptor (ppar)
1. Introduction
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Iodine in its different chemical forms is captured and used by practically all living beings and is considered a micronutrient in chordates. In vertebrates, iodine is a component of thyroid hormones that is essential for the proper development and functioning of several organs, primarily in the nervous system [1]. However, a significant amount of iodine in the body is non-hormonal and is concentrated in extra-thyroid tissues, where its biological function is barely understood [2]. Several groups have postulated that iodine may have an ancestral antioxidant function in all the cells that concentrate it, from primitive algae to the most recent vertebrates [3][4]. In these cells, oxidized iodine can act as an electron donor neutralizing reactive oxygen species (ROS) or attach to the double bonds of some polyunsaturated fatty acids in cell membranes, making them less reactive to ROS [5]. In addition, it has been shown that iodine binds to lipids, such as arachidonic acid (AA), exerts apoptosis, and/or has differentiation effects on diverse epithelial cells [6][7][8]. Moreover, iodine is uptake and metabolized by immune cells, and depending on the physiological context, this halogen can act as an anti-inflammatory or proinflammatory agent [9][10]. The distribution and action of iodine in organisms depend on the chemical form of iodine that is ingested. For example, molecular iodine (I
Iodine in its different chemical forms is captured and used by practically all living beings and is considered a micronutrient in chordates. In vertebrates, iodine is a component of thyroid hormones that is essential for the proper development and functioning of several organs, primarily in the nervous system [1]. However, a significant amount of iodine in the body is non-hormonal and is concentrated in extra-thyroid tissues, where its biological function is barely understood [2]. Several groups have postulated that iodine may have an ancestral antioxidant function in all the cells that concentrate it, from primitive algae to the most recent vertebrates [3,4]. In these cells, oxidized iodine can act as an electron donor neutralizing reactive oxygen species (ROS) or attach to the double bonds of some polyunsaturated fatty acids in cell membranes, making them less reactive to ROS [5]. In addition, it has been shown that iodine binds to lipids, such as arachidonic acid (AA), exerts apoptosis, and/or has differentiation effects on diverse epithelial cells [6,7,8]. Moreover, iodine is uptake and metabolized by immune cells, and depending on the physiological context, this halogen can act as an anti-inflammatory or proinflammatory agent [9,10]. The distribution and action of iodine in organisms depend on the chemical form of iodine that is ingested. For example, molecular iodine (I
2
) is not reduced to iodide (I
-
) in the blood before being absorbed in the gastrointestinal tract [11], induces differential effects [12], and its capture is 40% lower in the thyroid [13]. In fact, under conditions of iodine deficiency, I
-
appears to be more efficient than I
2
in restoring the thyroid gland to normality in goiter stages, while I
2
is more effective in decreasing mammary alterations secondary to iodine deficiency [14]. This article reviews different reports on the effects of iodine as an antioxidant, differentiator and immunomodulator, and does do not include the actions of thyroid hormones.
2. Safety Concentration
- Safety Concentration
Iodine is a structural component of thyroid hormones, which are essential for differentiation of the nervous system during development and crucial regulators of energetic metabolism. Public health policies have been established to guarantee that populations consume the required amount of iodine to eradicate iodine deficiency disorders. According to the International Council for Control of Iodine Deficiency Disorders (ICCIDD), the recommended dietary allowance of iodine is 150–299 μg/day for normal thyroid functioning, and the maximum limit of iodine intake with the lowest observed adverse effect level (LOAEL) is 1700−1800 μg/day [15][16]. In 1988, the joint of Food and Agriculture Organization of the United Nations and WHO Expert Committee on Food Additives suggested the maximal upper level from all iodine sources of 1 mg/day would be safe for most of the population except those with iodine sensitivity or underlying thyroid disorders. The increased intake of iodide can also have interactions with medications such as lithium or sulfisoxazole [17], but similar studies with molecular iodine do not exist; see Table 1 [15][16]. However, several studies report that iodine supplements at moderately high concentration are well tolerated in euthyroid subjects and that only high doses (>30 mg/day), mainly as I
