Hydrogels, three-dimensional (3D) polymer networks, present unique properties, like biocompatibility, biodegradability, tunable mechanical properties, sensitivity to various stimuli, the capacity to encapsulate different therapeutic agents, and the ability of controlled release of the drugs.
- hydrogel
- cellulose
- drug delivery
- drug delivery systems
- drug-release mechanisms
1. Introduction
The pharmaceutical field has experienced progressive development in the treatment of human diseases, the most recent achievement being the administration of biomolecules (drugs, proteins, etc.) by biomaterial carriers. The development of these carriers made the drugs release possible in certain places of the human body inaccessible by classical administration methods, allowing them to reach the target organs safely, without causing any harm [1].
It is crucial to control the release of drugs, as the pharmacological purpose is not achieved in the case of a rapid release. An “ideal” drug carrier system should deliver an exact amount of drug, at a certain preplanned rate, in order to provide the required drug level for treatment [2]. Thus, by using these systems, the bioavailability of the drugs is improved, the drug concentration is maintained relatively constant during the treatment and, last but not least, undesired side effects are considerably reduced by avoiding some issues such as the absorption in the gastrointestinal tract (GIT) and the hepatic first-pass metabolism [3].
2. Hydrogels as Potential Drug Delivery Systems
Hydrogels make an important contribution to the evolution of controlled drug delivery systems. In recent years, hydrogels have increasingly attracted the attention of researchers regarding the design and preparation of controlled drug-delivery systems, due to their special properties which certifies them for biomedical applications [4][295][306][317]. Their high swelling degree with the capacity to retain a high amount of liquid and soft consistency, make them similar to living tissues [128]. Hydrogels have a number of advantages which make them suitable for drug-delivery applications, such as (i) biocompatibility, (ii) the ability to design and control their properties, (iii) the capacity to encapsulate water-soluble compounds, and (iv) the possibility of sustained and local release of active ingredients [295].
Usually, the initial step is realized outside the body and consists of the incorporation of the drugs within hydrogels, and afterwards the hydrogel-drug complex is introduced into the body, directly at the affected site. However, the principal drawback of this type of polymeric system is represented by the implantation of the voluminous material within body, through surgery. Therefore, in recent years, there has been increasing interest in the preparation of formulations that are in a solution state outside the body, but which, once injected inside the body, turn into a gel [329].
2.1. Properties of Hydrogels
Hydrogels have gained special interest for applications such as drug administration due to their unique physical properties. Their potential to work as a drug delivery system is a combination of various factors, such as (Figure 1) [329][3310]: volume fraction of polymer—influences the amount of absorbed fluid by the hydrogel, and the crosslinking degree—have influence on the pore dimensions, thus directly on the structure of the hydrogel network, which can be correlated with the mechanical properties of the hydrogel, with its biodegradability, or with the processes of encapsulation/release of the drugs. Three of the most important parameters that characterize the structure of hydrogels are: morphology—their porous structure; swelling degree—which has a major influence on the mechanism of drug release from the polymer network; and elasticity—influence the mechanical properties of the network [911].
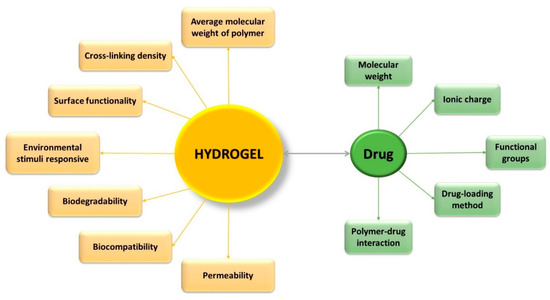
Figure 1. Characteristics which influence the effectiveness of controlled drug-delivery process.
The drugs are released from the polymer network only through a diffusion mechanism and in this sense the type of porous structure of hydrogels is particularly important [3412]. Depending on the pore size within the three-dimensional network of hydrogels, they can be classified as follows [1113]:
macroporous — average pore size: 0.1–1 μm—drug release: mechanism that depends on the matrix porosity and diffusion coefficient of the drug;
microporous — average pore size: 100–1000 Å—drug release: molecular diffusion and convection;
non-porous — average pore size: 10–100 Å—drug release: diffusion mechanism.
