Taro [Colocasia esculenta (L.) Schorms contatt] is an ancient tuberous crop that is cultivated in tropical and subtropical climates as staple food source. The edible part of taro widely used for human consumption is known as corm. Taro corms contain valuable bioactive molecules effective against cancer and cancer-related risk factors, such as carcinogens and biological agents, several pathophysiological conditions, including oxidative stress and inflammation, while controlling metabolic dysfunctions and boosting the immunological response. Such broad effects are achieved by the taro health-influencing compounds displaying antitumoral, antimutagenic, immunomodulatory, anti-inflammatory, antioxidant, anti-hyperglycemic, and anti-hyperlipidemic activities. Taro bioactivities are attributed to the combination of tarin, taro-4-I polysaccharide, taro polysaccharides 1 and 2 (TPS-1 and TPS-2), A-1/B-2 α-amylase inhibitors, monogalactosyldiacylglycerols (MGDGs), digalactosyldiacylglycerols (DGDGs), polyphenols, and nonphenolic antioxidants. Most of these compounds have been purified and successfully challenged in vitro and in vivo, proving their involvement in the aforementioned activities. Although thesAlthough these health-promoting effects have been recognized since ancient times, as well as other valuable features of taro for food profit, such as hypo-allergenicity, gluten-free, and carbohydrates with medium-glycemic index, taro crop remains underexploited. The popularization of taro intake should be considered a dietary intervention strategy to be applied to improve the overall health status of the organism and as supportive therapy to manage tumorigenesis.
- Taro Corms
- Colocasia esculenta
- tuber crop
- health-promoting compounds
- antitumoregenic
- im-munomodulator
- metabolic regulator
1. Introduction
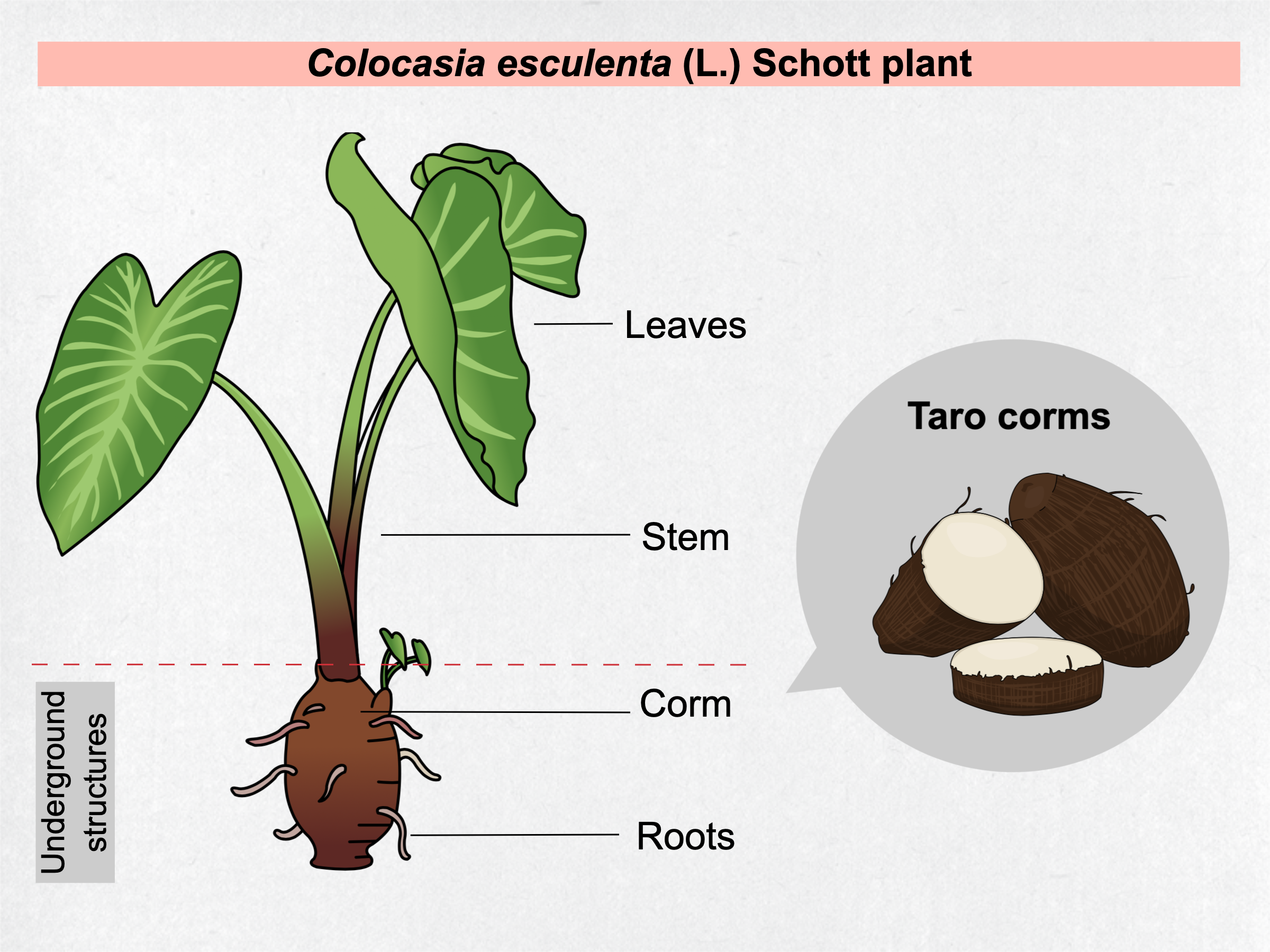
Taro [Colocasia esculenta (L.) Schott] is a perennial herbaceous plant of Asiatic origin that belongs to Araceae family and is classified as a monocotyledonous (Table 1). This herb can reach 1-2 m in height with large heart-shaped leaves, long petioles, an underground starchy structure called corm and the roots (Figure 1). Taro is cultivated in tropical and subtropical climates as staple food source due to its carbohydrate-rich nature. To avoid confusion with other similar plants, Colocasia esculenta species is called taro by the scientific community. Popularly, taro can have distinct names, such as eddoe, cocoyam, tannia, dasheen, inhame, and others, that vary according to the place it is cultivated and consumed \( \)[1].
Figure 1. General characterization of taro plant parts.
Table1. Classification of Colocasia esculenta (L.) Schott.
| Rank | Scientific name | Common name |
|
Kingdom
|
Plantae |
Plants
|
|
Subkingdom
|
Tracheobionta
|
Vascular plants
|
|
Superdivision
|
Spermatophyta
|
Seed plants
|
|
Division
|
Magnoliophyta
|
Flowering plants
|
|
Class
|
Liliopsida
|
Monocotyledons
|
|
Subclass
|
Arecidae
|
- |
|
Order
|
Arales
|
- |
|
Family
|
Araceae
|
Arum family
|
|
Genus
|
Colocasia Schott
|
Colocasia
|
|
Species
|
Colocasia esculenta (L.) Schott
|
Cocoyam and others |
Crops that have been neglected over the years are currently being revalued based on modern technologies used to extract, identify, estimate, and assay a great number of compounds displaying claimed pharmacological effects. The study of the composition of such food matrices has stimulated the recognition and reevaluation of so-called “orphan” crops, reaffirming the knowledge that traditional communities have practiced since ancient times by considering the vital role of those crops not only in supporting diets but also in promoting the health and treating these populations. In most cases, neglected or underutilized species have been substituted by those cultures in huge demand, although sometimes, those crops are poorer not only in nutritional aspects but mainly in bioactive compounds [12].
