The secondary structures of Scherer commonly known as perineuronal and perivascular satellitosis have been identified as a histopathological hallmark of diffuse, invasive, high-grade gliomas. They are recognised as perineuronal satellitosis when clusters of neoplastic glial cells surround neurons cell bodies and perivascular satellitosis when such tumour cells surround blood vessels infiltrating Virchow–Robin spaces.
- brain tumour
- satellitosis
- glioblastoma
- tumour heterogeneity
- perineuronal satellitosis
- perivascular satellitosis
- invasion
1. Introduction
One of the first histological descriptions of satellitosis in the nervous system was reported by Santiago Ramón y Cajal in 1899, when he described aggregates of glial cells surrounding both the cell body of neurons and its dendrites in healthy peripheral nervous tissue and only later in the 1930s this phenomenon was termed with “"perineuronal satellitosis”" [1,2][1][2]. Further contributions came later by Brownson [1], Critchley, Andrew and Brain [3], Riese [4], showing such a numerical increase in satellite cells in normal aged neurological specimens as well as in young normal brains [1,5][1][5]. This increase, which does not necessarily indicate metabolic failure or even necrobiosis of neurons, suggests a vitalizing-like event, a perfect example of the symbiotic relationship standing between neurons and glial cells [2]. Most of the time, it indicates a condition that characterizes aged but essentially normal brain specimens. The perineuronal satellitosis phenomenon has also been described in healthy tissues of diverse brain regions such as cerebral cortex, hippocampus, basal ganglia and thalamus [1,2,6][1][2][6]. Although these aggregations of glial cells are frequently reported in physiological conditions, they are more commonly known as a histological marker of various pathological conditions within the central nervous system (CNS), for example, those associated with type I neurofibromatosis (NF1) [5], and they have been mostly recognised as histopathological markers of diffuse neoplasms such as various grades of astrocytoma and oligodendroglioma [6,7,8,9,10][6][7][8][9][10].
The first description of perineuronal and perivascular satellitosis in brain tumours was made by Hans Joachim Scherer, a pioneer in the study of glioma growth patterns, in his pivotal paper “"Structural development in gliomas’’" [8]. After a careful revision of a certain number of glioblastomas (GB), he designated as secondary structure any form of tumour growth depending on a pre-existing tissue structure. He described thus seven types of secondary structures, plus an eighth type consisting of an eventual combination of two or more of the former ones: (I) perineural growth (also named neuronophagic growth), (II) surface growth, (III) perivascular growth, (IV) perifascicular growth, (V) intrafascicular growth, (VI) interfibrillar growth, (VII) white or grey matter growth. Each of these growth modalities, he observed, also had similar counterparts in specimens displaying findings of reactive rather than neoplastic processes and could potentially be related to glioma histological types and clinical prognosis.
Perineuronal growth, what we now define as perineuronal satellitosis (Figure 1A), is characterised by growth of neoplastic cells around a neuron’'s cell body and dendrites. Sometimes, this growth leads to the replacement of neurons by groups of tumour cells (Figure 1B,C), a highly characteristic feature which Scherer himself termed “"neuronophagic growth”" [8,9,10][8][9][10].