Researchers have designed and optimized the physical and chemical properties of the hydrogels for specific applications, such as sustained-release applications (permeability), pulsatile-release applications (environmental responsive nature), bioresorbable applications (biodegradability), and targeted release and bioadhesion applications (surface biorecognition sites) [3514].
Hydrogels intended for use in medicinal products must be non-toxic and have properties such as biocompatibility, biodegradability and adequate physical and mechanical integrity [911][3615].
Biocompatibility is sustained both by the high content of water within the hydrogel and by the similarities between the properties of hydrogels and those of the extracellular matrix [329]. The toxicity of hydrogels is mainly related to the toxicity of the unreacted components of hydrogels, such as monomers, oligomers, initiators, etc. To reduce the toxic effects of the hydrogels, the following must be taken into account: (i) to avoid the use of initiators, (ii) to remove the impurities by a powerful washing, or (iii) to use reactions with high rates of conversion, in order to eliminate the unreacted monomers and by-products [1]. Biodegradability is not always necessary for hydrogel formulations. This depends on the location where the drug delivery device is used. Therefore, it is not necessary for oral and transdermal drug administration, while it is absolutely necessary when hydrogels are used to various parts inside the body, in order to avoid unpleasant reactions of the human body to foreign bodies in the organism and even their surgical removal [3716][3817]. The hydrogels’ biodegradability can be achieved by different ways: as chemical and enzymatic oxidation, hydrolytic degradation, enzymatic degradation or thermal and mechanical degradation [329].
In the applications where biodegradability is not absolutely necessary, it is even more important to keep the integrity of the hydrogel, due to situations where the drugs need to be protected from the severe conditions within the body, until the drugs can be delivered to the target site [1]. The hydrogel strength can be improved either by incorporating of nanoparticles, or through raising the cross-linking degree. Nevertheless, the degree of cross-linking cannot be increased too much, due to the fact that a higher cross-linking degree induces a loss of elasticity, with the hydrogel becoming brittle. In addition, the elasticity is an important property of the gel because it gives flexibility to the 3D network, contributing to the circulation of the incorporated therapeutic agent within the polymeric network. That is why it is necessary to achieve a compromise between the mechanical strength and the elasticity of the hydrogels for a proper application of these [3918][4019].
2.2. Hydrogels Delivery Systems
The delivery systems based on hydrogels used for controlled drug release can be classified into reservoir and matrix devices [4019][4120][4221][4322][4423].
2.2.1. Reservoir System
Currently, the reservoir-based system is the most widely used for controlled drug delivery. In these types of system, the drug core is covered all around by a hydrogel membrane (Figure 2). The membrane thickness and the properties of the entrapped drug, like solubility, molecular weight and size of particle, control the release rate of the drug [4322][4423]. Upon contact with water, it diffuses through the hydrogel membrane and dissolves the drug until it reaches saturation solubility (Cs). As the drug diffuses through the membrane to the external medium, the drug concentration in the reservoir decreases below Cs, so that new amounts of the solid drug present in the core dissolve and the concentration Cs are restored. This ensures a constant rate of the release of the drug from a reservoir-based system that follows zero-order kinetics if the solid drug is still present in the core [4019].
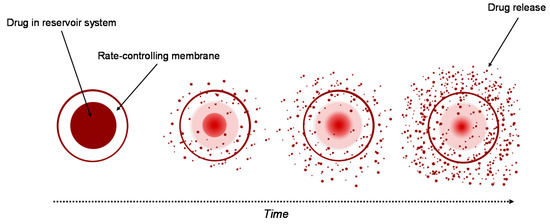
Figure 2. Drug delivery from reservoir device.
2.2.2. Matrix System
A similar system to the reservoir-based system is the matrix-type delivery system, with the observation that in this case, the drug is uniformly distributed as a solid into a hydrogel matrix (Figure 3) [4019][4221]. A matrix is composed of one or more drugs along with a hydrophilic polymer, as a gelling agent [4524]. In both types of delivery system, drugs are retained within the polymer matrices, but are non-covalent [4322].
