Carbon dots (CDs) are part of the nanocarbon family including quasi-spherical nanoparticles with sizes around 10 nm. They consist of amorphous and crystalline parts, mainly composed of carbon with a fringe spacing of 0.34 nm, which corresponds to the (002) interlayer spacing of graphite. Since their first discovery in 2006, CDs have gained ever-increasing attention due to their fascinating properties like distinctive optical behaviour, tunable emission, different functional groups, good biocompatibility, chemical and photo-stability, low toxicity, and low-cost production. More importantly, CDs properties can be changed by controlling their size, shape, and heteroatom doping and by modifying the surfaces. They are considered promising Green alternatives to traditional fluorescent dyes and have been proposed for different optoelectronic applications such as sensing, bioimaging, fingerprint detection, gene delivery, solar cells, or printing inks
- carbon dots
- organic light-emitting diode
- photoluminescence
- sustainable materials
- green chemistry
- optical properties
1. Introduction
The growing demand for electronic devices for an ever-increasing number of applications means that green and sustainable electronics are no longer just a dream but a pressing need [1].
In this context, the electronics and optoelectronics based on organic semiconductors showed, in the last few years, significant growth in many areas dominated by traditional electronics [2]. The foremost advantage of organic materials is that they are cheap, lightweight, easy to be processed, and flexible [3]. However, the synthetic techniques currently used for production of organic semiconductors [4] suffer from toxicity and environmental problems that can seriously prevent their large-scale production. In this regard, application of the principles of Green Chemistry for development of synthetic sustainable methods for the synthesis of semiconductors is essential to support the development of organic electronics, thus moving towards increasingly sustainable electronic devices [5].
In this context, the electronics and optoelectronics based on organic semiconductors showed, in the last few years, significant growth in many areas dominated by traditional electronics [2]. The foremost advantage of organic materials is that they are cheap, lightweight, easy to be processed, and flexible [3]. However, the synthetic techniques currently used for production of organic semiconductors [4] suffer from toxicity and environmental problems that can seriously prevent their large-scale production. In this regard, application of the principles of Green Chemistry for development of synthetic sustainable methods for the synthesis of semiconductors is essential to support the development of organic electronics, thus moving towards increasingly sustainable electronic devices [5].
In fact, the use of organic materials to build electronic devices [6] holds the promise that future electronic manufacturing methods will rely on safer and more abundant raw materials [7]. The vision is for resource-efficient synthetic methodologies, whereby both devices themselves and manufacturing of those devices use less and more safe materials.
In fact, the use of organic materials to build electronic devices [6] holds the promise that future electronic manufacturing methods will rely on safer and more abundant raw materials [7]. The vision is for resource-efficient synthetic methodologies, whereby both devices themselves and manufacturing of those devices use less and more safe materials.
The first pillar on which the new sustainable industrial revolution is based on is inevitably the development of new materials that are possibly safe and sustainable by design [8].
Among the emerging classes of materials able to meet these needs, carbon dots (CDs) are attracting considerable interest. They are part of the nanocarbon family, and differently from the best known carbon nanotube [9], they include quasi-spherical nanoparticles with sizes around 10 nm. Since their first discovery in 2006, CDs [10][11] have gained ever-increasing attention due to their fascinating properties like distinctive optical behaviour, tunable emission, different functional groups, good biocompatibility, chemical and photo-stability, low toxicity, and low-cost production. More importantly, CDs properties can be changed by controlling their size, shape, and heteroatom doping and by modifying the surfaces, thanks to the quantum confinement effect (QCE) [12] (
Among the emerging classes of materials able to meet these needs, carbon dots (CDs) are attracting considerable interest. They are part of the nanocarbon family, and differently from the best known carbon nanotube [9], they include quasi-spherical nanoparticles with sizes around 10 nm. Since their first discovery in 2006, CDs [10,11] have gained ever-increasing attention due to their fascinating properties like distinctive optical behaviour, tunable emission, different functional groups, good biocompatibility, chemical and photo-stability, low toxicity, and low-cost production. More importantly, CDs properties can be changed by controlling their size, shape, and heteroatom doping and by modifying the surfaces, thanks to the quantum confinement effect (QCE) [12] (
Figure 1). They are considered promising green alternatives to fluorescent dyes [13][14][15] and generally to toxic metallic colloidal semiconductor nanocrystals and have been proposed for optoelectronic applications in general [12][16][17][18][19] (
). They are considered promising green alternatives to fluorescent dyes [13,14,15] and generally to toxic metallic colloidal semiconductor nanocrystals and have been proposed for optoelectronic applications in general [12,16,17,18,19] (
), such as sensing, bioimaging, fingerprint detection, gene delivery, solar cells, or printing inks.
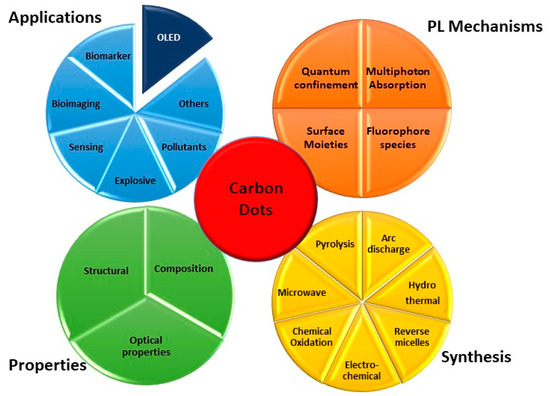
Figure 1.
In general, CD preparation methods can be grouped into two main approaches: top-down and bottom-up. The first strategy [20][21][22][23][24][25][26][27] involves the use of techniques such as arc discharge, laser ablation, chemical oxidation in strong acid, and electrochemical synthesis to break down carbon sources such as graphite, carbon nanotubes, and nanodiamonds to form fluorescent CDs. In contrast, in the bottom-up approach [28][29][30][31][32][33][34], CDs are synthesized from organic molecules by applying hydrothermal solvothermal methods, ultrasonic or microwave treatments, or simple thermal combustion.
In general, CD preparation methods can be grouped into two main approaches: top-down and bottom-up. The first strategy [20,21,22,23,24,25,26,27] involves the use of techniques such as arc discharge, laser ablation, chemical oxidation in strong acid, and electrochemical synthesis to break down carbon sources such as graphite, carbon nanotubes, and nanodiamonds to form fluorescent CDs. In contrast, in the bottom-up approach [28,29,30,31,32,33,34], CDs are synthesized from organic molecules by applying hydrothermal solvothermal methods, ultrasonic or microwave treatments, or simple thermal combustion.