Iodine is a structural component of thyroid hormones, which are essential for differentiation of the nervous system during development and crucial regulators of energetic metabolism. Public health policies have been established to guarantee that populations consume the required amount of iodine to eradicate iodine deficiency disorders. According to the International Council for Control of Iodine Deficiency Disorders (ICCIDD), the recommended dietary allowance of iodine is 150–299 μg/day for normal thyroid functioning, and the maximum limit of iodine intake with the lowest observed adverse effect level (LOAEL) is 1700−1800 μg/day [15,16]. In 1988, the joint of Food and Agriculture Organization of the United Nations and WHO Expert Committee on Food Additives suggested the maximal upper level from all iodine sources of 1 mg/day would be safe for most of the population except those with iodine sensitivity or underlying thyroid disorders. The increased intake of iodide can also have interactions with medications such as lithium or sulfisoxazole [17], but similar studies with molecular iodine do not exist; see Table 1 [15,16]. However, several studies report that iodine supplements at moderately high concentration are well tolerated in euthyroid subjects and that only high doses (>30 mg/day), mainly as I
-, generate hypothyroidism and goiter, which rapidly revert to normal when these individuals stop taking iodine at high concentrations, see Table 2 [18]. Other studies indicate that iodine per se participates in the physiopathology of various organs that uptake it, mainly the thyroid, mammary, prostate, pancreas, and ovaries, and potentially in the gastric, immune, and nervous systems. Moreover, in its molecular form, iodine acts as an antioxidant throughout the body if ingested at concentrations higher than 1 mg/day [19][20]. Dose-response studies in humans have demonstrated that I
generate hypothyroidism and goiter, which rapidly revert to normal when these individuals stop taking iodine at high concentrations, see Table 2 [15,18]. Other studies indicate that iodine per se participates in the physiopathology of various organs that uptake it, mainly the thyroid, mammary, prostate, pancreas, and ovaries, and potentially in the gastric, immune, and nervous systems [6]. Moreover, in its molecular form, iodine acts as an antioxidant throughout the body if ingested at concentrations higher than 1 mg/day [19,20]. Dose-response studies in humans have demonstrated that I
2 at concentrations of 1 to 6 mg/day exhibited significant beneficial actions in benign pathologies like fibrocystic breast disease [21][22], prostatic hyperplasia [23] and polycystic ovaries (unpublished results). The treatments in these studies lasted from five weeks up to two years and did not have any side effects at these concentrations. Some of the dose-response studies also analyzed the highest concentration of iodine (9 and 12 mg/day) and showed the same benefits but accompanied, in some cases, by transient hypothyroidism and/or minor side effects like headache, sinusitis, acne or diarrhea. These effects disappeared when the high dose of supplemental iodine was suspended [24]. Antineoplastic action of the I
at concentrations of 1 to 6 mg/day exhibited significant beneficial actions in benign pathologies like fibrocystic breast disease [21,22], prostatic hyperplasia [23] and polycystic ovaries (unpublished results). The treatments in these studies lasted from five weeks up to two years and did not have any side effects at these concentrations. Some of the dose-response studies also analyzed the highest concentration of iodine (9 and 12 mg/day) and showed the same benefits but accompanied, in some cases, by transient hypothyroidism and/or minor side effects like headache, sinusitis, acne or diarrhea. These effects disappeared when the high dose of supplemental iodine was suspended [24]. Antineoplastic action of the I
2 supplement without harmful effects on the thyroid has also been observed in mammary and prostatic pathologies in preclinical (rodents and canines) and clinical protocols [25][26][27][28]. Although the thyroid captures 40% less I
supplement without harmful effects on the thyroid has also been observed in mammary and prostatic pathologies in preclinical (rodents and canines) and clinical protocols [25,26,27,28]. Although the thyroid captures 40% less I
2
than I
−, the acceptable upper limits for iodine intake during pregnancy are not well defined, and the consequences of excess iodine in newborns are not well documented, so the iodine intake in any of its forms above the upper limits is not recommended in pregnant women or infants.
, the acceptable upper limits for iodine intake during pregnancy are not well defined, and the consequences of excess iodine in newborns are not well documented [15], so the iodine intake in any of its forms above the upper limits is not recommended in pregnant women or infants.
Table 1.
Predisposing Risk Factors Associated with permanent Iodine-Induced Thyroid Dysfunction.
|
Individuals with Underlying Thyroid Disease: |
|
Graves’s disease |
|
Hashimoto thyroiditis |
|
Euthyroid with a history of subacute thyroiditis |
|
Euthyroid with a history of postpartum thyroiditis |
|
Euthyroid with a history of type 2 amiodarone–induced thyroiditis |
|
Euthyroid with post-hemithyroidectomy |
|
Euthyroid after interferon-g therapy |
|
Individuals with a family history of goiter or thyroiditis |
|
Individuals with chronic iodine deficiency |
|
Fetuses, preterm neonates, and newborn infants exposed to high doses of iodine through the placenta and milk |
|
Elderly people with subclinical hypothyroidism |
|
Patients taking medications such as expectorants or amiodarone that contains high concentrations of iodine |
|
Patients with certain nonthyroidal disease such as chronic dialysis and cystic fibrosis, especially those taking sulfisoxazole. |
|
Patients taking lithium |
Table 2.