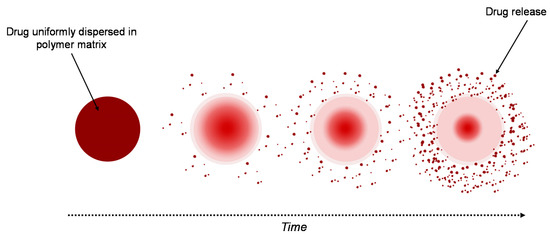
Figure 3. Drug delivery from a matrix system.
The drug release strongly depends on the matrix’s properties. When the system is placed into aqueous medium, water diffuses into the matrix hydrating it from the surface to the core. Three important processes control the release of drugs, these being: (i) the process of diffusion of water into the matrix, (ii) the process of dissolution of the drug, and (iii) the process of diffusion of the drug from the system. The polymer–drug interactions have an important role in the release process of the drug, in this case. Furthermore, the thickness of the hydrated matrix is included as the diffusional path length of the drug [4019]. A sustained-release matrix-type drug-delivery system has an important role in increasing the therapeutic effectiveness of the drugs by controlled and sustained release, and by selecting the desired site. For a specific period of time, a constant released level of the drug is maintained, so that the adverse effects are avoided [128].
2.3. Hydrogel Drug-Release Mechanisms
The main criterion for classifying the controlled drug-release processes is the mechanism which leads the release of the active pharmaceutical ingredient (API). The release mechanism is a result of three main processes: (i) drug diffusion, (ii) matrix swelling, and (iii) chemical reactivity of the drug/matrix [3716]. Various mathematical models were developed in order to estimate the drug release as a function of time, which are based on the limiting rate of the release process [911][1325][3310]. The mechanisms for the drug release and the corresponding hydrogel-type delivery systems can be classified as following [4626][4727][4828][4929][5030]:
diffusion-controlled — matrix and reservoir systems;
-
− swelling-controlled — systems that are osmotically controlled and are activated with different solvents;
-
− chemically controlled
— systems that can be biodegraded or eroded and pendant chain systems.
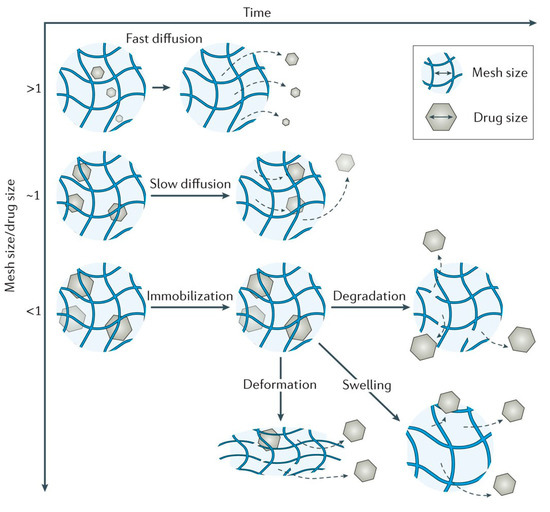
In Figure 4 are presented the schematic view of the drug release mechanism from hydrogels [5131].
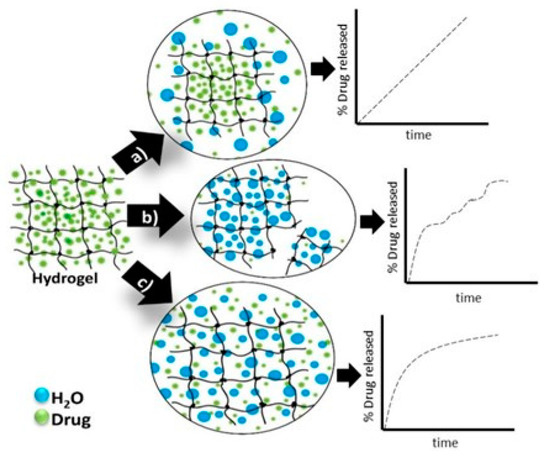
Figure 4. Hydrogels drug-release mechanisms and their respective kinetic profiles: (a) the case when the governing mechanism is given by the drug diffusion; (b) when it is imposed by the degradation of the polymeric matrix; (c) when the hydrogel swelling governs the process [5131].