Although CDs have been synthesized from different starting materials with a great variety of techniques, there is a huge effort to develop sustainable synthetic paths which adhere to the principles of Green Chemistry. It has been demonstrated that CDs can be obtained from any carbon-based materials. This, in particular, guarantees that most by-products of the food supply chain can be reused to produce CDs. Agriculture products contain a myriad of natural molecules that can make up a diverse source of surface functional groups in CD formation.
A relevant feature for CD sustainability has to do with synthetic methodologies for their fabrication. CDs can be produced hydrothermally, that is, by heating the starting materials in water under atmosphere or elevated pressure [28][29][30][31][34][35]. Consequently, the cost of production is low, and the operation is easy, relatively safe, and free from organic solvents (
A relevant feature for CD sustainability has to do with synthetic methodologies for their fabrication. CDs can be produced hydrothermally, that is, by heating the starting materials in water under atmosphere or elevated pressure [28,29,30,31,34,35]. Consequently, the cost of production is low, and the operation is easy, relatively safe, and free from organic solvents (
Figure 2). Furthermore, shorter processing time and lower energy consumption for CD manufacture can be obtained when microwaves are used as the heating source [36][37].
). Furthermore, shorter processing time and lower energy consumption for CD manufacture can be obtained when microwaves are used as the heating source [36,37].
Toxicity studies of the CDs were performed with both plants and animals (mice), revealing good biocompatibility [38][39][40], and opened the way not only to their bio-application but also to biodegradable electronics [19][40].
Toxicity studies of the CDs were performed with both plants and animals (mice), revealing good biocompatibility [38,39,40], and opened the way not only to their bio-application but also to biodegradable electronics [19,40].
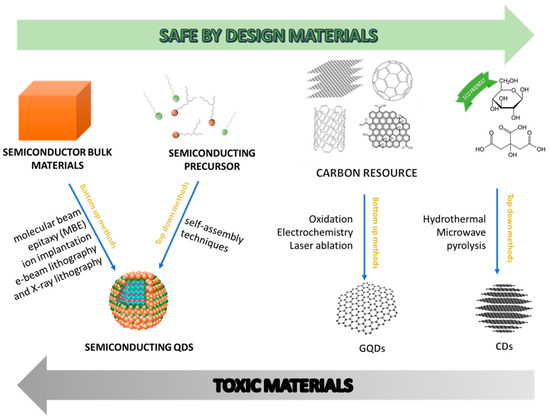
Figure 2.
With this perspective, in the present review, we will discuss CDs obtained with a bottom-up approach as it is the one that best adheres to the principles of green chemistry. They can be obtained from renewable sources or waste such as citric acid or agricultural waste [37]. The synthetic methodologies are simple and inexpensive, and they do not require the use of metal catalysts or chlorinated solvents. We will focalize on CDs as a sustainable new platform for organic light-emitting device (OLED) technology [42][43] without deepening the methods of synthesis on which relevant works have already been written [28][29][30][31][32][33][34][35][36][37][44]. In fact, among the various interesting applications of organic electronics, OLEDs are certainly those that have already carved out a slice of the market [42], and for this reason, the development of sustainable active materials and green technologies can already help the economy make a green turn.
With this perspective, in the present review, we will discuss CDs obtained with a bottom-up approach as it is the one that best adheres to the principles of green chemistry. They can be obtained from renewable sources or waste such as citric acid or agricultural waste [37]. The synthetic methodologies are simple and inexpensive, and they do not require the use of metal catalysts or chlorinated solvents. We will focalize on CDs as a sustainable new platform for organic light-emitting device (OLED) technology [42,43] without deepening the methods of synthesis on which relevant works have already been written [28,29,30,31,32,33,34,35,36,37,44]. In fact, among the various interesting applications of organic electronics, OLEDs are certainly those that have already carved out a slice of the market [42], and for this reason, the development of sustainable active materials and green technologies can already help the economy make a green turn.
2. Carbon Dots and Optical Properties
Since their fortuitous discovery in 2004 by Xu et al. [10] and subsequently by Sun et al. in 2006 [11], CDs attracted a great deal of attention. Generally, CDs are 0-dimension nanocarbons with a typical size of less than 10 nm, although approximately 60-nm-size CDs have also been reported. CDs are quasispherical nanoparticles consisting of amorphous and crystalline parts, mainly composed of carbon with a fringe spacing of 0.34 nm, which corresponds to the (002) interlayer spacing of graphite [12][13][14]. CDs are often confused, or associated, with graphene quantum dots (GQDs) [15], which are always part of the family of carbon-based nanomaterials but have different characteristics and origins. In fact, GQDs are nanofragments of graphene exhibiting a graphene structure inside the dots with a typical fringe spacing of 0.24 nm associated with the (100) in-plane lattice spacing of graphene. They have only one or a few layers of graphene with variable thickness between 2 and 10 nm and with usually 100 nm in lateral dimension, and they are generally produced by converting graphene or graphene oxide via top-down approaches [14].
Since their fortuitous discovery in 2004 by Xu et al. [10] and subsequently by Sun et al. in 2006 [11], CDs attracted a great deal of attention. Generally, CDs are 0-dimension nanocarbons with a typical size of less than 10 nm, although approximately 60-nm-size CDs have also been reported. CDs are quasispherical nanoparticles consisting of amorphous and crystalline parts, mainly composed of carbon with a fringe spacing of 0.34 nm, which corresponds to the (002) interlayer spacing of graphite [12,13,14]. CDs are often confused, or associated, with graphene quantum dots (GQDs) [15], which are always part of the family of carbon-based nanomaterials but have different characteristics and origins. In fact, GQDs are nanofragments of graphene exhibiting a graphene structure inside the dots with a typical fringe spacing of 0.24 nm associated with the (100) in-plane lattice spacing of graphene. They have only one or a few layers of graphene with variable thickness between 2 and 10 nm and with usually 100 nm in lateral dimension, and they are generally produced by converting graphene or graphene oxide via top-down approaches [14].