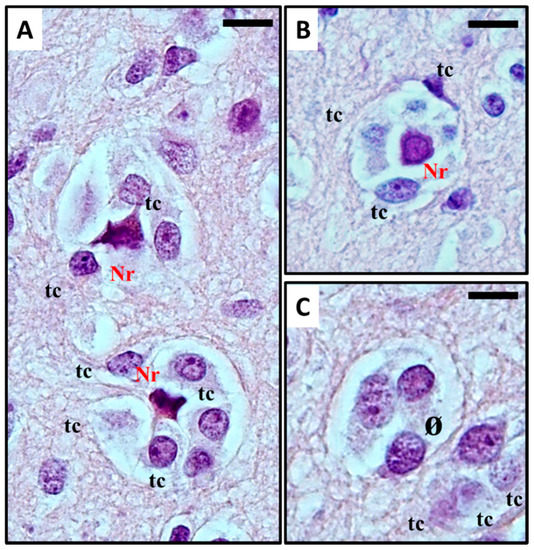
(
) Histological observation of
in glioblastoma. Haematoxylin and eosin stain show (
) Anaplastic, frankly malignant glial tumour cells (tc) induced neuronal apoptosis (
): only the picnotic remnant (Nr) of the neuron remains. In the end, (
) the vanishing neuron will be completely replaced by tumour cells (ø). What normally occurs is that neoplastic cells surround the neuronal cell body, which then results in neuronal cell death with intracellular degenerative change and appearance of ‘ghost cells' surrounded by the neoplastic cells in electron microscopy studies. The neoplastic cells can also phagocytose the remains of the neurons. For detailed information refer to [11][12]. Original images are collected at 400× magnification and relative region of interest (ROI) is reported. The scale bars are 20 µm.
Similar features were seen in Ethyl Nitrosourea (ENU)-induced gliomas in BD-IX rats where a range of neuronal degenerative changes whereby at the most severe manifestation “"ghost cells”" or dying neurons replaced normal neurons and these were surrounded by neoplastic glial cells [11,13][11][13]. Interestingly, both neuronal and perivascular satellitosis (Figure 2) have been demonstrated many years ago by sequential electron microscopic examination of the subventricular zone (SVZ, also known as the subependymal plate). In this rat model of glioma, pregnant rats were treated with a potent neuro-carcinogen, Ethyl Nitrosourea, on the 16th day of gestation and the resultant offspring developed glia neoplasms predominantly located adjacent to the lateral ventricles of the brain (a known ‘germinal’' zone). Thus the developmental genesis of such primary brain tumours could be followed and manifested both peri-neuronal and perivascular satellitosis as early hallmarks of developing brain tumours [7,12][7][12].
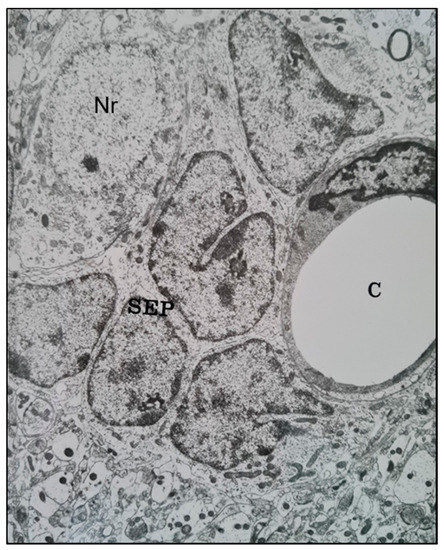
A transmission electron microscope micrograph of the sub-ventricular zone of the brain of a trans placentally ENU-treated BDIX rat showing neoplastically-transformed sub-ependymal plate glial stem cells (SEP) closely juxtaposed with a brain capillary (C) (right) and a neuronal cell body (Nr) (upper left). ×10,800.
Moreover, perivascular growth, what we now define as perivascular satellitosis (Figure 3) is characterized by the growth of neoplastic cells in Virchow–Robin spaces around blood vessels, typically small sized capillaries or precapillary vascular structures. As stated in an even earlier publication [8], Scherer observed how this form of growth was an early manifestation of tumour spread and probably one of the first pathways of neoplastic diffusion, yet tended to be present also in later stages, highly infiltrative areas of tumours, and usually was easier to detect in cortical and striatum grey matter than in white matter.
