Usually regarded as less evolved than their more recently diverged vascular sisters, which currently dominate vegetation landscape, bryophytes seem having nothing to envy to the defensive arsenal of other plants, since they had acquired a suite of chemical traits that allowed them to adapt and persist on land.
- bryophytes
- plants bioactive compounds from early-diverged land
- natural antifungals
- plant ex-tracts
- mosses
- liverworts
- hornworts
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Today as in the earliest terrestrial environment, plants are frequently found to manage their growth under adverse situations in terms of both abiotic stress and biotic interactions [1]: unfavorable conditions including temperature and UV radiation injuries, unpredictable water availability, predation and pathogens attack triggered—and still represent—an evolutionary pressure that challenges the plasticity of plants defense arsenal. The exploitation of their chemical weapons for pharmacological purposes dates back to several decades in a number of fields of application, relying on the observation that plant metabolites successfully evolved/selected for antagonistic activities against microbial pathogens could be employed as the bioactives reservoir, suitable in their native form or being specifically modified to tailoring their biological effect. So far, turning to compounds produced by a plant species to fight microorganisms belonging (and adapted) to its ecological niche has been a winning strategy, as countless are the plant-derived molecules actually present on the drug market in the form of pharmacophores, biocides and other antimicrobial agents. However, due to the incessant struggle between organisms for survival, an increasing occurrence of resistance phenomena is arising, making the race towards new and effective bioactive compounds more cogent. At the same time, the documented adverse effects on the environment and human health, the loss of efficacy by the most commonly commercialized synthetic pesticides, fungicides and insecticides, due to an indiscriminate use, has increased the need to provide new natural products as an alternative to hazardous chemicals.
Plants stand as an infinite resource for drug development: it has been estimated that the 11% of the 252 drugs considered as basic and essential by the WHO are obtained from flowering plants [2] but a wide amount of phytochemicals provided with antimicrobial properties was characterized and isolated in representatives of each taxa, from algae to pteridophytes to higher plants; bryophytes, non-vascular early land plants considered the link between seed and vascular plants to their algal ancestors, also contribute to this “phytochemical treasure chest”. Probably, because the necessity of defense against detrimental microorganisms has characterized the land life since the very beginning, and the lack of mechanical protection typical of the more equipped vascular plants was counterbalanced by the biochemical capability in devising active molecular architectures as a part of the survival strategy.
Bryophytes can be found in every type of habitat, except in the oceans, thus reflecting the positive species expansion occurred along the evolution. However, bryophytes rarely prevail in the ecosystems, probably due to the absence of special structures that prevent water loss (such as thick cuticles, epidermis and functional stomata), the lack of a highly specialized system for water transport and distribution inside the plant, and a water-dependent fecundation process.
More than a few biochemical adaptations provided the essential requirements for colonization and diversification on land: for example, the ability to establish close interactions with bacteria and fungi is thought a critical innovation to ensure access to limited and scattered nutrients, as revealed by the observation that arbuscular mycorrhizal fungi were found to regularly colonize not only flowering plants but also liverworts and hornworts [3]. Unprovided with mechanical protections typical of cormophytes, shielded by cuticle or bark against microbial pathogens, Bryophytes survived for more than 350 million years due to their alternative poikilohydric life strategy, taking advantage of chemical weapons: for example, the release of phenolic compounds from wetted thalli and phylloids is intended, and strongly effective, to