Passive diffusion is the most common release mechanism. In this mechanism, depending on the mesh size of the matrix, the biotherapeutic molecules entrapped within the matrix can diffuse freely. In the case of systems in which the release of active principles is based on an erosion-controlled mechanism, there is a close dependence between the rate of drug release and the rate of erosion. In the latter case of mechanism, chemically controlled release is based on a series of chemical reactions that produce either the degradation of the polymer in the hydrogel matrix by hydrolytic or enzymatic reactions or reactions of cleavage of the polymer–drug bonds [2732].
2.3.1. Diffusion-Controlled Drug Mechanism
As shown above, the diffusion-controlled release is the most common mechanism of drug release from hydrogels and it is used by reservoir or matrix devices [1325]. Reservoir-type delivery systems offers a constant and time-independent release of the drug, while the matrix system is one time-dependent drug release system and its working depends on the size of the open space or macromolecular mesh. For a time-dependent system, the initial release rate is variable and is proportional to the square root of time [5233].
Hydrogels are in fact cross-linked polymer networks with open spaces between polymer chains, called meshes, which permit the diffusion for liquids and small solutes. The most important feature is the mesh size because it influences the steric interactions between the network and the drug, and ultimately determines how the drug is released from the hydrogel. Depending on the ratio in which the mesh sizes (Rmesh) and the size of the drug molecules (Rdrug) are found, the following three situations can be identified (Figure 5) [5334]:
-
- Rmesh/Rdrug > 1, mesh size is larger than the drug molecules: the whole release process is controlled by diffusion. It is the case of small drug molecules which diffuse freely through the network, and their migration is not dependent on the mesh size;
-
- Rmesh/Rdrug ~ 1, mesh size reaches the drug size: the steric hindrance dominates the drug diffusion. The resulting effect is a slow drug diffusion, which is reflected by a slow and extended-release;
-
- Rmesh/Rdrug < 1, mesh size is very small and/or drug molecules are too large. The effect of steric hindrance causes a blockage of the drug within the network, until there is a degradation of the network or an increase in mesh size by swelling or deformation.
2.3.2. Swelling-Controlled Drug-Release Mechanism
Another possibility to release enclosed drugs is to control the swelling process of hydrogels. Swelling-controlled drug release could occur when the rate of drug diffusion is faster than the rate of hydrogel swelling, the higher the rate of hydrogel swelling, the higher the rate of drug release. Thus, the rate and ability of hydrogels to absorb water and the thickness of polymeric gels are important factors in swelling-controlled delivery systems [1325].
A shortcoming of controlled swelling systems is the too slow response of macroscopic hydrogels due to the slow diffusion of water. To obtain a faster reaction, the diffusion length can be reduced either by decreasing the size of the hydrogel or by designing a system of interconnected macropores into the volume of the hydrogel [5334].
2.3.3. Chemically-Controlled Drug-Release Mechanism
Chemically controlled delivery systems can release the encapsulated drug by breaking the polymer chains as an effect of surface or bulk erosion [1325]. In erodible drug delivery systems, drug release is controlled by either the dissolution or degradation process. Depending on the limiting process, the drug is released through a different mechanism: if erosion is the slower process then the rate of release will be controlled by diffusion; and if drug diffusion is a slow process, then degradation or erosion controls the drug release [911].
Erosion processes of hydrogels can take place in bulk or on the surface. Bulk erosion is the most common in the case of hydrogels because their network is permeable to the main actors of the degradation process, water and enzymes. Conversely, if the rate of cleavage of the bonds is higher than the rate of diffusion of water inside the hydrogel then erosion occurs at the surface [5334].
3. Cellulose-Based Hydrogels in Drug Delivery
Cellulose is the most known biodegradable polymer, being the main component of all vegetable fibers, with properties ranging from low cost and biocompatibility to high mechanical and thermal stability, which makes it a very promising and attractive polymer for various applications [54–57][35][36][37][38]. Cellulose-based hydrogels have properties that recommend them for various applications, such as targeted delivery of drugs and smart sensors, or hydrogels multi-receptive, injectable and self-healing [67,68][39][40]. Hydrogels based on cellulose derivatives have important applications as drug delivery systems (DDS) and are used in order to improve the controlled release of drugs, as a function of external stimuli, such as body temperature and variable pH ranges in different parts of the body [3].