2. Taro Consumption, Cultivation, and Nutritional Importance
Even though taro corm (or taro) is a rich source of health-promoting compounds, this crop, as well as tubercle consumption worldwide, is highly neglected probably because it is mainly associated with subsistence agriculture [23][34][45]. Moreover, due to poorness, unsustainable farming practices, and climate change, taro crops face many challenges in several underdeveloped countries, such as African Sub-Saharan nations and other countries in Central and South America [56]. In general, taro crops, as several subsistence crops, are cultivated in small farms, with low capital endowment, far from urban centers and with no access to capital markets, and low-off farm income [12]. The food processing sector can overcome these constraints and enhance taro crop availability and acceptance by urban populations by replacing corn and wheat in processed foods, enhancing raw product commercialization. In addition, this may also lead to attention regarding taro crops as a rich source of remarkable and unique compounds, whose pharmacological activities have been demonstrated both in in vitro and in animal models.
International research centers mainly dedicated to taro studies are still scarce, although they would be helpful to overcome many challenges that have remained unsolved for over ten years. Financial and scientific investments would aid in improving cultivation conditions, creating and maintaining germplasm collections of diverse regions, improving conservation methods, increasing food security, and enhancing the benefits of taro consumption. These efforts would increase the research field and shared information between countries, which might expand taro cultivation, sales, and consumption worldwide, especially in developing countries [67].
The most significant taro producers are the West African countries, i.e., Nigeria, Cameroon, and Ghana, followed by China, which contribute respectively 6.7 and 3.9 million tons of taro, corresponding to 83.6% of the worldwide taro production [78][89]. Other nations, such as the USA, Canada, Japan, Turkey, and Central and South American countries, produce about 2 million tons of taro. Brazil has not yet been internationally recognized as a taro producer country, since less than 1000 ha are planted and dispersed, which is probably due to the vast Brazilian territory, where relevant producers are spread throughout the Mid-South region (Figure 12) [910][1011]. However, Southeast Brazil boasts a germplasm bank, named INCAPER, which is used to collect and conserve taro cultivars, maintaining the diversity and characteristics of the Brazilian varieties, which include T37 (Macaquinho), T38 (Chinês), T39 (Japonês), T40 (Chinês Regional), T41 (Cem em Um), T42 (São Bento), and T43 (Branco) [1112]. The neglected and underutilized status of taro crops is noted by comparison to other tubercles, such as potatoes, which are widely consumed worldwide, although displaying superior nutritive importance. For example, in 2018, approximately 12.6 million tons of taro per annum were produced worldwide against 64.7 million tons of potato (Solanum tuberosum L.) (Figure 1) [7]. Taro is a healthy alternatiaro is a healthy alternative of carbohydrate source, as the cooking process does not interfere with their nutritional composition, causing only minimal modifications in nutrient contents, according to Food Data Central from the United States Department Agriculture (USDA) (https://fdc.nal.usda.gov/) [1213]. The proximate composition of crude, cooked, and baked taro is quite similar regarding vitamins and minerals, except for niacin and calcium levels, as well as protein and total fat amounts, which were lowered by thermal processing (Table 12).
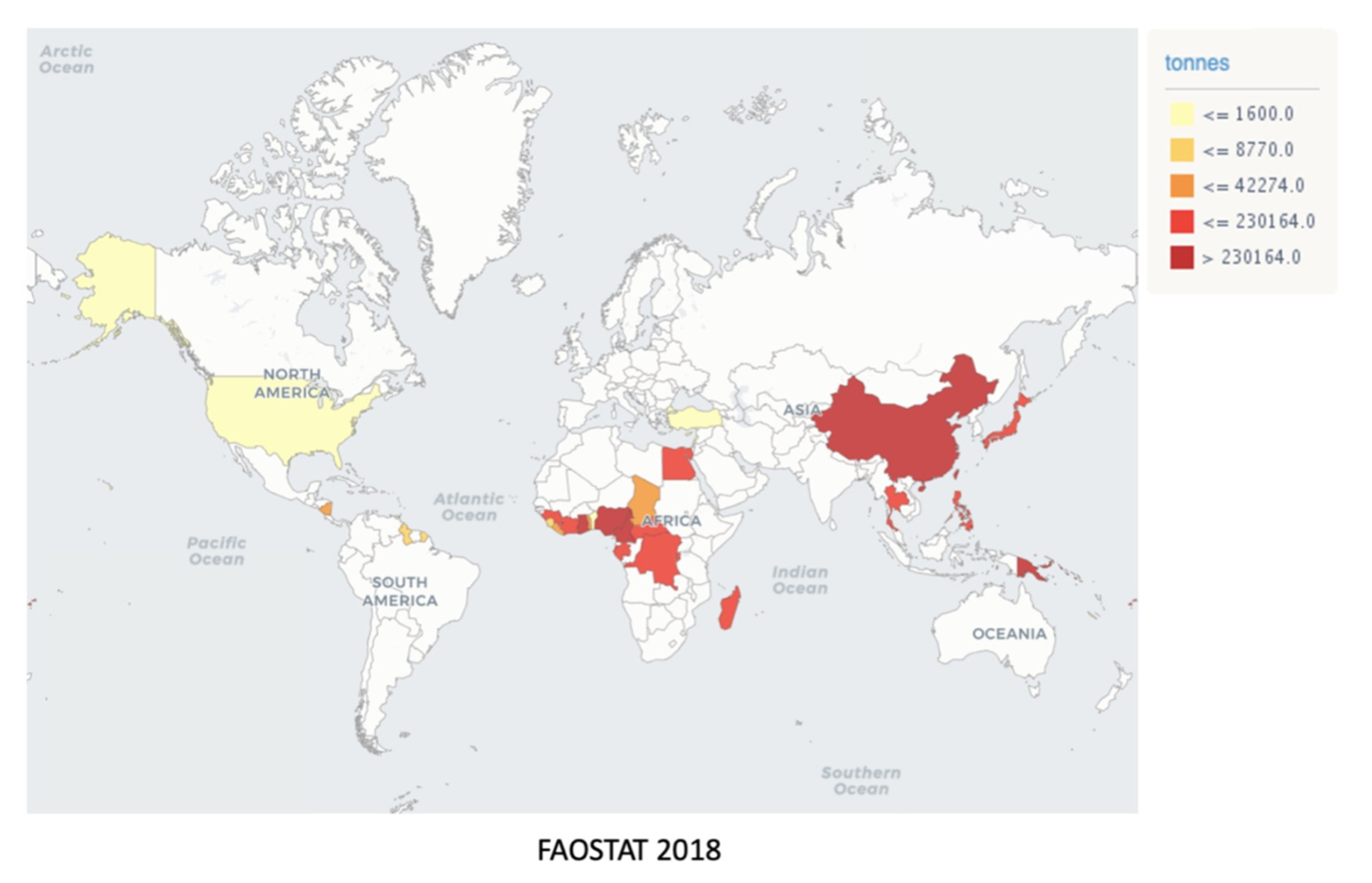
Table 12. Nutritional composition of taro analyzed raw, cooked and baked taro.