Over the last 15 years, CDs have been synthesized with different approaches (i.e., top-down and bottom-up). However, only recently, sustainable precursors and methodologies have been deeply investigated for their production [45][46]. These approaches look for sustainable materials which are low-cost, scalable, industrially and economically attractive, and based on renewable and highly abundant resources. This means that CD synthesis can meet the requirements of circular chemistry. Interestingly, CDs after synthesis can be further functionalized with various surface groups. In particular, oxygen-based functional groups, such as carboxyl and hydroxyl, give excellent solubility in water and are suitable for surface passivation and derivatization with various organic materials. Surface functionalization modifies both the physical properties of CDs such as their solubility in aqueous and non-aqueous solvents and their optical properties. For example, after surface passivation, the fluorescence properties of CDs can be improved [47][48]. In addition, the large conjugated structure endows CDs with some important characteristics, like good photostability, high surface area, and robust surface grafting [49]. Their electronic structures can be tuned by their size, shape, surface functional groups, and heteroatom doping, as theoretically investigated and experimentally confirmed by several groups [50][51][52][53][54]. The tunability of optoelectronic properties by modifying synthetic parameters and precursor strictly resembled the conjugated polymer features [55].
Over the last 15 years, CDs have been synthesized with different approaches (i.e., top-down and bottom-up). However, only recently, sustainable precursors and methodologies have been deeply investigated for their production [45,46]. These approaches look for sustainable materials which are low-cost, scalable, industrially and economically attractive, and based on renewable and highly abundant resources. This means that CD synthesis can meet the requirements of circular chemistry. Interestingly, CDs after synthesis can be further functionalized with various surface groups. In particular, oxygen-based functional groups, such as carboxyl and hydroxyl, give excellent solubility in water and are suitable for surface passivation and derivatization with various organic materials. Surface functionalization modifies both the physical properties of CDs such as their solubility in aqueous and non-aqueous solvents and their optical properties. For example, after surface passivation, the fluorescence properties of CDs can be improved [47,48]. In addition, the large conjugated structure endows CDs with some important characteristics, like good photostability, high surface area, and robust surface grafting [49]. Their electronic structures can be tuned by their size, shape, surface functional groups, and heteroatom doping, as theoretically investigated and experimentally confirmed by several groups [50,51,52,53,54]. The tunability of optoelectronic properties by modifying synthetic parameters and precursor strictly resembled the conjugated polymer features [55].
As mentioned above, the CD emission properties are their most amazing characteristics [41] and significant advances have been made in the last years, reaching photoluminescence (PL) quantum yields (PLQYs) up to 80% in CDs produced from citric acid as a renewable precursor or bright and stable PLQYs of 26% converting toxic cigarette butts [28][54].
As mentioned above, the CD emission properties are their most amazing characteristics [41] and significant advances have been made in the last years, reaching photoluminescence (PL) quantum yields (PLQYs) up to 80% in CDs produced from citric acid as a renewable precursor or bright and stable PLQYs of 26% converting toxic cigarette butts [28,54].
Mostly, CDs are blue emitters, but emissions from ultraviolet to near-infrared [55][56][57][58] as well as white light emission [59] were reported. In general, their PL spectra are symmetrical and broad, with large Stokes shifts (mainly due to the CD size distribution), and usually have an excitation-dependent behaviour, with the emission peak varying with the excitation wavelength [22][58].
Mostly, CDs are blue emitters, but emissions from ultraviolet to near-infrared [55,56,57,58] as well as white light emission [59] were reported. In general, their PL spectra are symmetrical and broad, with large Stokes shifts (mainly due to the CD size distribution), and usually have an excitation-dependent behaviour, with the emission peak varying with the excitation wavelength [22,58].
The emission mechanism of CDs is a longstanding debate, and several hypotheses have been proposed [41] (see
The emission mechanism of CDs is a longstanding debate, and several hypotheses have been proposed [41] (see
) such as (i) size-dependent emission, (ii) surface state-derived luminescence, and (iii) embedded molecular luminophore [60]. Regardless of the type of mechanism, it has been shown that CD emissions could be regulated by controlling their size (mainly referring to sp
2 carbon domains), their surface passivation and/or functionalization, and their doping due to the presence of heteroatom [61][62][63].
carbon domains), their surface passivation and/or functionalization, and their doping due to the presence of heteroatom [61,62,63].
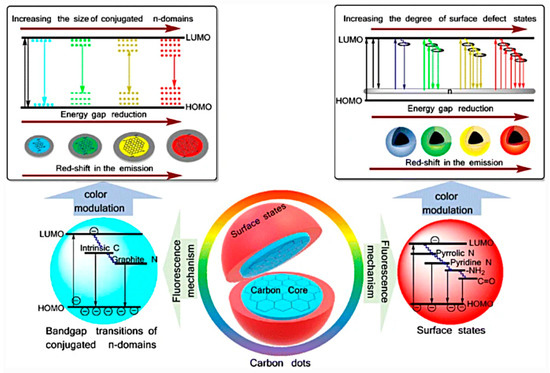
Figure 3.
The first hypothesis proposed is based on the size of CDs [60]. Yuan and coworkers synthetized multicolour-emitting CDs with different dimensions from citric acid (CA) and diaminonaphthalene (DAN) by controlling the process parameters. CDs showed average sizes of about 1.95, 2.41, 3.78, 4.90, and 6.68 nm (
A), with corresponding tunable absorption (
B) blue (430 nm), green (513 nm), yellow (535 nm), orange (565 nm), and red (565 nm) emissions, respectively (
C). In accordance with the results, they deduced that, by increasing the size of CDs and consequently the conjugated π-domain, the bandgap decreases (
D,E) [65].

Figure 4.
A
B
E
A
B
E
A second hypothesis is related to the surface states of CDs. Ding et al. [66] synthetized tunable photoluminescent CDs by one-pot hydrothermal synthesis (
A second hypothesis is related to the surface states of CDs. Ding et al. [66] synthetized tunable photoluminescent CDs by one-pot hydrothermal synthesis (
A). Noteworthy, these CDs had comparable particle size and carbon core but variable degree of oxidation of the surface state. Therefore, a gradual reduction in their band gaps and a red shift in their emission peaks from 440 to 625 nm (
B,C) was observed by increasing the incorporation of oxygen species into their surface structures (
Figure 5D) [66]. Also, Miao et al. [67] hypothesized a similar mechanism. They modulated the CD emission from 430 to 630 nm by controlling the degree of graphitization and the number of surface –COOH groups by changing the molar ratios of CA to urea at different temperatures (
D) [66]. Also, Miao et al. [67] hypothesized a similar mechanism. They modulated the CD emission from 430 to 630 nm by controlling the degree of graphitization and the number of surface –COOH groups by changing the molar ratios of CA to urea at different temperatures (
Figure 6). The increasing number of –COOH groups on the surface increases the electronic delocalization, and the emission wavelength is consequently red-shifted [64].