Sources and Effects of Excess Iodine.
|
Source of Iodine |
Iodine Dose (mg/day) |
Treatment Time |
Chemical Form of Iodine |
Effects on Thyroid Function |
Ref |
|
Iodopovidone (5% solution) mouthwash using 2–4 mL |
14–28 |
Days-weeks |
I2 |
Values remain within normal range |
[29] |
|
Amiodarone |
|
Months-years |
Iodide |
|
[30] |
|
1 tablet (100mg) |
3 |
Thyrotoxicosis (2%) Hyperthyroidism (1%) |
|||
|
1 tablet (600 mg) |
21 |
Hypothyroidism (2–10%) |
|||
|
Iodinated contrast medium (200 mL/dose) |
7–10 |
One dose |
Iodide |
Hyperthyroidism or Hypothyroidism (1–2%) |
[31] |
|
Seaweed Blended brown seaweed (1 bowl, 250 mL soup) |
1–3 |
Weeks-months |
Iodide, I2 |
Normal values or transient subclinical hypothyroidism (2–10%) |
|
|
High level of consumption (>6g seaweed/day) |
>20 |
Risk of papillary thyroid cancer (1–10%) |
|||
|
KI supplements Water solution (5–15mg)
|
>2
|
Days-weeks |
Iodide |
Transient subclinical hypothyroidism, |
[18] |
|
1 tablet (50mg) |
>30 |
Thyrotoxicosis (2–10%) TPOAb, TgAb (6–20%) |
|||
|
Purified water solutions (8mg/L per tablet) 1 tablet
|
1–5 |
Months-years |
I2 |
Normal values |
[18] |
|
4 tablets |
10–32 |
Transient hypothyroidism and goiter TPOAb, TgAb (3–16%) |
|||
|
Aqueous I2 solution I2 water solution; Lugol’s solution, or (1–2 tablets (3mg per tablet) |
1–6 |
Months -years |
I2, I2-iodide |
Values remain within normal range |
[26] |
|
3–4 tablets (3 mg per tablet)
|
9–12
|
Transient subclinical hypothyroidism, headache, sinusitis, diarrhea acne (6–20%)
|
|||
|
Mix yodica
|
1–3 |
Values remain within normal range
|
|||
|
Source of Iodine |
Iodine Dose (mg/day) |
Treatment Time |
Chemical Form of Iodine |
Effects on Thyroid Function |
Ref |
|
Iodopovidone (5% solution) mouthwash using 2–4 mL |
14–28 |
Days-weeks |
I2 |
Values remain within normal range |
[17,18,29] |
|
Amiodarone |
|
Months-years |
Iodide |
|
[15,30] |
|
1 tablet (100mg) |
3 |
Thyrotoxicosis (2%) Hyperthyroidism (1%) |
|||
|
1 tablet (600 mg) |
21 |
Hypothyroidism (2–10%) |
|||
|
Iodinated contrast medium (200 mL/dose) |
7–10 |
One dose |
Iodide |
Hyperthyroidism or Hypothyroidism (1–2%) |
[15,31] |
|
Seaweed Blended brown seaweed (1 bowl, 250 mL soup) |
1–3 |
Weeks-months |
Iodide, I2 |
Normal values or transient subclinical hypothyroidism (2–10%) |
[32,33] |
|
High level of consumption (>6g seaweed/day) |
>20 |
Risk of papillary thyroid cancer (1–10%) |
|||
|
KI supplements Water solution (5–15mg)
|
>2
|
Days-weeks |
Iodide |
Transient subclinical hypothyroidism, |
[15,17,18] |
|
1 tablet (50mg) |
>30 |
Thyrotoxicosis (2–10%) TPOAb, TgAb (6–20%) |
|||
|
Purified water solutions (8mg/L per tablet) 1 tablet
|
1–5 |
Months-years |
I2 |
Normal values |
[15,17,18] |
|
4 tablets |
10–32 |
Transient hypothyroidism and goiter TPOAb, TgAb (3–16%) |
|||
|
Aqueous I2 solution I2 water solution; Lugol’s solution, or (1–2 tablets (3mg per tablet) |
1–6 |
Months -years |
I2, I2-iodide |
Values remain within normal range |
[17,18,21,22,26] |
|
3–4 tablets (3 mg per tablet)
|
9–12
|
Transient subclinical hypothyroidism, headache, sinusitis, diarrhea acne (6–20%)
|
|||
|
Mix yodica
|
1–3 |
Values remain within normal range
|
KI, potassium iodide; I
2
, molecular iodine, TgAb, thyroglobulin antibody and TPOAb, thyroid peroxidase antibody positive titer. Modified from [6].