inhibit the germination of fungal spores that occasionally land on their surface. Thus, inevitably induced to biosynthesize secondary metabolites functional to limit biotic stressors, bryophytes are relatively unaffected by microbial diseases although usually growing in humid habitats, being able to elaborate constitutive or inducible small-molecule with antimicrobial properties [4]. Therefore, they represent a valuable source of interesting bioactive compounds: a number of terpenoids (the largest group of secondary metabolites from bryophytes with more than 1600 structures described) [5] and aromatic compounds (mainly flavonoids, phenylpropanoids, benzenoids and bibenzyl derivatives) have been isolated [6][7][8][6–8]. Some of them showed an interesting antimicrobial potential: several species of liverworts, including Plagiochila, Bazzania and Radula species, Conocephalum conicum, Dumortiera hirsuta, Marchantia polymorpha, Riccia gangetica, Metzgeria furcata, Lunularia cruciata and P. vernicosa complex, proved to contain metabolites, such as marchantin A and lunularin, that displays strong antifungal activity against various Aspergillus, Penicillium, Fusarium, Candida and Rhizoctonia spp. [9][10][11][9–11]. Others, Trichocolea mollissima, T. tomentella and T. lanata, possess prenyl-phenyl ethers that are mildly fungicidal [12][13][12,13]. Recently, a structural study aimed at understanding the structure of early land plants through the comparison of the tube and cell-sheet from Cambrian-to-Devonian microfossils with experimentally degraded modern liverworts, suggested that the presence of phenolic compounds was responsible for the protection of lower epidermal tissues from the soil microbe attack, and provided dimensional stability to the remains of early marchantioid liverworts similar in some ways to modern Marchantia and Conocephalum [14]. The class of bis(bibenzyl)s, characteristic constituents of liverworts, was proposed to be the main responsible for their antimicrobial effects; in particular, a significant antifungal activity was reported against the fluconazole-sensitive and resistant strains of Candida albicans, where the administration of some bis(bibenzyl)s also facilitated the accumulation of fluconazole in fungal cells when used in combination [15][16][17][18][15–18].
The efficacy of organic extracts against different fungal genera was reported for various mosses, at different extents: interesting inhibitory effects were reported for Syntrichia ruralis, Grimmia anodon and Pleurochaete squorrosa on S. cerevisiae, while C. albicans, which resulted in being insensitive to extracts obtained from these species, was strongly affected by the Tortella tortuosa acetone extract [19]. Aqueous crude extracts of Bryum argenteum and B. cellulare, cosmopolitan mosses, have been demonstrated to display a containment activity, at varying degrees, against the two phytopathogenic fungal species Curvularia lunata, the etiological cause of leaf spot of wheat, and Drechslera maydis, responsible for leaf blight in Zea mays [20][21][20,21]. The inhibitory effect was described not only in terms of hyphae elongation, but also in terms of spore germination [22], as previously reported for Philonotis revoluta against the fungus Helminthosporium turcicum, another etiological agent of “corn leaf blight”[11]. Other phytopathogenic fungi were found to be susceptible to crude extracts from moss species: Aspergillus niger, Fusarium moniliforme and Rhizoctonia bataticola development resulted in being highly impaired by the exposure to Thuidium delicatulum and T. cymbifolium extracts [23], hence highlighting the potential of such Bryopsida as a source of mycoherbicides to control plant and crop pathogens.
2. The (un)Covered Association
Bryophytes are non-vascular land plants exhibiting a peculiar haplodiplontic life cycle in which the gametophytic generation is dominant. Bryophytes macrogroup is composed of three different divisions: the hornworts (Anthocerophyta), liverworts (Marchantiophyta) and mosses (Bryophyta). The estimated number of bryophyte species is over 24,000 [24], making this macrogroup the second largest group of terrestrial plants, second only to the most recently evolved flowering plants. A recent classification described that 250, 7000 and 12,000 bryophyte species belong to the hornworts, liverworts and mosses divisions, respectively [25].