| Nutritional Composition | |||
|---|---|---|---|
| Principle * | Nutrient per 100 g of Dry Weight | ||
| Crude Taro | Cooked Taro | Baked Taro with Salt | |
| Water | 70.64 g | 63.8 g | 60.98 g |
| Energy | 112 kcal | 142 kcal | 144 kcal |
| Carbohydrates | 26.46 g | 34.6 g | 34.09 g |
| Protein | 1.5 g | 0.52 g | 1.93 g |
| Total fat | 0.20 g | 0.11 g | 0.26 g |
| Cholesterol | 0 mg | 0 mg | 0 mg |
| Dietary fibers | 4.1 g | 5.1 g | 5.3 g |
| Ash | 1.2 g | 0.97 g | na |
| Vitamins * | |||
| Folates | 0.022 mg | 0.019 mg | 0.023 mg |
| Niacin | 0.600 mg | 0.510 mg | 0.734 mg |
| Pantothenic acid | 0.303 mg | 0.336 mg | na |
| Pyridoxine | 0.283 mg | Na | na |
| Riboflavin | 0.025 mg | 0.028 mg | 0.031 mg |
| Thiamin | 0.095 mg | 0.107 mg | 0.110 mg |
| Vitamin A | 0.004 mg | 0.004 mg | 0.005 mg |
| Vitamin C | 4.5 mg | 5 mg | 4.3 mg |
| Vitamin E | 2.38 mg | 2.93 mg | 3.07 mg |
| Vitamin K | 0.001 mg | 0.0012 mg | 0.0013 mg |
| Electrolytes * | |||
| Sodium | 11 mg | 15 mg | 475 mg |
| Potassium | 591 mg | 484 mg | 762 mg |
| Minerals * | |||
| Calcium | 43 mg | 18 mg | 56 mg |
| Copper | 0.172 mg | 0.201 mg | 0.222 mg |
| Iron | 0.550 mg | 0.720 mg | 0.710 mg |
| Magnesium | 33 mg | 30 mg | 43 mg |
| Manganese | 0.383 mg | 0.449 mg | na |
| Selenium | 0.0007 mg | 0.0009 mg | 0.0009 mg |
| Zinc | 0.230 mg | 0.270 mg | 0.300 mg |
| Starch ** (g starch/100 g) | |||
| Total starch | 18.8 | 14.2 | na |
| Resistant Starch—RS | 5.2 | 2.1 | na |
| Slowly digestible starch—SDS | 13.6 (SDS+RDS) | 2.5 | na |
| Rapidly digestible starch—RDS | 9.6 | na | |
| Glycemic Index ** | na | Medium | Medium |
| Nutritional Composition | |||
| Principle * | Nutrients per 100 g of Dry Weight | ||
| Crude Taro | Cooked Taro | Baked Taro with Salt | |
| Water | 70.64 g | 63.8 g | 60.98 g |
| Energy | 112 kcal | 142 kcal | 144 kcal |
| Carbohydrates | 26.46 g | 34.6 g | 34.09 g |
| Protein | 1.5 g | 0.52 g | 1.93 g |
| Total fat | 0.20 g | 0.11 g | 0.26 g |
| Cholesterol | 0 mg | 0 mg | 0 mg |
| Dietary fibers | 4.1 g | 5.1 g | 5.3 g |
| Ash | 1.2 g | 0.97 g | na |
| Vitamins * | |||
| Folates | 0.022 mg | 0.019 mg | 0.023 mg |
| Niacin | 0.600 mg | 0.510 mg | 0.734 mg |
| Pantothenic acid | 0.303 mg | 0.336 mg | na |
| Pyridoxine | 0.283 mg | Na | na |
| Riboflavin | 0.025 mg | 0.028 mg | 0.031 mg |
| Thiamin | 0.095 mg | 0.107 mg | 0.110 mg |
| Vitamin A | 0.004 mg | 0.004 mg | 0.005 mg |
| Vitamin C | 4.5 mg | 5 mg | 4.3 mg |
| Vitamin E | 2.38 mg | 2.93 mg | 3.07 mg |
| Vitamin K | 0.001 mg | 0.0012 mg | 0.0013 mg |
| Electrolytes * | |||
| Sodium | 11 mg | 15 mg | 475 mg |
| Potassium | 591 mg | 484 mg | 762 mg |
| Minerals * | |||
| Calcium | 43 mg | 18 mg | 56 mg |
| Copper | 0.172 mg | 0.201 mg | 0.222 mg |
| Iron | 0.550 mg | 0.720 mg | 0.710 mg |
| Magnesium | 33 mg | 30 mg | 43 mg |
| Manganese | 0.383 mg | 0.449 mg | na |
| Selenium | 0.0007 mg | 0.0009 mg | 0.0009 mg |
| Zinc | 0.230 mg | 0.270 mg | 0.300 mg |
| Starch ** (g starch/100 g) | |||
| Total starch | 18.8 | 14.2 | na |
| Resistant Starch—RS | 5.2 | 2.1 | na |
| Slowly digestible starch—SDS | 13.6 (SDS+RDS) | 2.5 | na |
| Rapidly digestible starch—RDS | 9.6 | na | |
| Glycemic Index ** | na | Medium | Medium |
Additionally, taro is a rich source of antioxidants, mainly phenolic compounds, both regarding diversity and quantity, distributed in the edible portion of taro. In addition to antioxidants, taro phytochemicals display immunomodulatory, antioxidant, antitumoral, antimetastatic, antimutagenic, anti-hyperglycemic, and anti-hypercholesterolemic bioactivities. Moreover, taro is a potential alternative staple source, with a lower glycemic index than potato, and its consumption may decrease the incidence and prevalence of several diseases, including certain types of cancers [1314][15][16][17][18].
3. Taro Consumption Worldwide
Despite being considered an orphan crop, taro is a sacred food in some cultures, such as in Hawaii, Melanesia, and Micronesia, where it is known as a Gift of Ancient Gods. In these places, taro is consumed daily and included in several special occasions and rituals due to its symbolic importance [1011]. Taro is formulated according to the cultural traditions of each local population. For example, taro stems, petiole, corms, and leaves can be consumed as a common practice in Hawaii. However, taro corms are conventionally considered the edible portion of this plant, and they are consumed worldwide [23]. Some cultivars can exhibit high calcium oxalate contents, which is considered an antinutritional factor that confers an acrid taste to the tubercles, causes skin irritation, and can decrease calcium absorption [1819]. For this reason, taro should be preferentially consumed after cooking in order to avoid these undesired effects.