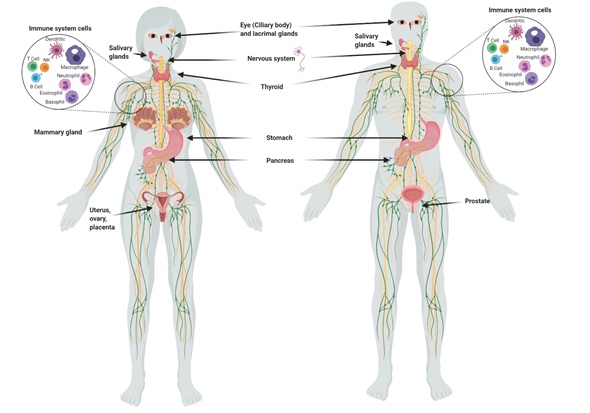
3. Iodine in Normal Tissues
Although the main uptake of iodine takes place in the thyroid, many other organs take it up (Figure 1), including the salivary glands, gastric mucosa, lactating mammary gland, nervous system, choroid plexus, ciliary body of the eye, lacrimal gland, thymus, skin, placenta, ovary, uterus, prostate, and pancreas, and they can maintain or lose this ability in pathological conditions . The I
Although the main uptake of iodine takes place in the thyroid, many other organs take it up (Figure 1), including the salivary glands, gastric mucosa, lactating mammary gland, nervous system, choroid plexus, ciliary body of the eye, lacrimal gland, thymus, skin, placenta, ovary, uterus, prostate, and pancreas, and they can maintain or lose this ability in pathological conditions [1]. The I
-
transport system in many of these extrathyroidal tissues involves the expression of the sodium iodide symporter (NIS) and/or the anion exchanger Pendrin (PDS/SLC26A4). Recent studies have also demonstrated the direct participation of other transporters including anoctamin 1 (ANO1), cystic fibrosis transmembrane conductance regulator (CFTR) and sodium multivitamin transporter (SMVT) that are capable to take up I
− . On the other hand, various studies have shown that I
[1]. On the other hand, various studies have shown that I
2
is captured by an independent mechanism of NIS, PDS, Na
+
and ATP, but it is saturable and depends on protein synthesis, suggesting a facilitated diffusion system [34]. This mechanism is similar to the one described in marine algae [35], indicating that I
2
absorption could be an evolutionary conserved system. Indeed, we demonstrated that the thyroid, mammary gland, and prostate can accumulate both types of iodine, which are captured by different mechanisms. The thyroid, lactating mammary gland, and prostate exhibit a significant uptake of I
-
, which is internalized by NIS (inhibited by KClO
4
). Molecular iodine is taken up by these tissues, but also by others like the nubile mammary gland, and NIS does not participate in its internalization [36]. These findings agree with the notion that I
2
contributes to maintaining the integrity of these organs. Iodine deficiency in rats is accompanied by ductal hyperplasia and perilobular fibrosis in the virgin mammary glands, and the supplement of I
2
but not I
- reverts these alterations. Similarly, the supplement of I
reverts these alterations [14]. Similarly, the supplement of I
2 (3–6 mg/day) in patients with fibrocystic breast disease is accompanied by remission of symptoms, as well as significant anti-inflammatory effects. Our group has found similar benefits in benign prostatic hyperplasia (BPH) in preclinical and clinical models [36]. In human patients with early BPH (Grade I and II), the supplement of 5 mg/day of Lugol’s solution (mix 1:3; I
(3–6 mg/day) in patients with fibrocystic breast disease is accompanied by remission of symptoms, as well as significant anti-inflammatory effects [21,22]. Our group has found similar benefits in benign prostatic hyperplasia (BPH) in preclinical and clinical models [36]. In human patients with early BPH (Grade I and II), the supplement of 5 mg/day of Lugol’s solution (mix 1:3; I
2:KI) for 8 months decreased the prostate-specific antigen (PSA) circulating levels and improved the urinary flow and symptoms scale. These studies agree with epidemiological data that associate the low incidence of breast and prostate pathologies with the moderately high dietary intake of iodine in Asian countries [37]. These populations consume marine algae daily in their diet, which contain high amounts of iodine in various chemical forms such as I
:KI) for 8 months decreased the prostate-specific antigen (PSA) circulating levels and improved the urinary flow and symptoms scale [23]. These studies agree with epidemiological data that associate the low incidence of breast and prostate pathologies with the moderately high dietary intake of iodine in Asian countries [3,4,37]. These populations consume marine algae daily in their diet, which contain high amounts of iodine in various chemical forms such as I
-
, I
2
and iodate (IO
3
)
-. The average consumption of iodine in the Japanese population is 1200 to 5280 µg/day compared to 166 and 209 µg/day in the United Kingdom and the United States, respectively [38]. However, despite the high nutritional intake of iodine, Asia does not differ from the rest of the world in the prevalence of thyroid disorders [37].
. The average consumption of iodine in the Japanese population is 1200 to 5280 µg/day compared to 166 and 209 µg/day in the United Kingdom and the United States, respectively [33,38]. However, despite the high nutritional intake of iodine, Asia does not differ from the rest of the world in the prevalence of thyroid disorders [37].
Figure 1.
Organs and tissues that take up iodine.