The long time accepted idea that early land plants were not interested by fungal association has been almost overwhelmed by the copious findings that over the years showed the contrary. Despite the persistence of such an erroneous misconception—sometimes attributed to the lack of collaboration between bryologists and mycologists, and to sampling bias for healthy specimens—fungal species forming associations with taxonomically various mosses have been reported since 1951, when Wilson individuated in some basidiomycetous pathogens the real cause of “fairy rings” in Antarctic moss prairies [26] and continued through the following decades [27][28][29][30][31][27-31]. As masterfully reviewed by Davey and Currah [32], various types of association, both pathogenic and beneficial, actually occur in the “mosses chamber” of the bryophyte’s world. In terms of their development, these interactions clearly differ from those occurring between fungi and higher plants, since characteristic targets for predation (such as storage organs and specialized nutrient transport tissues) and entrance (regulated stomata and lignified, reinforced cell walls around vessels) are likely missing in bryophytes: thus, the exploitation of degradative enzymes secreted from the penetration peg seems to represent the most common entry-ticket strategy, for example in Sphagnum pathogenic fungi [33][34][35][33-35]. The functional relationship with fungal symbionts, historically conceived as a distinctive trait of land plants, has been reviewed in 2010 by Pressel and colleagues, which investigated the phylogenetic implications of bryophyte–fungal associations unraveling the early evolution of fungal symbioses at the base of the land plant tree [36]: through the combination of both cytological and sequencing available data, a more than surprising endophytes diversity was noteworthily found as consistent with phylogenies. Despite several leafy liverworts families (Jungermanniideae), around 30% of liverwort species worldwide, are reportedly presenting ascomycete rhizoidal symbionts (while lesser than 100 species with basidiomycetes, glomeromycetes and mucoromycetes) [37], a demonstration of nutritional mutualisms have been achieved in a few of early-diverging thalloid and Haplomitriopsida liverworts only and, very recently, in the leafy Cephalozia bicuspidata colonized by the ascomycete fungus Pezoloma ericae [38]; the latter in particular was then established as a mycorrhiza-like symbiosis, sanctioning the Ascomycota as mycorrhizal fungal groups engaging in mutualisms with plants across the land plant phylogeny.
However, bryophyte-associated fungi must share with vascular plants-associated cousins the exigency to cope with the host defenses, evolving structural and functional adaptation to their ecological niche and host habit; questions like: how do bryopathogenic species overcome the occurrence of prolonged desiccation of their host? or what is the actual mechanism of host cells disruption exerted by these pathogens, when, in most cases, the absence of penetrating structures has been noticed? are still to be fully addressed. On the other hand, in a few species of mosses, some pathogen-response tools—such as resistance homologous genes and an oxidative burst triggered by hydrogen peroxidases activity during the fungal spread—have been found in common with higher plants [39][40][39,40] and, recently, similar but more detailed findings at both the transcriptional and proteomic level have been reported for the liverwort Marchantia polymorpha: Carella and coauthors, in fact, showed in 2019 that in M. polymorpha the infection by the phytopathogenic oomycete Phytophthora palmivora activates a phenylpropanoid metabolic pathway, displaying same deterrence strategy that, million years later, will evolve in the phenyl-propanoids-associated biochemical defenses so well characterized in Angiosperms [41]. Hence, it could be easily speculated that these compounds (namely polyphenols, flavonoids and anthocyanins), providing an ancestral layer of biochemical defenses in mitigating pathogen infection in liverworts, played a critical role for the terrestrialization of plants.
3. Phytochemistry of Bryophyte’s Antifungal Metabolites
Bryophytes evolved a vast chemical arsenal with more than 3000 different metabolites [42][43][44][42-44]; in particular, the most representative class with 2200 different molecules are terpenoids, followed by phenolic compounds (several hundred) and other molecules, such as saccharides, lipids, nitrogen- and sulfur-containing compounds [25]. During decades, as dedicated investigations have been carried out, the enumeration of antifungal compounds detected in bryophytes is continuously increasing. Biological activities were both reported from crude plant extracts in various solvents and purified compounds; in the first case, as diffusely described for higher plants, the effectiveness of a bryophyte extract in exerting the fungicide/fungistatic effect strongly depends on its balsamic period, and on seasonal, territorial (latitude and altitude and substrate composition) and climate variations that specifically influence the phytochemical composition of the plant tissues and, in turn, determine their pharmacological potential [45].