In Hawaii, taro is cooked and smashed with a little water to prepare a starchy paste, which may be consumed immediately (fresh poi) or after 2–3 days of fermentation producing a sour taste paste (sour poi), which is a typical Hawaiian dish [1920]. Achu, another ancient taro paste, preferentially prepared by women, is mostly consumed in Africa. Taro and bananas are boiled together, peeled, and pounded to form a smooth and homogeneous starchy paste. Then, typical sauces are mixed in, such as yellow sauce (achu soup), jaune sauce, black sauce (black soup), and pepper sauce [2021].
In other parts of the world, especially Brazil, taro can be served fried or steamed, prepared as a soup, or mashed. The corms are also marketed in a variety of commercial products such as flour, chips, fermented alcoholic beverage, ice bar, ice cream and canned taro, among others [2122][2223]. These taro derivatives are not globally available, as taro crops are concentrated in China, Taiwan, and Hawaii. Taro flour can be used as an ingredient for many other preparations including bread, cakes, cookies, noodles, and cereals, or even as a partial substitute for traditional whey flour [22][23][24][25].
The main carbohydrate present in taro is starch found in polygonal and small granules, averaging 1.3–2.2 µm in diameter, although granules measuring 5 µm can be observed [2526]. As a starchy vegetable, taro presents part of the starch in resistant form, which can escape small intestine digestion and be directed to colon fermentation. This resistant-starch results in several health effects, including the augmented absorption of minerals, contribution in controlling blood glycemia, and reduction in plasma triglycerides and cholesterol [2627].
Since taro is free of gluten and displays low protein and high -calorie content, as well as low fat levels, taro consumption can benefit individuals with dietary restrictions such as those presenting allergies, especially in children and gluten-intolerant individuals, while contributing to reduce the risk of obesity and type II diabetes. In addition, the presence of soluble and non-soluble dietary fibers can improve intestinal transit and possibly aid in colorectal cancer prevention. As a result of its gluten-free nature, taro flour has arisen as a promising substitute for wheat flour, boosting the Brazilian market for gluten-free derivatives [1314][15][16][2717][28][29][30].
To encourage and reinforce the importance of taro consumption, this study aims to discuss the benefits of the biofunctional compounds found in taro in promoting health, especially considering their potential against cancer, as well as in the control of other physiopathological conditions that compose the risk factors for cancer burden, including obesity and type II diabetes.
4. Bioactive Compounds and Pharmacological Properties of Taro corms
The use of taro to treat multiple unhealthy conditions and diseases such as diabetes, hemorrhage, diarrhea, arterial hypertension, alopecia, among others, dates from ancient times [31][1]. Taro’s health-promoting potential has been confirmed by in vitro and in vivo preclinical assays, by assaying raw or cooked corms and taro derivatives in the form of flour or extracts (Table 3) [14][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69].
The bioactivities found in taro corms include antitumoral, immunomodulatory, anti-mutagenic, anti-hyperglycemic, anti-hypercholesterolemic and antioxidant, which have been mainly attributed to its polyphenols, proteins, mucilage, polysaccharides, lipids, and non-polyphenol antioxidants. Many of these bioactive principles have already been identified and singly assayed, proving their participation in these claimed activities. Bioactive molecules identified in taro include tarin, taro-4-I polysaccharide, taro polysaccharides 1 and 2 (TPS-1/TPS-2), A-1/B-2 α-amylase inhibitors, monogalactosyldiacylglycerols (MGDGs), and digalactosyldiacylglycerols (DGDGs). Together, these data clearly indicate that the biological effects exerted by taro are possibly a synergic effect of multiple compounds displaying effectiveness not only against several cancer cell lines but also against some of the main external cancer risk factors, such as free radicals, mutagenic and carcinogenic agents, and physiopathological conditions such as obesity and type II diabetes (Table 3).
Interestingly, many of the bioactivities were demonstrated with cooked taro formulations, even following oral administration, suggesting that the recommendation to include taro in the daily human diets, alongside other healthy eating habits, could contribute to the efforts to reduce cancer risks.
Table 3. Protective and therapeutic potential of taro corms.
|
Taro corm preparation
|
Bioactive Compound Class
|
Active principle
|
Property
|
Reference
|
|
Poi extract
|
- | - |
Antitumoral and Antimetastatic
|
[32][33][34][35][36][37] |
|
Crude taro extract
|
Protein
|
Tarin
Tarin nano-liposomal capsules
|
||
|
Polysaccharide
|
Taro-4-I
|
|||
|
Ethanolic crude taro extract
|
- | - | ||
|
poi extract
|
- | - |
Immunomodulatory
|
[32][34][36][38][39][40][41][42] |
|
Crude taro extract
|
Polysaccharide
|
Taro-4-I
|
||
|
TPS-1 and TPS-2
|
||||
|
Protein
|
Tarin
|
|||
| - | - | |||
|
Taro flour
|
Flavonoid; Alkaloid; saponin; tannin
|
- |
Anti-hyperglycemic
|
[43][44][45][46][47][48] |
|
Methanolic extract of taro flour
|
Alkaloid; flavonoid; steroid
|
- | ||
|
Mucilage-rich extract from crude taro flour
|
Neutral sugar; protein, polyphenols
|
- | ||
|
Extract from defatted crude taro flour
|
Protein
|
A-1 and B-2
|
||
| Taro flour | Flavonoid; Alkaloid; saponin; tannin | - |
Anti-hypercholesterolemic or Anti-hyperlipidemic
|
[43][45][47][49][50][51] |
| Extract from cooked taro flour | - | - | ||
| Mucilage-rich extract from crude taro flour | Neutral sugar; protein, polyphenols | - | ||
| Ethanolic extract from crude taro | Lipid |
Extract
MGDG 1-3
DGDG 1-4
|
||
| Mucilage-rich extract from taro flour | Polysaccharide | Arabinogalactan | ||
|
Dietary fiber-rich extract from crude taro
|
Polysaccharide
|
- |
Anti-mutagenic
|
[52][53][54] |
|
Crude taro extract
|
- | - | ||
|
Heptane extract from cooked taro
|
- | - | ||
|
Distinct raw or cooked taro extracts in aqueous or organic solutions
|
Polyphenols and non-polyphenols
|
- |
Antioxidant
|
[14][43][44][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69] |
To date, there is a scarcity of clinical studies leaving gaps in the translation of the positive health effects identified in preclinical studies to human beings. The better exploitation and understanding of taro bioactivities, following dietary interventions in humans, could be helpful in developing new functional compounds.
Clinical trials on healthy non-diabetic young adults evidenced that taro shows a medium glycemic index, low glycemic load, and moderate glycemic response [70]. Moreover, the addition of other food components, such as vegetables, oils, and rich protein food, during cooking, can reduce the taro glycemic index to a lower rate [71].
Taro is reported to have anticancer potential through several preclinical analyses, as aforementioned. The Japanese population traditionally consumes starchy roots, such as taro, that are associated with a decrease in the risk of kidney cancer death [72][73][74][75][76].
Taken together, the intake of taro or their derivatives can provide bioactive compounds capable of promoting health benefits, especially in the control of hypercholesterolemia, which is not only a complication of diabetes and overweight but also a risk for cerebrovascular and cardiovascular diseases, which are important causes of death worldwide, along with cancer [75][76]. Moreover, the overall benefit against type II diabetes and obesity could certainly aid in reducing the risk factors for cancer.