References
- Gupta, A.K. Environmental responsive hydrogels: A novel approach in drug delivery system. J. Drug Deliv. Ther. 2012, 2, 1–8, doi:10.22270/jddt.v2i1.87.
- Moghanjoughi, A.A.; Khoshnevis, D.; Zarrabi, A. A concise review on smart polymers for controlled drug release. Drug Deliv. Transl. Res. 2016, 6, 333–340, doi:10.1007/s13346–015-0274-7.
- Ribeiro, A.M.; Magalhães, M.; Veiga, F.; Figueiras, A. Cellulose-based hydrogels in topical drug delivery: A challenge in medical devices. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Switzerland, 2018; Chapter 40, pp. 1–29.
- Vashist, A.; Gupta, Y.; Ahmad, S. Interpenetrating biopolymer network based hydrogels for an effective drug delivery system. Carbohydr. Polym. 2012, 87, 1433–1439, doi:10.1016/j.carbpol.2011.09.030.
- Kulkarni, R.V.; Biswanath, S. Electrically responsive smart hydrogels in drug delivery: A review. J. Appl. Biomater. Biomech. 2010, 5, 125–139.Onofrei, M.-D.; Filimon, A. Cellulose-based hydrogels: Designing concepts, properties, and perspectives for biomedical and environmental applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Mendez-Vilas, A., Solano, A., Eds.; Formatex Research Center S.L.: Badajoz, Spain, 2016; pp. 108–120.
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729, doi:10.1039/c8tb02491j.Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179, doi:10.1016/j.jconrel.2018.01.023.
- Ali, A.E.; El‐Rehim, H.A.A.; Kamal, H.; Hegazy, D.E.A. Synthesis of Carboxymethyl Cellulose Based Drug Carrier Hydrogel Using Ionizing Radiation for Possible Use as Site Specific Delivery System. J. Macromol. Sci. Part A 2008, 45, 628–634, doi:10.1080/10601320802168751.Hu, J.; Yang, L.; Yang, P.; Jiang, S.; Liu, X.; Li, Y. Polydopamine free radical scavengers. Biomater. Sci. 2020, 8, 4940–4950, doi:10.1039/d0bm01070g.
- Vlaia, L.; Coneac, G.; Olariu, I.; Lupuleasa, D. Cellulose-Derivatives-Based Hydrogels as Vehicles for Dermal and Transdermal Drug Delivery. In Emerging Concepts in Analysis and Applications of Hydrogels, 1st ed.; Majee, S.B., Ed.; IntechOpen: London, UK, 2016; Chapter 7, pp. 159–200.Kumar, A.R.; Aeila, A.S.S. Sustained release matrix type drug delivery system: An overview. World J. Pharm. Pharm. Sci. 2019, 9, 470–480.
- Simões, S.; Figueiras, A.; Veiga, F. Modular Hydrogels for Drug Delivery. J. Biomater. Nanobiotechnol. 2012, 3, 185–199, doi:10.4236/jbnb.2012.32025.Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007, doi:10.1016/j.polymer.2008.01.027.
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2014, 23, 748–770, doi:10.3109/10717544.2014.940091.Nazar, H.; Roldo, M.; Fatouros, D.G.; Van Der Merwe, S.M.; Tsibouklis, J. Hydrogels in mucosal delivery. Ther. Deliv. 2012, 3, 535–555, doi:10.4155/tde.12.16.
- Zhidong, L.; Li, J.; Nie, S.; Liu, H.; Ding, P.; Pan, W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int. J. Pharm. 2006, 315, 12–17, doi:10.1016/j.ijpharm.2006.01.029.Simões, S.; Figueiras, A.; Veiga, F. Modular Hydrogels for Drug Delivery. J. Biomater. Nanobiotechnol. 2012, 3, 185–199, doi:10.4236/jbnb.2012.32025.
- Kumar, A.R.; Aeila, A.S.S. Sustained release matrix type drug delivery system: An overview. World J. Pharm. Pharm. Sci. 2019, 9, 470–480.Amin, S.; Rajabnezhad, S.; Kohli, K. Hydrogels as potential drug delivery systems. Sci. Res. Essays 2009, 3, 1175–1183.