). The increasing number of –COOH groups on the surface increases the electronic delocalization, and the emission wavelength is consequently red-shifted [64].
Another relevant hypothesis to explain CD emission is molecular luminophore-derived emission or molecular state emission. Small molecules or oligomeric luminophores could be produced during CD synthesis, and these luminophores could be attached to the surface of CD backbones, allowing CDs to have bright emission properties [68]. Song et al. [69] studied the chemical structure and PL mechanism of CDs from CA and ethylenediamine (EDA). They proved the presence of a type of bright blue fluorophore and that CD emission was a result of small molecules, polymer clusters, and carbon cores. Indeed, the fluorophore may be attached to the carbon core, that may strongly affect the PL properties of the CDs.
Another relevant hypothesis to explain CD emission is molecular luminophore-derived emission or molecular state emission. Small molecules or oligomeric luminophores could be produced during CD synthesis, and these luminophores could be attached to the surface of CD backbones, allowing CDs to have bright emission properties [68]. Song et al. [69] studied the chemical structure and PL mechanism of CDs from CA and ethylenediamine (EDA). They proved the presence of a type of bright blue fluorophore and that CD emission was a result of small molecules, polymer clusters, and carbon cores. Indeed, the fluorophore may be attached to the carbon core, that may strongly affect the PL properties of the CDs.
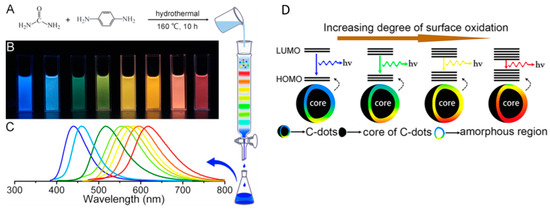
Figure 5.
A
B
C
D
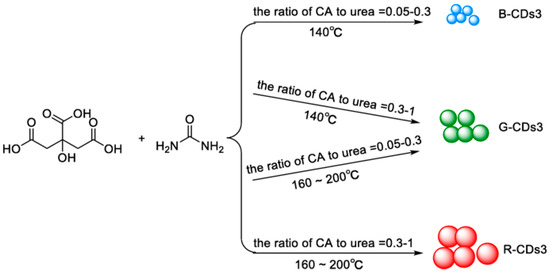
Figure 6. Multicolor-emitting CDs (called as CDs3) by using different molar ratios of CA to urea at different temperatures: the emission of CDs3 can be adjusted from 430 to 630 nm. The photoluminescence quantum yields (PLQYs) of the three CDs3 in blue, green, and red were 52.6%, 35.1%, and 12.9%, respectively. Reprinted with permission from [64], copyright (2019) Springer-Verlag.
Multicolor-emitting CDs (called as CDs3) by using different molar ratios of CA to urea at different temperatures: the emission of CDs3 can be adjusted from 430 to 630 nm. The photoluminescence quantum yields (PLQYs) of the three CDs3 in blue, green, and red were 52.6%, 35.1%, and 12.9%, respectively. Reprinted with permission from [64], copyright (2019) Springer-Verlag.
3. OLED-based Carbon Dots
CDs with amazing properties such as optical characteristics, intrinsic high stability, low-cost, and environment-friendliness, find natural and practical applications as crucial components in OLED technology. In the last decade, the interest in OLED based on CDs (hereafter CD-OLEDs) has been growth, and an increasing number of research groups have started to investigate in this field.
It is important to point out the dual employment of CDs as emitter and as a charge regulating interlayer (
Figure 7) in the typical OLED architecture glass/indium tin Oxide (ITO)/poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS)/emitting layer/interlayer/cathode. Besides, many groups used CDs also as a remote emitter, endowing blue commercial LEDs with a color converting filter based on CDs embedded in poly(methyl-methacrylate) (PMMA) or other matrices. The blue LED emission was tuned from blue to red by altering the film thickness of the filter or the doping concentration of CDs
) in the typical OLED architecture glass/indium tin Oxide (ITO)/poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS)/emitting layer/interlayer/cathode. Besides, many groups used CDs also as a remote emitter, endowing blue commercial LEDs with a color converting filter based on CDs embedded in poly(methyl-methacrylate) (PMMA) or other matrices. The blue LED emission was tuned from blue to red by altering the film thickness of the filter or the doping concentration of CDs [
].
.
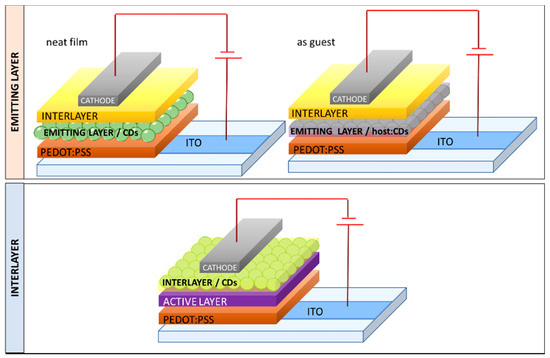
Figure 7.
Scheme of the possible device architectures that incorporate CD as an emitter or as an interlayer.
Table 1 gathers the main characteristics of the OLED in which the CDs are used as emitter or charge regulating interlayer. Starting materials, dimension and shape, electroluminescence peak (EL
PEAK
), maximum luminance (L
MAX
), current efficiency (η
c) and publication year are reported (where available).
) and publication year are reported (where available).
CDs as neat emitter are reported in the first 8 entries of the table, while entries 9-15 reported the main OLED performance with CDs as guest emitters in blend dispersion. In the last four entries the main parameters of devices featuring CDs as charge regulating interlayers are shown.
CDs as neat emitter are reported in the first 8 entries of the table, while entries 9-15 reported the main OLED performance with CDs as guest emitters in blend dispersion. In the last four entries the main parameters of devices featuring CDs as charge regulating interlayers are shown.