5. Conclusions and Prospects
Taro is a valuable source of several health-promoting compounds, such as taro lectin or tarin, bioactive-complex carbohydrates, and natural polyphenols and other antioxidants. In general, these molecules act through individual or synergic pathways, contributing to ameliorate systemic health status by managing oxidative stress imbalance, reducing systemic inflammation, modulating metabolic dysfunctions, and boosting the immune response. Many mechanisms remain to be elucidated to better exploit taro extracts, taro derivatives, or individual taro components. The non-toxicity of these molecules toward healthy cells turns taro components into potential candidates for supportive target therapies when associated with traditional drug treatments. In addition, since taro is a food matrix rich in bioactive compounds, spreading its benefits worldwide may enhance its consumption and consequently production while resulting in better population health maintenance.
References
- Abele, S.; Frohberg, K. Subsistence Agriculture in Central and Eastern Europe: How to Break the Vicious Circle? Oxford University Press: New York, NY, USA, 2004.T. K. Lim; Colocasia esculenta. Edible Medicinal and Non Medicinal Plants 2014, 9, 454-492, 10.1007/978-94-017-9511-1_13.
- Pereira, P.R.; Corrêa, A.C.N.T.F.; Vericimo, M.A.; Paschoalin, V.M.F. Tarin, a Potential Immunomodulator and COX-Inhibitor Lectin Found in Taro (Colocasia esculenta). Compr. Rev. Food Sci. Food Saf. 2018, 17, 878–891.Abele, S.; Frohberg, K. Subsistence Agriculture in Central and Eastern Europe: How to Break the Vicious Circle? Oxford University Press: New York, NY, USA, 2004.
- Temesgen, M.; Retta, N. Nutritional potential, health and food security benefits of taro Colocasia esculenta (L.): A Review. Food Sci. Qual. Manag. 2015, 36, 23–30.Pereira, P.R.; Corrêa, A.C.N.T.F.; Vericimo, M.A.; Paschoalin, V.M.F. Tarin, a Potential Immunomodulator and COX-Inhibitor Lectin Found in Taro (Colocasia esculenta). Compr. Rev. Food Sci. Food Saf. 2018, 17, 878–891.
- Siqueira, M.V. Yam: A neglected and underutilized crop in Brazil. Hortic. Bras. 2011, 29, 16–20.Temesgen, M.; Retta, N. Nutritional potential, health and food security benefits of taro Colocasia esculenta (L.): A Review. Food Sci. Qual. Manag. 2015, 36, 23–30.
- Akwee, P.; Netondo, G.; Palapala, V.A. A critical review of the role of taro Colocasia esculenta L.(Schott) to food security: A comparative analysis of Kenya and Pacific Island taro germplasm. Sci. Agric. 2015, 9, 101–108.Siqueira, M.V. Yam: A neglected and underutilized crop in Brazil. Hortic. Bras. 2011, 29, 16–20.
- Rao, V.R.; Hunter, D.; Eyzaguirre, P.B.; Matthews, P.J. Ethnobotany and global diversity of taro. In The Global Diversity of Taro: Ethnobotany and Conservation; Rao, V.R., Matthews, P.J., Eyzaguirre, P.B., Hunter, D., Eds.; Bioversity International: Rome, Italy, 2010; Volume 1.Akwee, P.; Netondo, G.; Palapala, V.A. A critical review of the role of taro Colocasia esculenta L.(Schott) to food security: A comparative analysis of Kenya and Pacific Island taro germplasm. Sci. Agric. 2015, 9, 101–108.
- FAO. Food and Agriculture Organization of the United Nations. Statistics Division: Production of Taro. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 17 February 2020).Rao, V.R.; Hunter, D.; Eyzaguirre, P.B.; Matthews, P.J. Ethnobotany and global diversity of taro. In The Global Diversity of Taro: Ethnobotany and Conservation; Rao, V.R., Matthews, P.J., Eyzaguirre, P.B., Hunter, D., Eds.; Bioversity International: Rome, Italy, 2010; Volume 1.
- Diop, N.; Jaffee, S.M. Fruits and vegetables: Global trade and competition in fresh and processed product markets. In Global Agricultural Trade and Developing Countries; Aksoy, M.A., Beghin, J.C., Eds.; The World Bank: Washington, DC, USA, 2005; pp. 237–254.FAO. Food and Agriculture Organization of the United Nations. Statistics Division: Production of Taro. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 17 February 2020).
- Vieira, G.H.S.; Peterle, G.; Loss, J.B.; Peterle, G.; Poloni, C.M.M.; Colombo, J.N.; Monaco, P.A.V.L. Strategies for taro (Colocasia esculenta) irrigation. J. Exp. Agric. Int. 2018, 1–9.Diop, N.; Jaffee, S.M. Fruits and vegetables: Global trade and competition in fresh and processed product markets. In Global Agricultural Trade and Developing Countries; Aksoy, M.A., Beghin, J.C., Eds.; The World Bank: Washington, DC, USA, 2005; pp. 237–254.
- Da Silva, E.E. A Cultura do Taro-Inhame (Colocasia esculenta (L.) Schott): Alternativa para o Estado de Roraima; Embrapa Roraima: Boa Vista, Roraima, Brazil, 2011.Vieira, G.H.S.; Peterle, G.; Loss, J.B.; Peterle, G.; Poloni, C.M.M.; Colombo, J.N.; Monaco, P.A.V.L. Strategies for taro (Colocasia esculenta) irrigation. J. Exp. Agric. Int. 2018, 1–9.
- Nunes, R.S.C.; Del Aguila, E.; Paschoalin, V.; da Silva, J. DNA barcoding assessment of the genetic diversity of varieties of taro, Colocasia esculenta (L.) Schott in Brazil. In Breeding and Genetic Engineering: The Biology and Biotechnology Research; iConcept Press Ltd.: Hong Kong, China, 2014.Da Silva, E.E. A Cultura do Taro-Inhame (Colocasia esculenta (L.) Schott): Alternativa para o Estado de Roraima; Embrapa Roraima: Boa Vista, Roraima, Brazil, 2011.
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 17 February 2020).Nunes, R.S.C.; Del Aguila, E.; Paschoalin, V.; da Silva, J. DNA barcoding assessment of the genetic diversity of varieties of taro, Colocasia esculenta (L.) Schott in Brazil. In Breeding and Genetic Engineering: The Biology and Biotechnology Research; iConcept Press Ltd.: Hong Kong, China, 2014.
- Simsek, S.; El, S.N. In vitro starch digestibility, estimated glycemic index and antioxidant potential of taro (Colocasia esculenta L. Schott) corm. Food Chem. 2015, 168, 257–261.USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 17 February 2020).
- Chen, L.; Liu, R.; Qin, C.; Meng, Y.; Zhang, J.; Wang, Y.; Xu, G. Sources and intake of resistant starch in the Chinese diet. Asia Pac. J. Clin. Nutr. 2010, 19, 274–282.Simsek, S.; El, S.N. In vitro starch digestibility, estimated glycemic index and antioxidant potential of taro (Colocasia esculenta L. Schott) corm. Food Chem. 2015, 168, 257–261.