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; Pros and cons. Trends Pharm. Sci. 2019, 5, 7–24.Zhidong, L.; Li, J.; Nie, S.; Liu, H.; Ding, P.; Pan, W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int. J. Pharm. 2006, 315, 12–17, doi:10.1016/j.ijpharm.2006.01.029.
- Kapoor, D.; Patel, M.; Vyas, R.B.; Lad, C.; Lal, B. Site Specific drug delivery through nasal route using bioadhesive polymers. J. Drug Deliv. Ther. 2015, 5, 1–9, doi:10.22270/jddt.v5i1.873.Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360, doi:10.1002/adma.200501612.
- Nokhodchi, A.; Raja, S.; Patel, P.; Asare-Addo, K. The Role of Oral Controlled Release Matrix Tablets in Drug Delivery Systems. BioImpacts 2012, 2, 175–187.Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36, doi:10.3390/gels3040036.
- Kumar, R.; Patil, M.B.; Patil, S.R.; Paschapur, M.S. Polysaccharides based colon specific drug delivery: A review. Int. J. Pharm. Tech. Res. 2009, 1, 334–346.Gonçalves, C.; Pereira, P.A.C.; Gama, M. Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 2010, 3, 1420–1460, doi:10.3390/ma3021420.
- Bai, J.P.; Burckart, G.J.; Mulberg, A.E. Literature Review of Gastrointestinal Physiology in the Elderly, in Pediatric Patients, and in Patients with Gastrointestinal Diseases. J. Pharm. Sci. 2016, 105, 476–483, doi:10.1002/jps.24696.Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373, doi:10.3390/ma2020353.
- Barba, A.A.; D’Amore, M.; Chirico, S.; Lamberti, G.; Titomanlio, G. Swelling of cellulose derivative (HPMC) matrix systems for drug delivery. Carbohydr. Polym. 2009, 78, 469–474, doi:10.1016/j.carbpol.2009.05.001.Cirillo, G.; Curcio, M.; Nicoletta, F.P.; Iemma, F. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics 2019, 11, 486, doi:10.3390/pharmaceutics11090486.
- Edgar, K.J. Cellulose esters in drug delivery. Cellulose 2006, 14, 49–64, doi:10.1007/s10570–006–9087–7.Pal, K.; Banthia, A.K.; Majumdar, D.K. Polymeric Hydrogels: Characterization and Biomedical Applications. Des. Monomers Polym. 2009, 12, 197–220, doi:10.1163/156855509x436030.
- Gharti, K.; Thapa, P.; Budhathoki, U.; Bhargava, A. Formulation and in vitro evaluation of floating tablets of hydroxypropyl methylcellulose and polyethylene oxide using ranitidine hydrochloride as a model drug. J. Young Pharm. 2012, 4, 201–208, doi:10.4103/0975–1483.104363.Bruschi, M.L. Classification of therapeutic systems for drug delivery. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L. Ed.; Elsevier: Cambridge, UK, 2015; Chapter 3, pp. 29–36.
- Dar, M.J.; Ali, H.; Khan, A.; Khan, G.M. Polymer-based drug delivery: The quest for local targeting of inflamed intestinal mucosa. J. Drug Target 2017, 25, 582–596, doi:10.1080/1061186x.2017.1298601.Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A.; Larsson, A. Swellable hydrogel-based systems for controlled drug delivery. In Smart Drug Delivery System; Sezer, A.D. Ed.; IntechOpen Limited: London, UK, 2015; Chapter 10, pp. 2712–2454.
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A. Buccal bioadhesive drug delivery—A promising option for orally less efficient drugs. J. Control. Release 2006, 114, 15–40, doi:10.1016/j.jconrel.2006.04.012.Yang, W.-W.; Pierstorff, E. Reservoir-Based Polymer Drug Delivery Systems. J. Lab. Autom. 2012, 17, 50–58, doi:10.1177/2211068211428189.
- Li, N.; Yu, M.; Deng, L.; Yang, J.; Wang, W. Thermosensitive hydrogel of hydrophobically-modified methylcellulose for intravaginal drug delivery. J. Mater. Sci. Mater. Med. 2012, 23, 1913–1919, doi:10.1007/s10856–012–4664–9.Sharma, K.; Singh, V.; Arora, A. Natural biodegradable polymers as matrices in transdermal drug delivery. Int. J. Drug Dev. Res. 2011, 3, 85–103.