4. Antioxidant Effects
Iodine is considered an ancestral antioxidant and its action is conserved throughout phylogeny [2]. Laminaria brown algae contain an iodine concentration 300,000 times higher than any other living organism, and inorganic iodine acts as a scavenger of various reactive oxygen species (ROS) [39]. Similar antioxidant effects have been described in other photosynthetic organisms, as well as in some invertebrates such as polyps of the jellyfish
Iodine is considered an ancestral antioxidant and its action is conserved throughout phylogeny [2]. Laminaria brown algae contain an iodine concentration 300,000 times higher than any other living organism, and inorganic iodine acts as a scavenger of various reactive oxygen species (ROS) [35,39]. Similar antioxidant effects have been described in other photosynthetic organisms, as well as in some invertebrates such as polyps of the jellyfish
Aurelia aurita and urchin larvae [40][41]. In vertebrates, micromolar amounts of iodine decrease damage by ROS, increasing the total antioxidant status in rat and human serum and preventing lipid peroxidation in the eyes of rabbits [42] and in several tissues of vertebrates [43][44][45]. The iodine released by deiodination of thyroxine has been shown to be an antioxidant agent and an inhibitor of lipoperoxidation [43]. Molecular iodine supplements decrease lipid peroxidation in normal and tumor mammary tissues from rats with methyl nitrosourea (MNU)-induced mammary cancer and prevent the cardiac damage induced by the antineoplastic agent doxorubicin when I
and urchin larvae [40,41]. In vertebrates, micromolar amounts of iodine decrease damage by ROS, increasing the total antioxidant status in rat and human serum [20] and preventing lipid peroxidation in the eyes of rabbits [42] and in several tissues of vertebrates [43,44,45]. The iodine released by deiodination of thyroxine has been shown to be an antioxidant agent and an inhibitor of lipoperoxidation [43]. Molecular iodine supplements decrease lipid peroxidation in normal and tumor mammary tissues from rats with methyl nitrosourea (MNU)-induced mammary cancer [13] and prevent the cardiac damage induced by the antineoplastic agent doxorubicin when I
2 (0.05% in drinking water) is administered 2 days before starting the antineoplastic treatment. Moderate iodine diets improve the lipid profile in mice, increasing low density lipoprotein receptors and scavenger receptor class B type 1 (SR-B1) in liver [46]. Moreover, iodine supplementation decreased hypercholesterolemia in overweight women [47]. More recently, our group showed that a moderate I
(0.05% in drinking water) is administered 2 days before starting the antineoplastic treatment [19]. Moderate iodine diets improve the lipid profile in mice, increasing low density lipoprotein receptors and scavenger receptor class B type 1 (SR-B1) in liver [46]. Moreover, iodine supplementation decreased hypercholesterolemia in overweight women [47]. More recently, our group showed that a moderate I
2 supplement prevented the pancreatic damage secondary to hypothyroidism by methimazole, normalizing thyroid hormone synthesis in the thyroid and preventing the oxidative status in pancreatic tissue [48]. Several studies suggest that iodine works by neutralizing ROS, or by acting as a free radical iodinating tyrosine, histidine, and double bonds of polyunsaturated fatty acids in cell membranes, making them less reactive to ROS [49]. However, the antioxidant effect of iodine could be more complex and include various mechanisms (Figure 2). In a model of prostatic hyperplasia, our group demonstrated that I
supplement prevented the pancreatic damage secondary to hypothyroidism by methimazole, normalizing thyroid hormone synthesis in the thyroid and preventing the oxidative status in pancreatic tissue [48]. Several studies suggest that iodine works by neutralizing ROS, or by acting as a free radical iodinating tyrosine, histidine, and double bonds of polyunsaturated fatty acids in cell membranes, making them less reactive to ROS [4,49]. However, the antioxidant effect of iodine could be more complex and include various mechanisms (Figure 2). In a model of prostatic hyperplasia, our group demonstrated that I
2
supplements prevent testosterone-induced oxidative stress, decreasing lipoperoxidation but also inhibiting the activity of both nitric oxide synthase (NOS) and type 2 cyclooxygenase (Cox2). The I
2 supplement also inhibit the formation of prostaglandins with equivalent intensity to that observed with Celecoxib (a specific Cox2 inhibitor). The effect on Cox2 inhibition can occur by deactivating the heme iron active site or as a competitor of its main substrate, arachidonic acid (AA). In the latter case, the formation of 6-iodolactone (6-IL) from AA can decrease the formation of prostaglandins, or 6-IL acts as a direct inhibitor of the enzyme [50]. Another recent proposal is the interaction of I
supplement also inhibit the formation of prostaglandins with equivalent intensity to that observed with Celecoxib (a specific Cox2 inhibitor). The effect on Cox2 inhibition can occur by deactivating the heme iron active site or as a competitor of its main substrate, arachidonic acid (AA). In the latter case, the formation of 6-iodolactone (6-IL) from AA can decrease the formation of prostaglandins, or 6-IL acts as a direct inhibitor of the enzyme [50]. Another recent proposal is the interaction of I
2
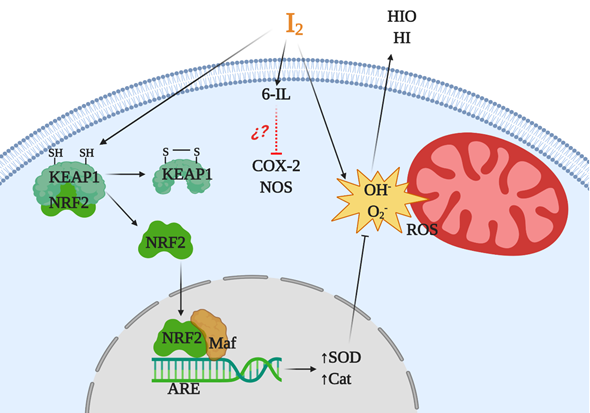
with the nuclear factor erythroid-2-related factor-2 (Nrf2) pathway [51]. Nrf2 is a promoter of the antioxidant response to endogenous and exogenous stressors that trigger the expression of phase II protective antioxidant enzymes such as superoxide dismutase (SOD) and catalase (Cat) [52]. Under basal conditions, Nrf2 is anchored to the cytoplasm through the actin cytoskeleton-binding protein 1 (Keap1). Iodination of Keap1 results in the release and translocation of Nrf2 to the nucleus. After Nrf2 heterodimerizes with small Maf proteins and binds to the antioxidant response element (ARE), SOD and Cat become overexpressed [51].