Table 1. Summary of performance [[42]] of Organic Light-Emitting Devices (OLEDs) incorporating a CD layer (2020 updated).
|
Entry |
Starting Materials |
Dimension (nm)/Shape |
ELPEAK (nm) |
LMAX (cd/m2) |
ηc (Cd/A) |
[Ref] (Year) |
|
|
CDs as neat emitter |
|
|
|
|
|
|
1 |
Citric acid with 2,3-diaminonaphthalene |
1.95 nm |
455 |
136 |
0.084 |
[65] (2017) |
|
2.41 nm |
536 |
93 |
0.045 |
|||
|
3.78 nm |
555 |
60 |
0.02 |
|||
|
4.9 nm |
585 |
65 |
0.027 |
|||
|
6.68 nm |
628 |
12 |
0.0028 |
|||
|
2 |
ethylenediamine and phthalic acid |
5.53 nm |
455 |
4.97 |
- |
[66] (2017) |
|
3 |
citric acid + octadecene + 1-hexadecylamine |
Spherical 5 nm |
550-670 |
35 |
0.022 |
[73] (2011) |
|
4 |
1-octadecene 1-hexadecylamine |
6 ± 1.9 nm |
460 |
21 |
0.06 |
[74] (2019) |
|
5 |
1-hexadecylamine and anhydrous citric acid |
3.3 nm |
426 |
24 |
0.018 |
[75] (2013) |
|
6 |
1-hexadecylamine and anhydrous citric acid |
426, 452, 588 |
61 |
|||
|
7 |
1-hexadecylamine and anhydrous citric acid + ZnO nps |
426, 452, 588 |
90 |
|||
|
8 |
anhydrous citric acid and hexadecylamine |
Spherical 2.0–2.5 nm lattice spacing 0.22 |
554 |
5.7 |
|
[76] (2018) |
|
|
CDs as guest emitter |
|
|
|
|
|
|
9 |
citric acid and diaminonaphthalene. |
quasi-spherical 2.4 lattice spacing 0.21 nm |
450 |
5240 |
2.6 |
[77] (2020) |
|
10 |
Phloroglucinol |
triangular 1.9 nm |
476 |
1882 |
1.22 |
[78] (2018) |
|
2.4 nm |
510 |
4762 |
5.11 |
|||
|
3 nm |
540 |
2784 |
2.31 |
|||
|
3.9 nm |
602 |
2344 |
1.73 |
|||
|
11 |
Citric acid with 2,3-diaminonaphthalene |
2.41 nm |
536 |
2050 |
1.1 |
[65] (2017) |
|
12 |
human hair |
2D array of CDs 2–6 nm |
498 |
350 |
0.22 |
[79] (2020) |
|
700 |
0.2 |
|||||
|
13 |
N,N-dimethyl-, N,N-diethyl-, and N,N-dipropyl-p-phenylenediamine |
quasi-spherical 2.2 ± 0.31. 2.3 ± 0.28. 2.3 ± 0.26 nm lattice spacing 0.21 nm |
605/434 612/435 616/435 |
5248–5909 |
3.65 3.85 |
[80] (2019) |
|
14 |
anhydrous citric acid and hexadecylamine |
spherical_2.0–2.5 nm lattice spacing 0.22 |
558-550 |
339.5–455.2 |
- |
[73] (2011) |
|
15 |
anhydrous citric acid and hexadecylamine |
- |
474 |
569.8 |
- |
[81] (2018) |
|
|
CDs as interlayer |
|
||||
|
16 |
ethylenediamine and citric acid |
- |
532 |
30 730 |
93.8 |
[82] (2020) |
|
17 |
banana leaves |
4–6 nm (quasi-spherical) |
486 |
- |
- |
[83] (2019) |
|
18 |
Ethanolamine |
- |
622 |
3500 |
0.63 |
[84] (2017) |
|
19 |
Citric acid and p-phenylendiamine |
|
|
13 |
9x10-4 |
[85] (2020) |
|
Citric acid and ethylenediamine |
|
|
70 |
2x10-3 |
||
|
Citric acid and urea |
|
|
146 |
2x10-3 |
||
|
Citric acid and 1- hexadecylamine |
|
|
174 |
8x10-4 |
||
|
Entry |
Starting Materials |
Dimension (nm)/Shape |
ELPEAK (nm) |
LMAX (cd/m2) |
ηc (Cd/A) |
[Ref] (Year) |
|
|
CDs as neat emitter |
|
|
|
|
|
|
1 |
Citric acid with 2,3-diaminonaphthalene |
1.95 nm |
455 |
136 |
0.084 |
[[65]] (2017) |
|
2.41 nm |
536 |
93 |
0.045 |
|||
|
3.78 nm |
555 |
60 |
0.02 |
|||
|
4.9 nm |
585 |
65 |
0.027 |
|||
|
6.68 nm |
628 |
12 |
0.0028 |
|||
|
2 |
ethylenediamine and phthalic acid |
5.53 nm |
455 |
4.97 |
- |
[[66]] (2017) |
|
3 |
citric acid + octadecene + 1-hexadecylamine |
Spherical 5 nm |
550-670 |
35 |
0.022 |
[[73]] (2011) |
|
4 |
1-octadecene 1-hexadecylamine |
6 ± 1.9 nm |
460 |
21 |
0.06 |
[[74]] (2019) |
|
5 |
1-hexadecylamine and anhydrous citric acid |
3.3 nm |
426 |
24 |
0.018 |
[[75]] (2013) |
|
6 |
1-hexadecylamine and anhydrous citric acid |
426, 452, 588 |
61 |
|||
|
7 |
1-hexadecylamine and anhydrous citric acid + ZnO nps |
426, 452, 588 |
90 |
|||
|
8 |
anhydrous citric acid and hexadecylamine |
Spherical 2.0–2.5 nm lattice spacing 0.22 |
554 |
5.7 |
|
[[76]] (2018) |
|
|
CDs as guest emitter |
|
|
|
|
|
|
9 |
citric acid and diaminonaphthalene. |
quasi-spherical 2.4 lattice spacing 0.21 nm |
450 |
5240 |
2.6 |
[[77]] (2020) |
|
10 |
Phloroglucinol |
triangular 1.9 nm |
476 |
1882 |
1.22 |
[[78]] (2018) |
|
2.4 nm |
510 |
4762 |
5.11 |
|||
|
3 nm |
540 |
2784 |
2.31 |
|||
|
3.9 nm |
602 |
2344 |
1.73 |
|||
|
11 |
Citric acid with 2,3-diaminonaphthalene |
2.41 nm |
536 |
2050 |
1.1 |
[[65]] (2017) |
|
12 |
human hair |
2D array of CDs 2–6 nm |
498 |
350 |
0.22 |
[[79]] (2020) |
|
700 |
0.2 |
|||||
|
13 |
N,N-dimethyl-, N,N-diethyl-, and N,N-dipropyl-p-phenylenediamine |
quasi-spherical 2.2 ± 0.31. 2.3 ± 0.28. 2.3 ± 0.26 nm lattice spacing 0.21 nm |
605/434 612/435 616/435 |
5248–5909 |
3.65 3.85 |
[[80]] (2019) |
|
14 |
anhydrous citric acid and hexadecylamine |
spherical_2.0–2.5 nm lattice spacing 0.22 |
558-550 |
339.5–455.2 |
- |
[[73]] (2011) |
|
15 |
anhydrous citric acid and hexadecylamine |
- |
474 |
569.8 |
- |
[[81]] (2018) |
|
|
CDs as interlayer |
|
||||
|
16 |
ethylenediamine and citric acid |
- |
532 |
30 730 |
93.8 |
[[82]] (2020) |
|
17 |
banana leaves |
4–6 nm (quasi-spherical) |
486 |
- |
- |
[[83]] (2019) |
|
18 |
Ethanolamine |
- |
622 |
3500 |
0.63 |
[[84]] (2017) |
|
19 |
Citric acid and p-phenylendiamine |
|
|
13 |
9x10-4 |
[[85]] (2020) |
|
Citric acid and ethylenediamine |
|
|
70 |
2x10-3 |
||
|
Citric acid and urea |
|
|
146 |
2x10-3 |
||
|
Citric acid and 1- hexadecylamine |
|
|
174 |
8x10-4 |
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85]
References
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P.; International Telecommunication Union; United Nations University; International Solid Waste Association. The Global E-Waste Monitor 2017: Quantities, Flows, and Resources; United Nations University: Tokyo, Japan; International Telecommunication Union: Geneva, Switzerland; International Solid Waste Association: Rotterdam, The Netherlands, 2017; ISBN 9789280890532.