- Prajapati, N.B.; Goyal, A. Thermoreversible mucoadhesive insitu gel: A review. Int. J. Innov. Drug Discov. 2013, 3, 67–84.Patel, H.; Panchal, D.R.; Patel, U.; Brahmbhatt, T.; Suthar, M. Matrix type drug delivery system: A review. J. Pharm. Sci. Bio-Sci. Res. 2011, 1, 143–151.
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608, doi:10.1016/j.msec.2014.05.050.Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; Pros and cons. Trends Pharm. Sci. 2019, 5, 7–24.
- Vicario-De-La-Torre, M.; Forcada, J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels 2017, 3, 16, doi:10.3390/gels3020016.Gavasane, A.J.; Pawar, H.A. Synthetic Biodegradable Polymers Used in Controlled Drug Delivery System: An Overview. Clin. Pharmacol. Biopharm. 2014, 3, 1–7, doi:10.4172/2167–065x.1000121.
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999, doi:10.1016/j.jsps.2019.08.001.Siegel, R.A.; Rathbone, M.J. Overview of controlled release mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R., Rathbone, M., Eds.; Springer: Boston, MA, USA, 2012; Chapter 2, pp. 19–43.
- Bawa, P.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 022001, doi:10.1088/1748–6041/4/2/022001.Bruschi, M.L. Main mechanisms to control the drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L. Ed.; Elsevier: Cambridge, UK, 2015; Chapter 4, pp. 37–62.
- Onofrei, M.-D.; Filimon, A. Cellulose-based hydrogels: Designing concepts, properties, and perspectives for biomedical and environmental applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Mendez-Vilas, A., Solano, A., Eds.; Formatex Research Center S.L.: Badajoz, Spain, 2016; pp. 108–120.Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408, doi:10.1016/j.addr.2006.09.004.
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179, doi:10.1016/j.jconrel.2018.01.023.Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173.
- Hu, J.; Yang, L.; Yang, P.; Jiang, S.; Liu, X.; Li, Y. Polydopamine free radical scavengers. Biomater. Sci. 2020, 8, 4940–4950, doi:10.1039/d0bm01070g.Rocha-García, D.; Guerra-Contreras, A.; Rosales-Mendoza, S.; Palestino, G. Role of porous silicon/hydrogel composites on drug delivery. Open Mater. Sci. 2016, 3, 93–101, doi:10.1515/mesbi-2016–0011.
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007, doi:10.1016/j.polymer.2008.01.027.Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999, doi:10.1016/j.jsps.2019.08.001.
- Nazar, H.; Roldo, M.; Fatouros, D.G.; Van Der Merwe, S.M.; Tsibouklis, J. Hydrogels in mucosal delivery. Ther. Deliv. 2012, 3, 535–555, doi:10.4155/tde.12.16.Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603, doi:10.3390/molecules24030603.
- Amin, S.; Rajabnezhad, S.; Kohli, K. Hydrogels as potential drug delivery systems. Sci. Res. Essays 2009, 3, 1175–1183.Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17, doi:10.1038/natrevmats.2016.71.
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360, doi:10.1002/adma.200501612.Sezer, S.; Şahin, İ.; Öztürk, K.; Şanko, V.; Koçer, Z.; Sezer, U.A. Cellulose-based hydrogels as biomaterials. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Switzerland, 2018; Chapter 39, pp. 1–27.
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36, doi:10.3390/gels3040036.Barman, A.; Das, M. Cellulose-based hydrogels for pharmaceutical and biomedical applications. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Switzerland, 2018; Chapter 36, pp. 1–28.
- Gonçalves, C.; Pereira, P.A.C.; Gama, M. Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 2010, 3, 1420–1460, doi:10.3390/ma3021420.Kayra, N.; Aytekin, A.Ö. Synthesis of cellulose-based hydrogels: preparation, formation, mixture, and modification. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing: Basel, Switzerland, 2018; Chapter 14, pp. 1–28.
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373, doi:10.3390/ma2020353.Rusu, D.; Ciolacu, D.; Simionescu, B.C. Cellulose-Based hydrogels in tissue engineering applications. Cellul. Chem. Technol. 2019, 53, 907–923, doi:10.35812/cellulosechemtechnol.2019.53.88.