Figure 2.
Antioxidant mechanisms of molecular iodine (I
2
). I
2
acts as a scavenger of a reactive oxygen species (ROS) like hydroxyl radicals (OH) or superoxide anions (O
2
) generating neutral components hypoiodous acid (HIO) or hydroiodic acid (HI). I
2
in combination with arachidonic acid (AA), and generating the iodolipid 6-iodolactone (6-IL), inhibits the activity of proinflammatory enzymes like nitric oxide synthase (NOS) and cyclooxygenase type 2 (Cox2). In addition, the iodination of the cysteine-rich protein Keap1 releases and promotes the nuclear translocation of nuclear factor erythroid-2-related factor-2 (Nrf2) that with Maf activates the antioxidant response element (ARE), inducing overexpression of antioxidant enzymes type II like superoxide dismutase (SOD) and catalase (Cat).
5. Antiproliferative and Apoptotic Actions
Since the 1940s it has been known that iodine, in addition to be a structural part of thyroid hormones, also participates in the function and proliferation of thyroid cells. An excess of I
- causes inhibitory actions that include decreased iodine organification and hormonal secretion, thyroglobulin proteolysis, decreased glucose and amino acid transport, protein and RNA biosynthesis, and significant inhibition of thyrocyte proliferation under both in vitro and in vivo conditions. The specific mechanism by which iodine performs all these modifications has not been fully elucidated, but a multifaceted mechanism has been postulated and includes the participation of transforming growth factor beta-1 (TGF-β1), triiodothyronine (T3) and iodolipids such as 6-IL or 2-iodohexadecanal (2-IHDA). Moreover, all the inhibitory actions of iodine can be reversed with drugs that block the enzyme thyroid peroxidase (TPO), such as methylmercaptoimidazole (MMI) or propylthiouracil (PTU). In the presence of H
causes inhibitory actions that include decreased iodine organification and hormonal secretion, thyroglobulin proteolysis, decreased glucose and amino acid transport, protein and RNA biosynthesis, and significant inhibition of thyrocyte proliferation under both in vitro and in vivo conditions [49]. The specific mechanism by which iodine performs all these modifications has not been fully elucidated, but a multifaceted mechanism has been postulated and includes the participation of transforming growth factor beta-1 (TGF-β1), triiodothyronine (T3) and iodolipids such as 6-IL or 2-iodohexadecanal (2-IHDA). Moreover, all the inhibitory actions of iodine can be reversed with drugs that block the enzyme thyroid peroxidase (TPO), such as methylmercaptoimidazole (MMI) or propylthiouracil (PTU) [49]. In the presence of H
2
O
2
, TPO oxidizes I
-
and covalently binds it to proteins or lipids. The specific species of iodine generated by TPO has not been identified, but there are several candidates, such as I
-,
I0 (free radical of iodine), IO
−
(hypoiodite) and I
2. Vitale et al. [53] showed that an excess of KI (10–50 mM) induces apoptosis in primary thyrocyte cells, but if TPO activity is blocked with PTU, the apoptotic effect of I
[49]. Vitale et al. [53] showed that an excess of KI (10–50 mM) induces apoptosis in primary thyrocyte cells, but if TPO activity is blocked with PTU, the apoptotic effect of I
-
is eliminated. In addition, lung cancer cells (without absorption of natural iodide) transfected with NIS or NIS/TPO, a supplement of KI (30 mM), induced apoptosis only in cells transfected with NIS/TPO, indicating that oxidation of I
-
by TPO is required to exert apoptotic effects [54].