- Ostroverkhova, O. Organic Optoelectronic Materials: Mechanisms and Applications. Chem. Rev. 2016, 116, 13279–13412.
- Forrest, S.R.; Thompson, M.E. Introduction: Organic electronics and optoelectronics. Chem. Rev. 2007, 107, 923–925.
- Hadziioannou, G.; Malliaras, G.G.M. Semiconducting Polymers: Chemistry, Physics and Engineering, 2nd Edition, Two-Volume Set; Hadziioannou, G., Malliaras, G.G.M., Eds.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-3-527-31271-9.
- Zvezdin, A.; Di Mauro, E.; Rho, D.; Santato, C.; Khalil, M. En route toward sustainable organic electronics. MRS Energy Sustain. 2020, 7, doi:10.1557/mre.2020.16.
- Giovanella, U.; Betti, P.; Bolognesi, A.; Destri, S.; Melucci, M.; Pasini, M.; Porzio, W.; Botta, C. Core-type polyfluorene-based copolymers for low-cost light-emitting technologies. Org. Electron. 2010, 11, 2012–2018, doi:10.1016/j.orgel.2010.09.009.
- Irimia-Vladu, M. “Green” electronics: Biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 2014, 43, 588–610.
- Yan, L.; Zhao, F.; Wang, J.; Zu, Y.; Gu, Z.; Zhao, Y. A Safe-by-Design Strategy towards Safer Nanomaterials in Nanomedicines. Adv. Mater. 2019, 31, 1805391.
- Gomulya, W.; Derenskyi, V.; Kozma, E.; Pasini, M.; Loi, M.A. Polyazines and polyazomethines with didodecylthiophene units for selective dispersion of semiconducting single-walled carbon nanotubes. Adv. Funct. Mater. 2015, 25, 5858–5864, doi:10.1002/adfm.201502912.
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737, doi:10.1021/ja040082h.
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757, doi:10.1021/ja062677d.
- Xiao, L.; Sun, H. Novel properties and applications of carbon nanodots. Nanoscale Horiz. 2018, 3, 565–597.
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and graphene quantum dots for optoelectronic and energy devices: A review. Adv. Funct. Mater. 2015, 25, 4929–4947, doi:10.1002/adfm.201501250.
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.H.; Sain, M. Future perspectives and review on organic carbon dots in electronic applications. ACS Nano 2019, 13, 6224–6255, doi:10.1021/acsnano.9b00688.
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634.
- Liu, M.L.; Chen, B. Bin; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471.
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939, doi:10.1039/c4tc00988f.
- Gayen, B.; Palchoudhury, S.; Chowdhury, J. Carbon dots: A mystic star in the world of nanoscience. J. Nanomater. 2019, 2019, 3451307.
- Chan, K.K.; Yap, S.H.K.; Yong, K.T. Biogreen synthesis of carbon dots for biotechnology and nanomedicine applications. Nano-Micro Lett. 2018, 10, 72.
- Zuo, J.; Jiang, T.; Zhao, X.; Xiong, X.; Xiao, S.; Zhu, Z. Preparation and application of fluorescent carbon dots. J. Nanomater. 2015, 2015, doi:10.1155/2015/787862.
- Pal, A.; Sk, M.P.; Chattopadhyay, A. Recent advances in crystalline carbon dots for superior application potential. Mater. Adv. 2020, 1, 525–553, doi:10.1039/d0ma00108b.
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J.; et al. Full-color fluorescent carbon quantum dots. Sci. Adv. 2020, 6, eabb6772, doi:10.1126/sciadv.abb6772..
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2011, 47, 932–934, doi:10.1039/c0cc03552a.
- Yang, Z.C.; Wang, M.; Yong, A.M.; Wong, S.Y.; Zhang, X.H.; Tan, H.; Chang, A.Y.; Li, X.; Wang, J. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem. Commun. 2011, 47, 11615–11617, doi:10.1039/c1cc14860e.
- Hu, S.; Liu, J.; Yang, J.; Wang, Y.; Cao, S. Laser synthesis and size tailor of carbon quantum dots. J. Nanoparticle Res. 2011, 13, 7247–7252, doi:10.1007/s11051-011-0638-y.
- Tarasenka, N.; Stupak, A.; Tarasenko, N.; Chakrabarti, S.; Mariotti, D. Structure and optical properties of carbon nanoparticles generated by laser treatment of graphite in liquids. ChemPhysChem 2017, 18, 1074–1083, doi:10.1002/cphc.201601182.
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290, doi:10.1039/c3ra00088e.
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chemie Int. Ed. 2013, 52, 3953–3957, doi:10.1002/anie.201300519.
- Dong, Y.; Pang, H.; Yang, H. Bin; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chemie Int. Ed. 2013, 52, 7800–7804, doi:10.1002/anie.201301114.