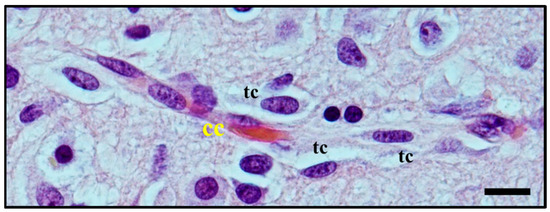
Histological observation of
. Atypical glial tumour cells (tc) moving and stretching along capillary vessels: increase in nuclear size of endothelial capillary cells (cc) is a sign of their metabolic reaction to tumour cells aggression and makes nuclei bulge into vessel lumen. Original image collected at 400× magnification and relative regions of interest (ROI) are reported. The scale bar is 50 µm.
Given these preliminary studies on the invasive pattern of glioma, he postulated that a complete surgical excision of infiltrative glial neoplasms was technically impossible, thus explaining the high and fast recurrence rates not only of GB but also of lower grade glial tumours: this concept could be applied even at a time when hemispherectomies pioneered by Walter Dandy [14] were still being practised as standard treatment for glial neoplasms, and recurrences were observed in the contralateral hemisphere as early as six months postoperatively [15]. Despite these early speculations on the functional significance of satellitosis, the phenomenon has received very little attention in more recent years.
2. Satellitosis in Neurological Disease
2.1. Histological Characterisation of Perineuronal Satellitosis in Brain Tumours
The term satellitosis usually refers to an increase in the number of cells encircling a neuron. The term has been applied to both reactive and neoplastic processes. Sherer himself preferred the term growth to define the neoplastic ones in order to avoid confusion [8]. In everyday practice of neuropathology, neoplastic satellitosis (Figure 4A) is more commonly seen than reactive satellitosis (Figure 4B), typically in association with diffuse astrocytic neoplasms [15] and easily found when the tumour infiltrates the grey matter [16]. A common example of reactive process instead, can be exemplified by neuron degeneration, where the satellite cells are usually represented by microglial cells (Figure 4B) [17]. In order to recognise these patterns as microscopic features of neoplastic or reactive pathological conditions and to distinguish perineuronal malignant satellitosis from reactive satellitosis and microglial neuronophagia, the recognition of atypical nuclear morphology of infiltrating neoplastic astrocytes or even their glial nature could be difficult in the absence of immunohistochemical or ultrastructural analyses (Figure 1A). Also in other secondary structures described by Scherer, such as perivascular, subependymal, and subpial spread, satellitosis mirrors the ability for the infiltrating tumour cells to breach the glia limitans and enter the subarachnoid space: even at gross observation, typically during neurosurgeries for high grade glioma resections, neurosurgeons can easily recognize mounds of tumour on the surface of the brain, and clearly see the presence of a subjacent tumour [18].
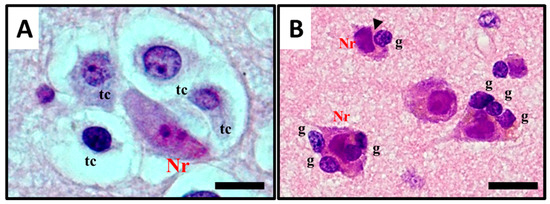
Figure 4. Histological observation and description of (A) perineuronal satellitosis in glioblastomas (GB) and (B) perineuronal satellitosis in non-neoplastic brain damage condition. (A). Polymorphic, markedly atypical glial tumour cells (tc) surrounding a neuron (Nr), which nevertheless shows only mild sign of cellular, mostly hypoxic stress (darkened colour of nucleus and cytoplasm, focal vacuolisation, chromatic dispersion); (B) Slightly atypical, non-neoplastic glial cells (g) are surrounding neurons (Nr) with little changes, mostly due to hypoxic stress, in the collateral brain tissue of an active plaque (patient with relapsing multiple sclerosis). The contact between the cell’'s shapes, not only cytoplasmic but also nucleus structure, creating nuclear membrane indentations (►). All images are shown at 400× magnification and relative regions of interest (ROI) are reported. The scale bars are 50 µm.