Aside from abiotic factors, particular, even poorly investigated, biotic interactions have been revealed to be connected with the antifungal potential of bryophytes, as in the case of the association between bacteria and Tortula ruralis, Aulacomnium palustre and Sphagnum rubellum, typical moss species belonging to nutrient-poor plant communities. Through the analysis of the antagonistic activity of bacterial isolates against the phytopathogenic fungus Verticillium dahliae, a high percentage (99%) of moss-associated bacteria was found to produce antifungal compounds [46], suggesting genus Sphagnum as a promising source of antagonistic molecules against human fungal pathogens [47]. Although their acknowledged antimicrobial potential, the bryophytes have not been fully characterized chemically and pharmaceutically, also due to the difficulty of classification and collection of a significant number of species; however, to date, the various attempts to fill this gap have successfully determined notable progress, shedding light on a plethora of metabolites with acknowledged, or potentially even not assessed yet, antifungal properties. As follows, the major secondary metabolites of these early-diverged land plants, grouped depending on their chemical features, are reported and discussed.
3.1. Terpenes
Plant-derived bioactives are mostly labeled as an antioxidant: these phytochemicals are redox-active molecules, and, dynamically involved in maintaining the redox balance in the cell, have been frequently proven to exert an inhibitory effect on the fungal growth and development. Terpenoids, flavonoids and bibenzyls are the most widely described antioxidants found in the secondary metabolism of bryophytes [42], showing a high level of variability, in terms of relative abundance, within different species; for example, amongst liverworts, the chemical profile of Radula species is very distinctive with respect to other species: in fact, if they mainly elaborate bibenzyls (including bibenzyl cannabinoids and prenyl bibenzyl derivatives) and bis-bibenzyls, the distribution of terpenoids is generally almost limited, with some exceptions for Portuguese species that appear to be a rich source of sesquiterpenoids [48]. On the other hand, 264 of 679 liverwort species so far examined proved to contain α-tocopherol, which antioxidative properties are assumed to be pivotal for the constituents of oil bodies of liverworts and supposedly the cause of its activity against plant pathogenic fungi [49].
Terpenes and terpenoids constitute a group of natural and biochemically active metabolites that have been found in all living organisms [50]. For example, steroids are essentials components of cell membranes in eukaryotic cells and carotenoids are pivotal in photosynthetic organisms. Therefore, it has been speculated that the terpene and terpenoid biosynthetic pathway might be very ancient. Bryophytes are also capable to synthesize a vast variety of terpenes and terpenoids and many of these metabolites resemble those observed in flowering plants, while others are peculiar of some bryophytes [42][51][42,51]. In particular, it has been reported that liverworts synthesize around 1600 terpenoids [5] and one reason of this high structure variety might lie in the high presence of oil bodies—discussed above—a distinctive cellular compartment of liverworts [52]. These intracellular organelles are surrounded by a single membrane layer and include proteins, lipophilic materials, some phenolic compounds (marchantins) and terpenoids [53]. Neither mosses nor hornworts possess these compartments, but they have the ability to produce different types of terpenoids as well. From a structural point of view, terpenes are simple hydrocarbons, while terpenoids (also called isoprenoids) are terpenes in which the structure has undergone rearrangements and modifications with the addiction of one or more oxygen residues. However, the terms “terpene” and “terpenoid” are often used interchangeably [54]. The classification of terpenoids is based on the number of carbon atoms included in the structure and in bryophytes the most common and characteristics are monoterpenoids (C10), sesquiterpenoids (C15), diterpenoids (C20) and triterpenoids (C30).
Monoterpenoids are peculiar metabolites that contribute to the characteristic fragrances of the plant material when crushed. In particular, sabinene, α-pinene, limonene and the acetate derivatives of borneol, nerol, geraniol and myrtenol are accumulated in liverwort [55], whereas the most mosses synthetize ß-citrocitral, α- and ß-pinene, limonene, camphene and bornyl acetate [42]. For some of them, an antifungal activity is well documented [56]. The few data reported for the hornworts suggest that limonene is the most abundant monoterpenoid produced in Anthoceros caucasicus, followed by α- and ß-pinene, myrcene and terpinolene [57].