- Ramdath, D.D.; Isaacs, R.L.; Teelucksingh, S.; Wolever, T.M. Glycaemic index of selected staples commonly eaten in the Caribbean and the effects of boiling v. crushing. Br. J. Nutr. 2004, 91, 971–977.Chen, L.; Liu, R.; Qin, C.; Meng, Y.; Zhang, J.; Wang, Y.; Xu, G. Sources and intake of resistant starch in the Chinese diet. Asia Pac. J. Clin. Nutr. 2010, 19, 274–282.
- Bahado-Singh, P.; Wheatley, A.; Ahmad, M.; Morrison, E.S.A.; Asemota, H. Food processing methods influence the glycaemic indices of some commonly eaten West Indian carbohydrate-rich foods. Br. J. Nutr. 2006, 96, 476–481.Ramdath, D.D.; Isaacs, R.L.; Teelucksingh, S.; Wolever, T.M. Glycaemic index of selected staples commonly eaten in the Caribbean and the effects of boiling v. crushing. Br. J. Nutr. 2004, 91, 971–977.
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56.Bahado-Singh, P.; Wheatley, A.; Ahmad, M.; Morrison, E.S.A.; Asemota, H. Food processing methods influence the glycaemic indices of some commonly eaten West Indian carbohydrate-rich foods. Br. J. Nutr. 2006, 96, 476–481.
- Bsc, S.N.; Bsc, G.S. Oxalate content of foods and its effect on humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74.Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56.
- Brown, A.C.; Ibrahim, S.A.; Song, D. Poi history, uses, and role in health. In Fruits, Vegetables, and Herbs; Elsevier: Amsterdam, The Netherlands, 2016; pp. 331–342.Bsc, S.N.; Bsc, G.S. Oxalate content of foods and its effect on humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74.
- Grimaldi, I.M.; Leke, W.N.; Nkeabeng, I.M.L.; van Andel, T. Traditional preparation of Achu, a cultural keystone dish in western Cameroon. Int. J. Gastron. Food Sci. 2018, 13, 25–28.Brown, A.C.; Ibrahim, S.A.; Song, D. Poi history, uses, and role in health. In Fruits, Vegetables, and Herbs; Elsevier: Amsterdam, The Netherlands, 2016; pp. 331–342.
- Maga, J.A. Taro: Composition and food uses. Food Rev. Int. 1992, 8, 443–473.Grimaldi, I.M.; Leke, W.N.; Nkeabeng, I.M.L.; van Andel, T. Traditional preparation of Achu, a cultural keystone dish in western Cameroon. Int. J. Gastron. Food Sci. 2018, 13, 25–28.
- Kaushal, P.; Kumar, V.; Sharma, H. Utilization of taro (Colocasia esculenta): A review. J. Food Sci. Technol. 2015, 52, 27–40.Maga, J.A. Taro: Composition and food uses. Food Rev. Int. 1992, 8, 443–473.
- Hyacinthe, A.A.; Bedel, F.J.; Constant, Y.J.; Soumaila, D.; Patrice, K.L. Bread characteristics and descriptive analysis of taro (Colocasia esculenta, Cv Fouê): Wheat composite bread and some fritters. Int. J. Food Sci. Nutr. 2018, 3, 41–45.Kaushal, P.; Kumar, V.; Sharma, H. Utilization of taro (Colocasia esculenta): A review. J. Food Sci. Technol. 2015, 52, 27–40.
- Ammar, M.; Hegazy, A.; Bedeir, S. Using of taro flour as partial substitute of wheat flour in bread making. World J. Dairy Food Sci. 2009, 4, 94–99.Hyacinthe, A.A.; Bedel, F.J.; Constant, Y.J.; Soumaila, D.; Patrice, K.L. Bread characteristics and descriptive analysis of taro (Colocasia esculenta, Cv Fouê): Wheat composite bread and some fritters. Int. J. Food Sci. Nutr. 2018, 3, 41–45.
- Tattiyakul, J.; Asavasaksakul, S.; Pradipasena, P. Chemical and physical properties of flour extracted from taro Colocasia esculenta (L.) Schott grown in different regions of Thailand. Sci. Asia 2006, 32, 279–284.Ammar, M.; Hegazy, A.; Bedeir, S. Using of taro flour as partial substitute of wheat flour in bread making. World J. Dairy Food Sci. 2009, 4, 94–99.
- Simsek, S.; El, S.N. Production of resistant starch from taro (Colocasia esculenta L. Schott) corm and determination of its effects on health by in vitro methods. Carbohydr. Polym. 2012, 90, 1204–1209.Tattiyakul, J.; Asavasaksakul, S.; Pradipasena, P. Chemical and physical properties of flour extracted from taro Colocasia esculenta (L.) Schott grown in different regions of Thailand. Sci. Asia 2006, 32, 279–284.
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283.Simsek, S.; El, S.N. Production of resistant starch from taro (Colocasia esculenta L. Schott) corm and determination of its effects on health by in vitro methods. Carbohydr. Polym. 2012, 90, 1204–1209.
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437.Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283.
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17.Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437.
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17.
- Rakesh Prajapati; Manisha Kalariya; Rahul Umbarkar; Sachin Parmar; Navin Sheth; Prajapati R; Kalariya M; Parmar S; Sheth N; Colocasia esculenta: A potent indigenous plant. International Journal of Nutrition, Pharmacology, Neurological Diseases 2010, 1, 90, 10.4103/2231-0738.84188.
- Amy C. Brown; Jonathan E. Reitzenstein; Jessie Liu; Martin R. Jadus; The anti-cancer effects of poi(Colocasia esculenta) on colonic adenocarcinoma cellsIn vitro. Phytotherapy Research 2005, 19, 767-771, 10.1002/ptr.1712.
- Namita Kundu; Patricia Campbell; Brian Hampton; Chen-Yong Lin; Xinrong Ma; Nicholas Ambulos; X. Frank Zhao; Olga Goloubeva; Dawn Holt; Amy Fulton; et al. Antimetastatic activity isolated from Colocasia esculenta (taro). Anti-Cancer Drugs 2012, 23, 200-211, 10.1097/cad.0b013e32834b85e8.
- Yau Sang Chan; Jack Ho Wong; T.B. Ng; A cytokine-inducing hemagglutinin from small taros.. Protein & Peptide Letters 2010, 17, 823-830, 10.2174/092986610791306742.
- Anna Carolina Nitzsche Teixeira Fernandes Corrêa; Maurício Afonso Verícimo; Andriy Dashevskiy; Patrícia Ribeiro Pereira; Vania Margaret Flosi Paschoalin; Liposomal Taro Lectin Nanocapsules Control Human Glioblastoma and Mammary Adenocarcinoma Cell Proliferation. Molecules 2019, 24, 471, 10.3390/molecules24030471.