2.2. Molecular Mechanisms Involved in Perineuronal Satellitosis Phenomena
Many studies investigated the role of non-neoplastic glial cells in GB growth and evolution [34[19][20][21][22][23],35,36,37,38], proposing different mechanisms [32[24][25],33], which may even contribute to drug resistance [33][25]. However, increasing evidence supports the hypothesis that precursor glial cells, glial cells and glioma cells may mutually benefit the close relationship with surrounding neurons [28][26]. Among glial cells, astrocytes play a pivotal role in CNS tumours [34,35][19][20] as well as in physiological events such as neurotransmission processes [36][21] water homeostasis, defense against oxidative/nitrosative stress, energy storage, mitochondria biogenesis, scar formation, tissue repair via angiogenesis and neurogenesis, synapse modulation by regulation of neurotransmitters and ions concentrations [37][22]. Astrocytic cells are actively involved in neurotransmitter recycling, clearance of extracellular potassium ions, and in the propagation of Ca2+ waves [38][23]. The additional role of astrocytes as a source of energetic fuel for neurons was originally proposed by Pellerin and co-workers (1994), who described an activation of neurons with subsequent release of the neurotransmitter glutamate stimulating glycolysis in nearby astrocytes: the lactate produced in this glycolytic burst is released back to neurons and pushes neuronal metabolism further. The hypothesis has been termed the “"Astrocyte-Neuron Lactate Shuttle Hypothesis”" (ANLSH): such a model does not exclude a direct neuronal glucose uptake. However, it suggests that lactate produced by astrocytic glycolysis is the main metabolic fuel of glutamatergic neurons during neurotransmission [39][27]. Although the hypothesis focuses on the mechanism by which astrocytes may “"talk”" with neurons, we could pave the way for the hypothesis that neoplastic astrocytes can interact with neurons in the same way. Pei et al. [40][28] reviewed the implication of neurotransmitter molecules on glioma progression, underlining how glutamatergic and calcium (Ca+) signaling exerts a positive feedback on glioma development by metabolic reprogramming, which accelerates glioma growth.
2.3. Histological Characterisation of Perivascular Satellitosis in Brain Tumours
Tumour cells have long been known to connect with vascular structures, both from the tissues they stem from and from infiltrated or metastasized ones. The first morphological evidence of this interaction was reported at least as far back as 400 BC and 192 AD, respectively, by Hippocrates and Galen [71][29]. It was John Hunter in 1787 that introduced the term angiogenesis and related it to inflammatory processes [72][30]. While the first report of blood vessels apparently stemming within tumours was reported by Rudolf Virchow in 1863 [73][31], when he showed that solid tumours have their own blood supply, a few years later Thiersch (1865) and subsequently Goldmann (1908) provided more precise descriptions of tumoral vessels as vascular neo-formations featuring high proliferation and both chaotic and irregular growth [74[32][33],75], a description that may fit with the concept of tumour angiogenesis.
Early observations of perivascular patterns surrounding the Virchow–Robin spaces of pre-existing brain vessels were reported by Scherer [8,9][8][9]. In his studies he reported that 35% of gliomas, both in earlier and later disease stages, form cuffs of glial neoplastic cells surrounding capillaries and small vessels of the brain. Later, he went on to recognise this process of “"perivascular gliosis”" as one of the distinctive features of glial tumours [9]. Moreover, he pointed out how the organisation of glioma cells tends to turn into the formation of cell cuffs around normal micro-vessels typically found in areas of apparently normal brain parenchymal tissue at some distance from the original tumour mass, and he underlined the precocity of the process in the biological history of the neoplasm [8]. This early finding about perivascular satellitosis has inspired many groups over the years, which described the phenomenon using a wide range of different terminologies, particularly “"vessel co-option”". Even though researchers use this term to describe the molecular mechanism where glioma cells reach and subsequently encircle vessels, the term perivascular satellitosis is still used by pathologists and is currently reported in textbooks to teach the main histological features of vasculature in pathological conditions.