- Cirillo, G.; Curcio, M.; Nicoletta, F.P.; Iemma, F. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics 2019, 11, 486, doi:10.3390/pharmaceutics11090486.Liu, H.; Rong, L.; Wang, B.; Xie, R.; Sui, X.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. Facile fabrication of redox/pH dual stimuli responsive cellulose hydrogel. Carbohydr. Polym. 2017, 176, 299–306, doi:10.1016/j.carbpol.2017.08.085.
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Polymeric Hydrogels: Characterization and Biomedical Applications. Des. Monomers Polym. 2009, 12, 197–220, doi:10.1163/156855509x436030.Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562, doi:10.1039/c8tb02331j.
- Bruschi, M.L. Classification of therapeutic systems for drug delivery. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L. Ed.; Elsevier: Cambridge, UK, 2015; Chapter 3, pp. 29–36.
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A.; Larsson, A. Swellable hydrogel-based systems for controlled drug delivery. In Smart Drug Delivery System; Sezer, A.D. Ed.; IntechOpen Limited: London, UK, 2015; Chapter 10, pp. 2712–2454.
- Yang, W.-W.; Pierstorff, E. Reservoir-Based Polymer Drug Delivery Systems. J. Lab. Autom. 2012, 17, 50–58, doi:10.1177/2211068211428189.
- Sharma, K.; Singh, V.; Arora, A. Natural biodegradable polymers as matrices in transdermal drug delivery. Int. J. Drug Dev. Res. 2011, 3, 85–103.
- Patel, H.; Panchal, D.R.; Patel, U.; Brahmbhatt, T.; Suthar, M. Matrix type drug delivery system: A review. J. Pharm. Sci. Bio-Sci. Res. 2011, 1, 143–151.
- Gavasane, A.J.; Pawar, H.A. Synthetic Biodegradable Polymers Used in Controlled Drug Delivery System: An Overview. Clin. Pharmacol. Biopharm. 2014, 3, 1–7, doi:10.4172/2167–065x.1000121.
- Siegel, R.A.; Rathbone, M.J. Overview of controlled release mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R., Rathbone, M., Eds.; Springer: Boston, MA, USA, 2012; Chapter 2, pp. 19–43.
- Bruschi, M.L. Main mechanisms to control the drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L. Ed.; Elsevier: Cambridge, UK, 2015; Chapter 4, pp. 37–62.
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408, doi:10.1016/j.addr.2006.09.004.
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173.
- Rocha-García, D.; Guerra-Contreras, A.; Rosales-Mendoza, S.; Palestino, G. Role of porous silicon/hydrogel composites on drug delivery. Open Mater. Sci. 2016, 3, 93–101, doi:10.1515/mesbi-2016–0011.
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603, doi:10.3390/molecules24030603.
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17, doi:10.1038/natrevmats.2016.71.
- Sezer, S.; Şahin, İ.; Öztürk, K.; Şanko, V.; Koçer, Z.; Sezer, U.A. Cellulose-based hydrogels as biomaterials. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Switzerland, 2018; Chapter 39, pp. 1–27.
- Barman, A.; Das, M. Cellulose-based hydrogels for pharmaceutical and biomedical applications. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Switzerland, 2018; Chapter 36, pp. 1–28.
- Kayra, N.; Aytekin, A.Ö. Synthesis of cellulose-based hydrogels: preparation, formation, mixture, and modification. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing: Basel, Switzerland, 2018; Chapter 14, pp. 1–28.
- Rusu, D.; Ciolacu, D.; Simionescu, B.C. Cellulose-Based hydrogels in tissue engineering applications. Cellul. Chem. Technol. 2019, 53, 907–923, doi:10.35812/cellulosechemtechnol.2019.53.88.
- Liu, H.; Rong, L.; Wang, B.; Xie, R.; Sui, X.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. Facile fabrication of redox/pH dual stimuli responsive cellulose hydrogel. Carbohydr. Polym. 2017, 176, 299–306, doi:10.1016/j.carbpol.2017.08.085.
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562, doi:10.1039/c8tb02331j.