- Hye-Ryung Park; Hyun-Sun Lee; Sun Young Cho; Yoon-Sook Kim; Kwang-Soon Shin; Anti-metastatic effect of polysaccharide isolated from Colocasia esculenta is exerted through immunostimulation. International Journal of Molecular Medicine 2012, 31, 361-368, 10.3892/ijmm.2012.1224.
- Hisahiro Kai; Ena Akamatsu; Eri Torii; Hiroko Kodama; Chizuko Yukizaki; Yoichi Sakakibara; Masahito Suiko; Kazuhiro Morishita; Hiroaki Kataoka; Koji Matsuno; et al. Inhibition of proliferation by agricultural plant extracts in seven human adult T-cell leukaemia (ATL)-related cell lines. Journal of Natural Medicines 2011, 65, 651-655, 10.1007/s11418-011-0510-5.
- Huixian Li; Zhou Dong; Xiaojia Liu; Huamin Chen; Furao Lai; Mengmeng Zhang; Structure characterization of two novel polysaccharides from Colocasia esculenta (taro) and a comparative study of their immunomodulatory activities. Journal of Functional Foods 2018, 42, 47-57, 10.1016/j.jff.2017.12.067.
- Patrícia Ribeiro Pereira; Eduardo Mere Del Aguila; Maurício Afonso Verícimo; Russolina Benedeta Zingali; Vania Margaret Flosi Paschoalin; Joab Trajano Silva; Purification and Characterization of the Lectin from Taro (Colocasia esculenta) and Its Effect on Mouse Splenocyte Proliferation In Vitro and In Vivo. The Protein Journal 2014, 33, 92-99, 10.1007/s10930-013-9541-y.
- Edgardo E. Tulin; Zenaida T. Ecleo; Cytokine-Mimetic Properties of Some Philippine Food and Medicinal Plants. Journal of Medicinal Food 2007, 10, 290-299, 10.1089/jmf.2006.067.
- Lyris A. D. Mérida; Érika Bertozzi De Aquino Mattos; Anna Carolina Nitzsche Teixeira Fernandes Corrêa; Patrícia Ribeiro Pereira; Vania Margaret Flosi Paschoalin; Maria F. B. Pinho; Mauricio A. Vericimo; Tarin stimulates granulocyte growth in bone marrow cell cultures and minimizes immunosuppression by cyclo-phosphamide in mice. PLoS ONE 2018, 13, e0206240, 10.1371/journal.pone.0206240.
- Patrícia Ribeiro Pereira; Joab T. Silva; Maurício Afonso Verícimo; Vania Margaret Flosi Paschoalin; Gerlinde A.P.B. Teixeira; Crude extract from taro (Colocasia esculenta) as a natural source of bioactive proteins able to stimulate haematopoietic cells in two murine models. Journal of Functional Foods 2015, 18, 333-343, 10.1016/j.jff.2015.07.014.
- Chinedum Ogbonnaya Eleazu; Kate Chinedum Eleazu; Mercy Amarachi Iroaganachi; Effect of cocoyam (Colocasia esculenta), unripe plantain (Musa paradisiaca) or their combination on glycated hemoglobin, lipogenic enzymes, and lipid metabolism of streptozotocin-induced diabetic rats. Pharmaceutical Biology 2015, 54, 91-97, 10.3109/13880209.2015.1016181.
- C. O. Eleazu; M. Iroaganachi; K. C. Eleazu; Ameliorative Potentials of Cocoyam (Colocasia esculentaL.) and Unripe Plantain (Musa paradisiacaL.) on the Relative Tissue Weights of Streptozotocin-Induced Diabetic Rats. Journal of Diabetes Research 2013, 2013, 1-8, 10.1155/2013/160964.
- Chinedum Ogbonnaya Eleazu; Polycarp Nnaecheta Okafor; Ijeh Ifeoma; Biochemical basis of the use of cocoyam (Colocassia esculenta L.) in the dietary management of diabetes and its complications in streptozotocin induced diabetes in rats. Asian Pacific Journal of Tropical Disease 2014, 4, S705-S711, 10.1016/s2222-1808(14)60711-8.
- Islam, M.H.; Mostafa, M.N.; Rahmatullah, M.; Antihyperglycemic activity of methanolic extracts of corms of Colocasia esculenta var esculenta. European Journal of Pharmaceutical and Medical Research 2018, 5, 129–132.
- Chika I. Chukwuma; Shahidul Islam; Eric Oscar Amonsou; A comparative study on the physicochemical, anti-oxidative, anti-hyperglycemic and anti-lipidemic properties of amadumbe (Colocasia esculenta ) and okra (Abelmoschus esculentus ) mucilage. Journal of Food Biochemistry 2018, 42, e12601, 10.1111/jfbc.12601.
- McEwan, R.; Madivha, R.; Djarova, T.; Oyedeji, O.; Opoku, A.; Alpha-amylase inhibitor of amadumbe (Colocasia esculenta): Isolation, purification and selectivity toward-amylases from various sources. African Journal of Biochemistry Research 2010, 4, 220–224..
- Fidyasari, A.; Raharjo, S.J.; Widiarto, E. Instant Tiwul Made of Colocasia esculenta (L.) Schott as A Current Functional Food Development for Hypercholesterolemic Patients. In Proceedings of the Innovation of Food Technology to Improve Food Secu-rity and Health, Surabaya, Indonesia, 20–21 October 2016; pp. 57–64.
- Yuichi Sakano; Motoh Mutsuga; Rie Tanaka; Hiroyuki Suganuma; Takahiro Inakuma; Masatake Toyoda; Yukihiro Goda; Masaaki Shibuya; Yutaka Ebizuka; Inhibition of Human Lanosterol Synthase by the Constituents of Colocasia esculenta (Taro). Biological & Pharmaceutical Bulletin 2004, 28, 299-304, 10.1248/bpb.28.299.
- Puthenpura T. Boban; Bala Nambisan; Perumana R. Sudhakaran; Hypolipidaemic effect of chemically different mucilages in rats: a comparative study. British Journal of Nutrition 2006, 96, 1021-1029, 10.1017/bjn20061944.
- Lynnette R. Ferguson; Anthony M. Roberton; Robert J. McKenzie; Mark E. Watson; P. J. Harris; Adsorption of a hydrophobic mutagen to dietary fiber from taro (Colocasia esculenta),an important food plant of the south pacific. Nutrition and Cancer 1991, 17, 85-95, 10.1080/01635589209514175.
- Yasushi Nakamura; Emi Suganuma; Naomi Kuyama; Kenji Sato; Kozo Ohtsuki; Comparative Bio-antimutagenicity of Common Vegetables and Traditional Vegetables in Kyoto. Bioscience, Biotechnology, and Biochemistry 1997, 62, 1161-1165, 10.1271/bbb.62.1161.
- K J Botting; M M Young; A E Pearson; P J Harris; L R Ferguson; Antimutagens in food plants eaten by Polynesians: micronutrients, phytochemicals and protection against bacterial mutagenicity of the heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline.. Food and Chemical Toxicology 1999, 37, 95-103, 10.1016/s0278-6915(98)00121-5.