Perivascular satellitosis has been reviewed in many tumours by Kuczynski et al. [76][34] highlighting the key histopathological traits associated with this process in cancer, while also clarifying the terminology used for different processes.
In glioma, the term perivascular satellitosis is used to refer to a non-vasculogenic process whereby blood vessels hijack glioma cells to migrate towards pre-existing vasculature (Figure 3 and Figure 5) [77][35]. This feature has commonly been found in both high and low-grade glioma [15]. In high-grade glioma cells entirely surround the blood brain barrier (BBB) capillaries (perivascular growth pattern), modulating the function of pericytes and the properties of the BBB, while, in low grade glioma, vessels are surrounded when the cancer cells infiltrate the brain parenchyma (diffuse infiltrating pattern) as single cells in proximity to vessels.
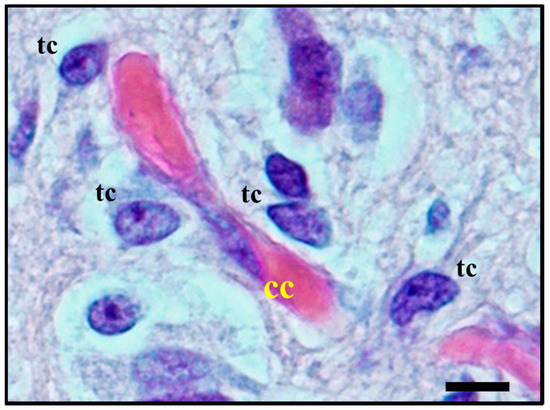
Figure 5. Histological observation of perivascular satellitosis shows atypical glial tumour cells (tc) moving and stretching along capillary vessels: a marked increase in nuclear size of endothelial cells (cc) is a sign of metabolic reaction to tumour cells aggression. The original image is captured at 400× magnification with a relative region of interest (ROI) reported. The scale bars are 50 µm.
2.4. Molecular Mechanisms Involved in Perivascular Satellitosis Phenomenon
The mechanisms that trigger neoplastic “"satellite”" glial cells to migrate and invade are multiple and interdependent [78][36].
Recent works [79,80][37][38] adopting new advanced intravital microscopy imaging technologies have recorded patient-derived GB cell movement in mouse brain, showing by time-lapse how GB cells closely interact with the microvasculature of BBB and they move along the pre-existing vasculature of the brain. Here, neoplastic cells can travel several millimetres or even centimetres away from the main tumour mass. Interestingly, during this invasion process the infiltrative cells, so-called guerilla cells, protected from cytotoxic agents by an intact BBB, invade normal brain; in particular, it has been seen that neoplastic cells adhere to vascular basal laminae, where mitogenic growth factors are sequestered within the extracellular matrix components stimulating glioma cells to divide, suggesting that this process that takes place around capillaries is a possible form of ‘pseudoinvasion’' [78][36].
A recent review by Seano et al. [81][39] discussed this phenomenon proposing two mechanisms: (I) individual-cell co-option; and (II) collective-cell: vessel co-option mechanism [82][40]. The same group also demonstrated the process behind the collective-vessel co-option that causes disruption of the astrocyte: vascular coupling and blood-brain barrier (BBB) breach, with consequent blood vessel leakage, abnormal vasculature (large lumen and tortuous architecture) and later inflammation. While individual cell co-option is led by astrocyte-like GB cells (i.e., Olig2- and Wnt7-negative) via Olig2-Wnt7, a signalling axis [80][38] does not involve an inflammatory process and spread in association with blood vessels; this later process is undetectable even with advanced imaging techniques [83][41]. Moreover, the authors raised the possibility that these mechanisms, although still unclear, might be related to the failure of current anti-angiogenic treatments.
References
- Brownson, R.H. Perineuronal satelite cells in the motor cortex of aging brains. J. Neuropathol. Exp. Neurol. 1956, doi:10.1097/00005072-195604000-00004.