In terms of carcinogenesis, the overproduction of ROS, such as single oxygen (O
2
), superoxide anions (O
2−
), hydrogen peroxide (H
2
O
2
) and hydroxyl radicals (°OH), is a hallmark related to the etiology and progression of cancer [55]. ROS have a wide range of cellular and molecular effects resulting in mutagenicity, cytotoxicity, and changes in gene expression. The notion that I
2 is the chemical form responsible for antineoplastic effects originates from the first descriptions of the consumption of seaweed or Lugol’s solution. Previously, we mentioned that seaweeds contain iodine in several chemical forms although the exact proportion is not known. Traditional Eastern breast cancer medicine has long used iodine-rich seaweeds as a cancer treatment to “soften” tumors and “reduce” nodulation [56]. The addition of small proportions (2 to 5%) of Laminaria
is the chemical form responsible for antineoplastic effects originates from the first descriptions of the consumption of seaweed or Lugol’s solution [4]. Previously, we mentioned that seaweeds contain iodine in several chemical forms although the exact proportion is not known [33]. Traditional Eastern breast cancer medicine has long used iodine-rich seaweeds as a cancer treatment to “soften” tumors and “reduce” nodulation [56]. The addition of small proportions (2 to 5%) of Laminaria
angustata, porphyra tenera
or
Laminaria religiosa to the diet significantly delays the occurrence of tumors in rats treated with the chemical carcinogen, 7,12-dimethylbenzanthracene (DMBA) [57][58]. The first report demonstrating that iodine exhibited an antineoplastic effect in extrathyroidal tissues was in the rat mammary cancer model induced by DMBA, using 0.05% Lugol’s solution [59]. Later, our group reported that in this model, KI, I
to the diet significantly delays the occurrence of tumors in rats treated with the chemical carcinogen, 7,12-dimethylbenzanthracene (DMBA) [57,58]. The first report demonstrating that iodine exhibited an antineoplastic effect in extrathyroidal tissues was in the rat mammary cancer model induced by DMBA, using 0.05% Lugol’s solution [59]. Later, our group reported that in this model, KI, I
2
, or Lugol’s solution can induce antineoplastic actions. The protective effect of 0.1% KI is lost when the enzyme lactoperoxidase (LPO), which is present in mammary cancers, is inhibited by MMI, indicating that I
-
from KI needs to be oxidized to have the apoptotic effect [60]. In this study, our group also demonstrated that I
2
prevents DMBA-induced DNA adduct formation in pre-malignant and cancer tissues. This finding is particularly relevant since LPO can oxidize natural or synthetic estrogens to catechol estrogens [61]. The resulting estrogenic quinones have been shown to react with DNA to form mutagenic adducts that can initiate or promote cancer [62]. This notion agrees with the report of Cavalieri’s group showing that higher levels of E2-DNA adducts are present in the urine of breast cancer patients and women at high risk for this disease [63].
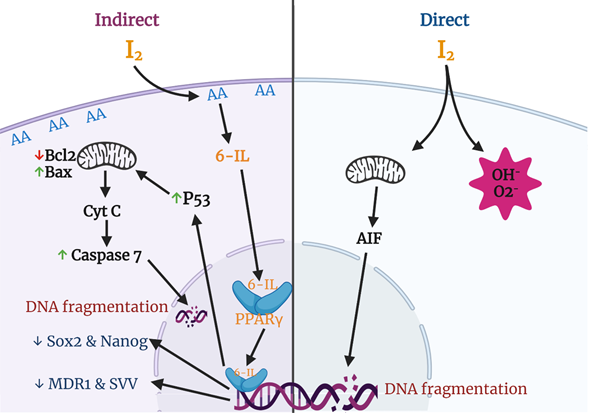
Various groups have described apoptosis effects of iodine in several cancer cell lines and proposed different mechanisms and pathways (Figure 3). The most studied effects include a direct action, where the oxidized iodine dissipates the mitochondrial membrane potential, thereby triggering mitochondrion-mediated apoptosis [64], and an indirect effect through iodolipids formation and the activation of peroxisome proliferator-activated receptors type gamma (PPARγ) [65].
It is well known that the mitochondrial membrane potential (MMP) is required for a variety of mitochondrial functions including protein import, ATP production, and regulation of metabolite transport. The mitochondrial intermembrane space contains proteins that can induce apoptosis involving caspases (e.g., cytochrome c) or execute a caspase-independent apoptotic death program through the apoptosis-inducing factor (AIF) or through the release and degradation of the antiapoptotic protein Survivin (SVV). The release of these factors requires abatement of the MMP, and thiol depletion is a powerful trigger [66][67]. Molecular iodine treatment is accompanied by depletion of cellular thiol content and dissipation of the MMP in estrogen-responsive (MCF-7) and non-responsive (MDA-MB-231) human cell lines. In addition, the pre-incubation of MCF-7 cells with N-acetylcysteine (NAC), a thiol-containing agent, prevents the apoptotic effect of I
It is well known that the mitochondrial membrane potential (MMP) is required for a variety of mitochondrial functions including protein import, ATP production, and regulation of metabolite transport. The mitochondrial intermembrane space contains proteins that can induce apoptosis involving caspases (e.g., cytochrome c) or execute a caspase-independent apoptotic death program through the apoptosis-inducing factor (AIF) or through the release and degradation of the antiapoptotic protein Survivin (SVV). The release of these factors requires abatement of the MMP, and thiol depletion is a powerful trigger [66,67]. Molecular iodine treatment is accompanied by depletion of cellular thiol content and dissipation of the MMP in estrogen-responsive (MCF-7) and non-responsive (MDA-MB-231) human cell lines. In addition, the pre-incubation of MCF-7 cells with N-acetylcysteine (NAC), a thiol-containing agent, prevents the apoptotic effect of I
2
[64]. Comparative studies of mitochondria isolated from tumoral (TT) versus extra-tumoral (ET) human breast tissue showed that the I
2
treatment increased mitochondrial permeability in TT and decreased it in ET, suggesting a differential sensitivity toward iodine in both physiological conditions [68].