- Tom Agbor-Egbe; June E Rickard; Identification of phenolic compounds in edible aroids. Journal of the Science of Food and Agriculture 1989, 51, 215-221, 10.1002/jsfa.2740510209.
- Mia Isabelle; Bee Lan Lee; Meng Thiam Lim; Woon-Puay Koh; Dejian Huang; Choon Nam Ong; Antioxidant activity and profiles of common vegetables in Singapore. Food Chemistry 2010, 120, 993-1003, 10.1016/j.foodchem.2009.11.038.
- Amal El- Dardiry; Amal M. Ewis; M. M. Abo-Srea; Impact of Taro Corms on Functional Low Fat Ice Cream Properties. Journal of Food and Dairy Sciences 2018, 9, 399-402, 10.21608/jfds.2018.36100.
- Yeon Soo Kim; Damilare Adeyemi; Ponijese Korovulavula; Dong Wook Jang; Mi-Kyung Park; Effect of steaming on the functional compounds and antioxidant activity of Fijian taro (Colocasia esculenta L. Schott) corms. Korean Journal of Food Preservation 2019, 26, 449-454, 10.11002/kjfp.2019.26.4.449.
- Tuti, M.; Pal, R.; Arun Kumar, R.; Bist, J.; Bhatt, J.; Colocasia based cropping systems affects the antioxidant properties and productivity of colocasia [Colocasia esculenta (l.) schott] tuber. Bioscan Int. Q. J. Life Sci. 2015, 10, 117–123.
- Chakraborty, P.; Deb, P.; Chakraborty, S.; Chatterjee, B.; Abraham, J.; Cytotoxicity and antimicrobial activity of Colocasia esculenta. Journal of Chemical and Pharmaceutical Research 2015, 7, 627–635.
- Akshatha; Smitha Kavadikeri; Nagashree N Rao; In vitro Micropropagation and Antioxidant Assay in Colocasia esculenta. Plant Tissue Culture and Biotechnology 2018, 28, 183-190, 10.3329/ptcb.v28i2.39677.
- Vivek Kumar; Harish Kumar Sharma; Process optimization for extraction of bioactive compounds from taro (Colocasia esculenta), using RSM and ANFIS modeling. Journal of Food Measurement and Characterization 2016, 11, 704-718, 10.1007/s11694-016-9440-y.
- Jun Takebayashi; Tomoyuki Oki; Jun Watanabe; Koji Yamasaki; Jianbin Chen; Maki Sato-Furukawa; Megumi Tsubota-Utsugi; Kyoko Taku; Kazuhisa Goto; Teruki Matsumoto; et al.Yoshiko Ishimi Hydrophilic antioxidant capacities of vegetables and fruits commonly consumed in Japan and estimated average daily intake of hydrophilic antioxidants from these foods. Journal of Food Composition and Analysis 2013, 29, 25-31, 10.1016/j.jfca.2012.10.006.
- Lee, S.; Wee, W.; Yong, J.; Syamsumir, D.; Antimicrobial, antioxidant, anticancer property and chemical composition of different parts (corm, stem and leave) of Colo-casia esculenta extract. Ann. Univ. Mariae Curie-Skłodowska 2011, 24, 9–16.
- Ezinne Awa; Chinedum Eleazu; Bioactive constituents and antioxidant activities of raw and processed cocoyam (Colocasia esculenta). Nutrafoods 2015, 14, 133-140, 10.1007/s13749-015-0033-x.
- Chinedum Eleazu; Characterization of the natural products in cocoyam (Colocasia esculenta) using GC–MS. Pharmaceutical Biology 2016, 54, 2880-2885, 10.1080/13880209.2016.1190383.
- Richelle M Alcantara; Wilma A Hurtada; Erlinda I Dizon; The Nutritional Value and Phytochemical Components of Taro [Colocasia esculenta (L.) Schott] Powder and its Selected Processed Foods. Journal of Nutrition & Food Sciences 2012, 3, 3, 10.4172/2155-9600.1000207.
- K.L. Lindsey; M.L. Motsei; A.K. Jäger; Screening of South African Food Plants for Antioxidant Activity. Journal of Food Science 2002, 67, 2129-2131, 10.1111/j.1365-2621.2002.tb09514.x.
- Mehmet Akyüz; Gölevez [(Colocasia esculenta (L.)] Yumrularının Etanol Ekstresinin Antioksidan Aktivitesinin Belirlenmesi. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi 2019, 22, 388-394, 10.18016/ksutarimdoga.vi.589216.
- Amadi, J.A.; Glycaemic index of three cocoyam varieties consumed in imo state, Nigeria. J. Dietit. Assoc. Niger. 2017, 8, 96–103.
- Chinedum Ogbonnaya Eleazu; The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus; prospects, challenges and solutions. African Health Sciences 2016, 16, 468-479, 10.4314/ahs.v16i2.15.
- Masakazu Washio; Mitsuru Mori; Fumio Sakauchi; Yoshiyuki Watanabe; Kotaro Ozasa; Kyohei Hayashi; Tsuneharu Miki; Masahiro Nakao; Kazuya Mikami; Yoshinori Ito; et al.Kenji WakaiAkiko Tamakoshi Risk Factors for Kidney Cancer in a Japanese Population: Findings from the JACC Study. Journal of Epidemiology 2004, 15, S203-S211, 10.2188/jea.15.s203.
- Masakazu Washio; Mitsuru Mori; Risk Factors for Renal Cell Cancer in a Japanese Population. Clinical medicine. Oncology 2008, 3, CMO.S2669-5, 10.4137/cmo.s2669.
- Masakazu Washio; Mitsuru Mori; Kazuya Mikami; Tsuneharu Miki; Yoshiyuki Watanabe; Masahiro Nakao; Tatsuhiko Kubo; Koji Suzuki; Kotaro Ozasa; Kenji Wakai; et al.Akiko Tamakoshi Risk Factors for Renal Cell Carcinoma in a Japanese Population. Asian Pacific Journal of Cancer Prevention 2014, 15, 9065-9070, 10.7314/apjcp.2014.15.21.9065.
- Joint Committee for Comprehensive Risk Management Chart for the Prevention of Cerebro- Cardiovascular Diseases; Tamio Teramoto; Masayuki Yokode; Hiroyasu Iso; Akihiko Kitamura; Hiroki Shiomi; Tsuyoshi Kimura; Masayasu Matsumoto; Mami Iida; Jun Sasaki; et al.Shigeru InoueRyouichi NagatomiTetsuya ShojiHidenori AraiHiromi RakugiHirohito SoneShizuya YamashitaShigeru Mizyzaki Comprehensive risk management for the prevention of cerebro- cardiovascular diseases in Japan. Hypertension Research 2017, 40, 847-855, 10.1038/hr.2016.155.
- Peter Kraft; Michael K. Schuhmann; Cornelia Garz; Solveig Jandke; Daniela Urlaub; Stine Mencl; Alma Zernecke; Hans-Jochen Heinze; Roxana O. Carare; Christoph Kleinschnitz; et al.Stefanie Schreiber Hypercholesterolemia induced cerebral small vessel disease. PLoS ONE 2017, 12, e0182822, 10.1371/journal.pone.0182822.