- Vijayan, V.K.; Zhou, S. ‐S; Russell, M.J.; Geddes, J.; Ellis, W.; Cotman, C.W. Perineuronal satellitosis in the human hippocampal formation. Hippocampus 1993, doi:10.1002/hipo.450030215.
- Brain, W.R.; Greenfield, J.G. Late infantile metachromatic leuco-encephalopathy, with primary degeneration of the interfascicular oligodendroglia. Brain 1950, doi:10.1093/brain/73.3.291.
- Riese, W. The Cerebral Cortex in the Very Old Human Brain1. J. Neuropathol. Exp. Neurol. 1946, 5, 160–164, doi:10.1097/00005072-194604000-00006.
- Yokota, O.; Tsuchiya, K.; Hayashi, M.; Kakita, A.; Ohwada, K.; Ishizu, H.; Takahashi, H.; Akiyama, H. Glial clusters and perineuronal glial satellitosis in the basal ganglia of neurofibromatosis type 1. Acta Neuropathol. 2008, 116, 57–66, doi:10.1007/s00401-008-0390-2.
- Johnsen, S.D. Book Review: Clinical Neuropathology. Text and Color Atlas. By Catherine Haberland. Demos Publishing, New York, NY, 2007. J. Child Neurol. 2008, doi:10.1177/0883073808315421.
- Pilkington, G.J.; Lantos, P.L. The development of experimental brain tumours a sequential light and electron microscope study of the subependymal plate - II. Microtumours. Acta Neuropathol. 1979, doi:10.1007/BF00702669.
- Scherer, H.J. Structural development in gliomas. Am. J. Cancer 1938, doi:10.1158/ajc.1938.333.
- Scherer, H.J. The forms of growth in gliomas and their practical significance. Brain 1940, doi:10.1093/brain/63.1.1.
- Scherer, H.J. p. I’étude du cancer; 1937; Vol. 26;.
- P. L. Lantos and G. J. Pilkington Neuronal changes in experimental gliomas. Neuropathol. Appl. Neurobiol. 1980, 6, 255–66.
- Lantos, P.L.; Pilkington, G.J. The development of experimental brain tumours a sequential light and electron microscope study of the subependymal plate - I. Early lesions (abnormal cell clusters). Acta Neuropathol. 1979, doi:10.1007/BF00702668.
- Lantos, P.L.; Pilkington, G.J. Cell degeneration and necrosis in experimental gliomas. Br. J. Exp. Pathol. 1978, 59, 85—92.
- Dandy, W.E. Removal of right cerebral hemisphere for certain tumors with hemiplegia: Preliminary report. J. Am. Med. Assoc. 1928, doi:10.1001/jama.1928.02690380007003.
- Wesseling, P.; Kros, J.M.; Jeuken, J.W.M. The pathological diagnosis of diffuse gliomas: Towards a smart synthesis of microscopic and molecular information in a multidisciplinary context. Diagnostic Histopathol. 2011, 17, 486–494, doi:10.1016/j.mpdhp.2011.08.005.
- Saul, R.A.; Sturner, R.A.; Burger, P.C. Hyperplasia of the Myenteric Plexus: Its Association With Early Infantile Megacolon and Neurofibromatosis. Am. J. Dis. Child. 1982, doi:10.1001/archpedi.1982.03970450094023.
- Garman, R.H. Histology of the Central Nervous System. Toxicol. Pathol. 2011, 39, 22–35, doi:10.1177/0192623310389621.
- Yamamoto, J.; Kitagawa, T.; Akiba, D.; Nishizawa, S. 5-aminolevulinic acid-induced fluorescence in cerebellar primary central nervous system lymphoma: A case report and literature review. Turk. Neurosurg. 2015, doi:10.5137/1019-5149.JTN.10594-14.1.
- O’Brien, E.; Howarth, C.; Sibson, N.R. The role of astrocytes in CNS tumours: Pre-clinical models and novel imaging approaches. Front. Cell. Neurosci. 2013.