The indirect action of I
2 could be exerted by the formation 6-IL previously detected in thyroid tissue of rat, pig, horse, and human [69][70]. Although the specific iodinated components have not yet been characterized in other tissues, several studies have reported elevated prostaglandin levels in cancerous tissues compared to normal tissues [71]. Prostaglandins are produced from AA by Cox2, indicating high levels of AA in several tumors [72]. In relation to the mammary gland, we reported that MNU-induced tumors contain four times higher basal concentrations of AA, and after 0.05% I
could be exerted by the formation 6-IL previously detected in thyroid tissue of rat, pig, horse, and human [69,70]. Although the specific iodinated components have not yet been characterized in other tissues, several studies have reported elevated prostaglandin levels in cancerous tissues compared to normal tissues [71]. Prostaglandins are produced from AA by Cox2, indicating high levels of AA in several tumors [72]. In relation to the mammary gland, we reported that MNU-induced tumors contain four times higher basal concentrations of AA, and after 0.05% I
2
treatment, 6-IL levels were 15-fold higher than in normal mammary tissue, suggesting a role for 6-IL in the antiproliferative effect of I
2
[65]. These findings have been corroborated in human cancer cell lines where lipids like 6-IL were identified after I
2
treatment [73] or where the addition of I
2 or 6-IL triggered apoptosis [74][75]. In this regard, the consistent observations that cancer cells are more sensitive to I
or 6-IL triggered apoptosis [74,75]. In this regard, the consistent observations that cancer cells are more sensitive to I
2 than normal cells led us to propose that the high concentration of AA in tumoral cells is the crucial component that, when iodinated, is responsible for the antiproliferative effect of I
than normal cells [73,74,75] led us to propose that the high concentration of AA in tumoral cells is the crucial component that, when iodinated, is responsible for the antiproliferative effect of I
2.
[65].
In the search for cellular mechanisms associated with iodine effects, studies from our laboratory demonstrated that both I
2
and 6-IL supplementation significantly modified the expression of PPARs [76]. These receptors, originally associated with lipid metabolism regulation, are widely expressed and form part of the nuclear receptor family that binds thyroid hormones, steroids, and vitamins. To date, three isotypes called PPARα, PPARβ/δ, and PPARγ have been identified. These three subtypes display differential tissue distribution, and each is involved in specific functions such as early development, cell proliferation, differentiation, apoptosis, and metabolic homeostasis [77]. In our experiments, 20–200 μM I
2 increased the expression of PPARγ mRNA and protein, decreased the expression of mRNA for PPARα, and had no effect on PPARβ/δ expression in MCF-7 cells. We also showed that 6-IL is a specific agonist of PPARγ with an in vitro affinity 6 times higher than AA. These findings agree with the observation that the affinity and selectivity of the PPARγ isoform for some fatty acids is increased by the conformational changes resulting from the incorporation of halogens (phenyl acetate < phenyl butyrate < p-chlorophenyl acetate < p-iodophenyl butyrate) [78]. Moreover, recent reports have shown that antineoplastic effects of iodine or iodolipids are exerted on different types of cells that can take up I
increased the expression of PPARγ mRNA and protein, decreased the expression of mRNA for PPARα, and had no effect on PPARβ/δ expression in MCF-7 cells. We also showed that 6-IL is a specific agonist of PPARγ with an in vitro affinity 6 times higher than AA [76]. These findings agree with the observation that the affinity and selectivity of the PPARγ isoform for some fatty acids is increased by the conformational changes resulting from the incorporation of halogens (phenyl acetate < phenyl butyrate < p-chlorophenyl acetate < p-iodophenyl butyrate) [78]. Moreover, recent reports have shown that antineoplastic effects of iodine or iodolipids are exerted on different types of cells that can take up I
2
and exhibit apoptotic induction by PPARγ agonists. Such cells include prostate, lung carcinoma, pancreas carcinoma, melanoma, glioblastoma, and neuroblastoma cells [79].
Figure 3.
Apoptotic and differentiation mechanisms of molecular iodine (I
2
). In the direct pathway the oxidized iodine dissipates the mitochondrial membrane potential triggering mitochondrion-mediated apoptosis. The indirect pathway includes the iodination of arachidonic acid (AA), generating 6- iodolactone (6-IL), and the activation of peroxisome proliferator-activated receptors type gamma (PPARγ). The activation of PPARγ could induce the p53-caspase apoptotic pathway and/or the inhibition of markers related to stem cell maintenance (Sox2, Nanog), chemoresistance (multidrug resistance protein 1; MDR1), and survival (Survivin; SVV).