- Chen, Y.; Lian, H.; Wei, Y.; He, X.; Chen, Y.; Wang, B.; Zeng, Q.; Lin, J. Concentration-induced multi-colored emissions in carbon dots: Origination from triple fluorescent centers. Nanoscale 2018, 10, 6734–6743, doi:10.1039/c8nr00204e.
- Ehrat, F.; Bhattacharyya, S.; Schneider, J.; Löf, A.; Wyrwich, R.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Tracking the source of carbon dot photoluminescence: aromatic domains versus molecular fluorophores. Nano Lett. 2017, 17, 7710–7716, doi:10.1021/acs.nanolett.7b03863.
- Bao, L.; Liu, C.; Zhang, Z.L.; Pang, D.W. Photoluminescence-tunable carbon nanodots: Surface-state energy-gap tuning. Adv. Mater. 2015, 27, 1663–1667, doi:10.1002/adma.201405070.
- Zhang, J.; Su, Z.C.; Cui, Y.; Hu, G.; Tang, Y.L.; Gan, Z.X.; Yang, L.; Lao, X.Z.; Bao, Y.T.; Xu, S.J. The roles of self-absorption and radiative energy transfer in photoluminescence of N-doped carbon nanodots in solution. AIP Adv. 2019, 9, 035135, doi:10.1063/1.5078443.
- Khan, S.; Gupta, A.; Verma, N.C.; Nandi, C.K. Time-resolved emission reveals ensemble of emissive states as the origin of multicolor fluorescence in carbon dots. Nano Lett. 2015, 15, 8300–8305, doi:10.1021/acs.nanolett.5b03915.
- Zhu, S.; Zhang, J.; Liu, X.; Li, B.; Wang, X.; Tang, S.; Meng, Q.; Li, Y.; Shi, C.; Hu, R.; et al. Graphene quantum dots with controllable surface oxidation, tunable fluorescence and up-conversion emission. RSC Adv. 2012, 2, 2717, doi:10.1039/c2ra20182h.
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J. Mater. Chem. 2011, 21, 2445, doi:10.1039/c0jm02963g.
- Yang, S.T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.H.; Liu, Y.; Chen, M.; et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114, doi:10.1021/jp9085969.
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res. Lett. 2013, 8, 1–9.
- Dias, C.; Vasimalai, N.; P. Sárria, M.; Pinheiro, I.; Vilas-Boas, V.; Peixoto, J.; Espiña, B. Biocompatibility and bioimaging potential of fruit-based carbon dots. Nanomaterials 2019, 9, 199, doi:10.3390/nano9020199.
- Sendão, R.; Yuso, M. del V.M. de; Algarra, M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Comparative life cycle assessment of bottom-up synthesis routes for carbon dots derived from citric acid and urea. J. Clean. Prod. 2020, 254, doi:10.1016/j.jclepro.2020.120080.
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381.
- Giovanella, U.; Pasini, M.; Botta, C. Organic Light-Emitting Diodes (OLEDs): Working Principles and Device Technology; Springer: Cham, Switzerland, 2016; pp. 145–196.
- Squeo, B.M.; Pasini, M. BODIPY platform: A tunable tool for green to NIR OLEDs. Supramol. Chem. 2020, 32, 56–70, doi:10.1080/10610278.2019.1691727.
- De Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195, doi:10.1039/c9tc01640f.
- Huang, C.C.; Hung, Y.S.; Weng, Y.M.; Chen, W.; Lai, Y.S. Sustainable development of carbon nanodots technology: Natural products as a carbon source and applications to food safety. Trends Food Sci. Technol. 2019, 86, 144–152, doi:10.1016/j.tifs.2019.02.016.
- Ludmerczki, R.; Mura, S.; Carbonaro, C.M.; Mandity, I.M.; Carraro, M.; Senes, N.; Garroni, S.; Granozzi, G.; Calvillo, L.; Marras, S.; et al. Carbon dots from citric acid and its intermediates formed by thermal decomposition. Chem. A Eur. J. 2019, 25, 11963–11974, doi:10.1002/chem.201902497.
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, doi:10.1016/j.nantod.2016.08.006.
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci. Rep. 2014, 4, 1–8, doi:10.1038/srep04976.
- Kandasamy, G. Recent advancements in doped/co-doped carbon quantum dots for multi-potential applications. C J. Carbon Res. 2019, 5, 24, doi:10.3390/c5020024.
- Mandal, B.; Sarkar, S.; Sarkar, P. Exploring the electronic structure of graphene quantum dots. J. Nanoparticle Res. 2012, 14, doi:10.1007/s11051-012-1317-3.
- Li, Y.; Shu, H.; Niu, X.; Wang, J. Electronic and optical properties of edge-functionalized graphene quantum dots and the underlying mechanism. J. Phys. Chem. C 2015, 119, 24950–24957, doi:10.1021/acs.jpcc.5b05935.
- Yamijala, S.S.; Bandyopadhyay, A.; Pati, S.K. Structural stability, electronic, magnetic, and optical properties of rectangular graphene and boron nitride quantum dots: Effects of size, substitution, and electric field. J. Phys. Chem. C 2013, 117, 23295–23304, doi:10.1021/jp406344z.
- Güttinger, J.; Stampfer, C.; Frey, T.; Ihn, T.; Ensslin, K. Graphene quantum dots in perpendicular magnetic fields. Phys. status solidi 2009, 246, 2553–2557, doi:10.1002/pssb.200982312.
- Li, X.; Lau, S.P.; Tang, L.; Ji, R.; Yang, P. Sulphur doping: A facile approach to tune the electronic structure and optical properties of graphene quantum dots. Nanoscale 2014, 6, 5323–5328, doi:10.1039/c4nr00693c.
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, doi:10.1016/j.mtchem.2017.09.001.
- Guo, W.; Luo, Y.; Wei, K.; Gao, X. A cellular level biocompatibility and biosafety evaluation of mesoporous SiO2-based nanocomposite with lanthanum species. J. Mater. Sci. 2011, 47, 1514–1521, doi:10.1007/s10853-011-5938-1.
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 1–8, doi:10.1038/ncomms5596.
- Tang, L.; Ji, R.; Li, X.; Bai, G.; Liu, C.P.; Hao, J.; Lin, J.; Jiang, H.; Teng, K.S.; Yang, Z.; et al. Deep ultraviolet to near-infrared emission and photoresponse in layered n-doped graphene quantum dots. ACS Nano 2014, 8, 6312–6320, doi:10.1021/nn501796r.