- Guan, X.; Hasan, M.N.; Maniar, S.; Jia, W.; Sun, D. Reactive Astrocytes in Glioblastoma Multiforme. Mol. Neurobiol. 2018.
- Allen, N.J. Astrocyte Regulation of Synaptic Behavior. Annu. Rev. Cell Dev. Biol. 2014, doi:10.1146/annurev-cellbio-100913-013053.
- Kim, Y.; Park, J.; Choi, Y.K. The role of astrocytes in the central nervous system focused on BK channel and heme oxygenase metabolites: A review. Antioxidants 2019, doi:10.3390/antiox8050121.
- Bélanger, M.; Magistretti, P.J. The role of astroglia in neuroprotection. Dialogues Clin. Neurosci. 2009.
- Matarredona, E.R.; Pastor, A.M. Extracellular Vesicle-Mediated Communication between the Glioblastoma and Its Microenvironment. Cells 2019, doi:10.3390/cells9010096.
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, doi:10.1038/nature16071.
- Venkatesh, H.; Monje, M. Neuronal Activity in Ontogeny and Oncology. Trends in Cancer 2017.
- Mangia, S.; Simpson, I.A.; Vannucci, S.J.; Carruthers, A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: Evidence from modeling of measured lactate levels during visual stimulation. In Proceedings of the Journal of Neurochemistry; 2009.
- Pei, Z.; Lee, K.C.; Khan, A.; Erisnor, G.; Wang, H.Y. Pathway analysis of glutamate-mediated, calcium-related signaling in glioma progression. Biochem. Pharmacol. 2020.
- Lytton, D.G.; Resuhr, L.M. Galen on abnormal swellings. J. Hist. Med. Allied Sci. 1978, doi:10.1093/jhmas/XXXIII.4.531.
- Lenzi, P.; Bocci, G.; Natale, G. John Hunter and the origin of the term “angiogenesis.” Angiogenesis 2016, doi:10.1007/s10456-016-9496-7.
- Virchow, R. Die krankhaften Geschwülste; 1978;
- Thiersch, C. Der Epithelialkrebs, namentlich der Haut: eine anatomisch-klinische Untersuchung; Engelmann, 1865; Vol. 1;.
- Goldmann, E. The Growth of Malignant Disease in Man and the Lower Animals, with Special Reference to the Vascular System. J. R. Soc. Med. 1908, 1, 1–13, doi:10.1177/003591570800101201.
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019.
- Diksin, M.; Smith, S.J.; Rahman, R. The molecular and phenotypic basis of the glioma invasive perivascular niche. Int. J. Mol. Sci. 2017.
- Bolteus, A.J.; Berens, M.E.; Pilkington, G.J. Migration and invasion in brain neoplasms. Curr. Neurol. Neurosci. Rep. 2001.
- Winkler, F.; Kienast, Y.; Fuhrmann, M.; Von Baumgarten, L.; Burgold, S.; Mitteregger, G.; Kretzschmar, H.; Herms, J. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 2009, doi:10.1002/glia.20850.
- Griveau, A.; Seano, G.; Shelton, S.J.; Kupp, R.; Jahangiri, A.; Obernier, K.; Krishnan, S.; Lindberg, O.R.; Yuen, T.J.; Tien, A.C.; et al. A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell 2018, doi:10.1016/j.ccell.2018.03.020.
- Seano, G.; Jain, R.K. Vessel co-option in glioblastoma: emerging insights and opportunities. Angiogenesis 2020.
- Lu-Emerson, C.; Duda, D.G.; Emblem, K.E.; Taylor, J.W.; Gerstner, E.R.; Loeffler, J.S.; Batchelor, T.T.; Jain, R.K. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with Glioblastoma. J. Clin. Oncol. 2015, doi:10.1200/JCO.2014.55.9575.
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, doi:10.1038/ncomms5196.