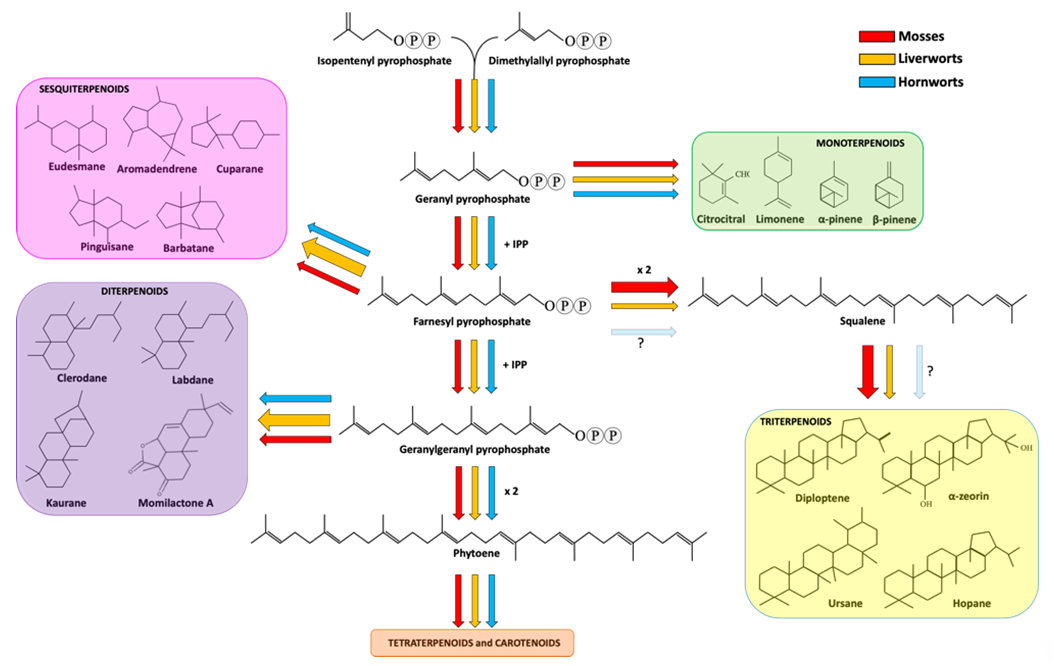
Sesquiterpenoids includes three isoprene units and display a variety of forms, spacing from linear to mono-, bi- and tricyclic structures. Sesquiterpenoids represent the widest group of terpenoids produced by bryophytes. As well explained in a recent review [44], liverwort produces around 900 types of sesquiterpenoids deriving from 60 different skeletal groups. Eudesmane and aromadendrene structures are the two most representative backbones, followed by cuparane, pinguisane and barbatane [42] (Figure 1).
Figure 1. Terpenoid biosynthetic pathway in bryophytes. Red, orange and cyan arrows indicate the metabolic fluxes observed in mosses, liverworts and hornworts, as suggested in various reports (see references in the main text). Arrow thickness represents the amount of carbon streams (resources) committed for the production of specific metabolites in a specific bryophyte division. The question marks next to the faint arrows state that no information is available about the production of squalene and triterpenoid in hornworts.