- Shamsipur, M.; Barati, A.; Karami, S. Long-wavelength, multicolor, and white-light emitting carbon-based dots: Achievements made, challenges remaining, and applications. Carbon N. Y. 2017, 124, doi:10.1016/j.carbon.2017.08.072.
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984, doi:10.1039/C5TC00813A.
- Wang, Y.; Li, Y.; Yan, Y.; Xu, J.; Guan, B.; Wang, Q.; Li, J.; Yu, J. Luminescent carbon dots in a new magnesium aluminophosphate zeolite. Chem. Commun. 2013, 49, 9006–9008, doi:10.1039/c3cc43375g.
- Xu, Q.; Kuang, T.; Liu, Y.; Cai, L.; Peng, X.; Sreenivasan Sreeprasad, T.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-doped carbon dots: Synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219.
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970, doi:10.1039/c7tc05878k.
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583.
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv. Mater. 2017, 29, doi:10.1002/adma.201604436.
- Ding, Y.; Zhang, F.; Xu, J.; Miao, Y.; Yang, Y.; Liu, X.; Xu, B. Synthesis of short-chain passivated carbon quantum dots as the light emitting layer towards electroluminescence. RSC Adv. 2017, 7, 28754–28762, doi:10.1039/c7ra02421e.
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 2018, 30, 1704740, doi:10.1002/adma.201704740.
- Shi, L.; Yang, J.H.; Zeng, H.B.; Chen, Y.M.; Yang, S.C.; Wu, C.; Zeng, H.; Yoshihito, O.; Zhang, Q. Carbon dots with high fluorescence quantum yield: The fluorescence originates from organic fluorophores. Nanoscale 2016, 8, 14374–14378, doi:10.1039/c6nr00451b.
- Song, Y.; Zhu, S.; Yang, B. Bioimaging based on fluorescent carbon dots. RSC Adv. 2014, 4, 27184–27200.
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491.
- Zheng, X.; Wang, H.; Gong, Q.; Zhang, L.; Cui, G.; Li, Q.; Chen, L.; Wu, F.; Wang, S. Highly luminescent carbon nanoparticles as yellow emission conversion phosphors. Mater. Lett. 2015, 143, doi:10.1016/j.matlet.2014.12.138.
- Joseph, J.; Anappara, A.A. White-light-emitting carbon dots prepared by the electrochemical exfoliation of graphite. ChemPhysChem 2017, 18, 292–298, doi:10.1002/cphc.201601020.
- Wang, F.; Chen, Y.; Liu, C.; Ma, D. White light-emitting devices based on carbon dots’ electroluminescence. Chem. Commun. 2011, 47, 3502, doi:10.1039/c0cc05391k.
- Paulo-Mirasol, S.; Martínez-Ferrero, E.; Palomares, E. Direct white light emission from carbon nanodots (C-dots) in solution processed light emitting diodes. Nanoscale 2019, 11, 11315–11321, doi:10.1039/c9nr02268f.
- Zhang, X.; Zhang, Y.; Wang, Y.; Kalytchuk, S.; Kershaw, S.V.; Wang, Y.; Wang, P.; Zhang, T.; Zhao, Y.; Zhang, H.; et al. Color-switchable electroluminescence of carbon dot light-emitting diodes. ACS Nano 2013, 7, 11234–11241, doi:10.1021/nn405017q.
- Xu, J.; Miao, Y.; Zheng, J.; Wang, H.; Yang, Y.; Liu, X. Carbon dot-based white and yellow electroluminescent light emitting diodes with a record-breaking brightness. Nanoscale 2018, 10, 11211–11221, doi:10.1039/c8nr01834k.
- Yuan, F.; Wang, Y.-K.; Sharma, G.; Dong, Y.; Zheng, X.; Li, P.; Johnston, A.; Bappi, G.; Fan, J.Z.; Kung, H.; et al. Bright high-colour-purity deep-blue carbon dot light-emitting diodes via efficient edge amination. Nat. Photonics 2020, 14, doi:10.1038/s41566-019-0557-5.
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.; Chen, A.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 1–11, doi:10.1038/s41467-018-04635-5
- Singh, A.; Wolff, A.; Yambem, S.D.; Esmaeili, M.; Riches, J.D.; Shahbazi, M.; Feron, K.; Eftekhari, E.; Ostrikov, K. (Ken); Li, Q.; et al. Biowaste‐derived, self‐organized arrays of high‐performance 2d carbon emitters for organic light‐emitting diodes. Adv. Mater. 2020, 32, 1906176, doi:10.1002/adma.201906176
- Jia, H.; Wang, Z.; Yuan, T.; Yuan, F.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Electroluminescent warm white light‐emitting diodes based on passivation enabled bright red bandgap emission carbon quantum dots. Adv. Sci. 2019, 6, 1900397, doi:10.1002/advs.201900397.
- Xu, J.; Miao, Y.; Zheng, J.; Yang, Y.; Liu, X. Ultrahigh brightness carbon dot–based blue electroluminescent leds by host–guest energy transfer emission mechanism. Adv. Opt. Mater. 2018, 6, 1800181, doi:10.1002/adom.201800181.
- Zhang, X.; Zeng, Q.; Xiong, Y.; Ji, T.; Wang, C.; Shen, X.; Lu, M.; Wang, H.; Wen, S.; Zhang, Y.; et al. Energy level modification with carbon dot interlayers enables efficient perovskite solar cells and quantum dot based light‐emitting diodes. Adv. Funct. Mater. 2020, 30, 1910530, doi:10.1002/adfm.201910530
- Alam, M.B.; Yadav, K.; Shukla, D.; Srivastava, R.; Lahiri, J.; Parmar, A.S. Carbon quantum dot as electron transporting layer in organic light emitting diode. ChemistrySelect 2019, 4, 7450–7454, doi:10.1002/slct.201901551
- Park, Y.R.; Jeong, H.Y.; Seo, Y.S.; Choi, W.K.; Hong, Y.J. Quantum-dot light-emitting diodes with nitrogen-doped carbon nanodot hole transport and electronic energy transfer layer. Sci. Rep. 2017, 7, 1–13, doi:10.1038/srep46422.
- Paulo-Mirasol, S.; Gené-Marimon, S.; Martínez-Ferrero, E.; Palomares, E. Inverted hybrid light-emitting diodes using carbon dots as selective contacts: the effect of surface ligands. ACS Appl. Electron. Mater. 2020, 2, 1388–1394, doi:10.1021/acsaelm.0c00163.