A peculiarity of liverwort sesquiterpenoids is that many are enantiomers of those found in seed plants [5], with few exceptions regarding germacrane- and guaiane-type sesquiterpenoids [43]. For example, it was seen that both Conocephalum conicum (liverwort) and Leptospermum scoparium (angiosperm) are able to produce cadina-3,5-diene, but the former synthesizes the (+)-enantiomer, whereas the latter the (-)-enantiomer [58]. Moreover, frullanolide and b-caryophyllene can be found in both enantiomeric forms in the liverworts Frullania tamarisci and Pellia epiphylla, respectively [59][60][61][59-61]. Another interesting feature of some liverwort sesquiterpenoids lies in the occurrence of these metabolites in nature: in fact, while some sesquiterpenoids such as the pinguisanes were only found in liverworts [42], others, despite appearing also in other organisms, are almost rare (such as 1,10-seco- and 2,3-seco-aromadendranes and africane-type sesquiterpenoids [6][42][55][62][63][6,42,55,62,63]). Finally, some sesquiterpenoids are liverwort species-specific [5]. In mosses, the number of sequiterpenoids was estimated around 100, a value substantially lower than that encountered in liverworts [42]. A possible explanation, beside the presence of oil bodies in liverworts, is that liverworts have been much more studied than mosses, resulting in a deeper knowledge of the chemical composition. Sesquiterpenoids are also produced in hornworts, as reported by Sonwa and Konig (2003) [57], but the available information on this division is still almost limited. Among the sesquiterpenoids, Neves and coworkers (1999) investigated the ability of Targionia lorbeeriana (a liverwort) extracts against two fungal pathogens: Cladosporium cucumerinum and Candida albicans [64].[64] The former infects many species belonging to the Cucurbitaceae family, whereas the latter is a well-known animal pathogen. In that report, three guaianolide-type sesquiterpene lactones and two germacrane-type sesquiterpene lactones were extracted, isolated and tested, revealing that the main sesquiterpene lactone constituent of T. lobeeriana, the dehydrocostus lactone, was particularly effective against C. cucumerinum. Moreover, the acetyltrifloculoside lactone and 11-αH-dihydrodehydrocostus lactone exerted antifungal activity towards C. cucumerinum as well, even in a less extent. On the other hand, among the five tested compounds, only the 8,15-acetylsalonitenolide (a germacranolide) showed activity against C. albicans. The fifth compounds, i.e., the 8-acetylsalonitenolide (a germacranolide), did not show any biological activity in the bioassays. In the report of Scher et al. (2004), different compounds extracted from Bazzania trilobata (a liverwort) were tested against different fungal pathogens, such as Botrytis cinerea, Cladosporium cucumerinum, Phythophthora infestans, Pyricularia oryzae and Septoria tritici [65]. More in detail, six sesquiterpenes, such as 5- and 7-hydroxycalamenene, drimenol, drimenal, viridiflorol and gymnomitrol, showed different growth inhibitor activities. 5-hydroxycalamenenes was particularly effective in the inhibition of P. oryzae, whereas the 7-hydroxycalamenenes towards all the pathogens. Furthermore, this last was also tested in vivo in a glasshouse environment and it showed a significant reduction in the infection of grape vine leaves caused by Plasmopara viticola when used at 250 ppm. Drimenol was active against C. cucumerinum, whereas the aldehydic form, i.e., drimenal, showed a strong inhibiting activity against S. tritici and P. infestans. Viridiflorol showed weak activity against C. cucumerinum and P. oryzae. Gymnomitrol exerted strong activity against P. infestans and moderate against C. cucumerinum, P. oryzae and S. tritici.
Diterpenoids are specialized metabolites deriving from the condensation of four isoprenyl units. They are the second largest group of terpenoids accumulated in liverworts. More in detail, about 500 compounds have been reported in liverworts so far, including acyclic, di-, tri-, tetra- and pentacyclic diterpenes. From a structural point of view, the most part of diterpenoids are based on clerodane, kaurane and labdane skeletons [5][42][5,42] (Figure 1). Interestingly, succulatane, infuscane, abeo-labdane, spiroclerodane, 5,10-seco-clerodane or 9,10-seco-clerodane backbones were found to be liverwort-specific [42]. In mosses, the number of identified diterpenoids is lower and non-exclusive of this division. For example, the momilactone diterpenoids extracted from Hypnum plumaeforme (moss) were also found in rice [66]. As for sesquiterpenoids, diterpenoids were also found in hornworts [57]. Among the diterpenoids, momilactones A, B and F, and acrenol were extracted from the moss Hypnum plumaeforme Wilson and they strongly inhibited the growth of the fungal pathogens P. oryzae, Colletotrichum acutatum and Ustilago maydis [67]. More in detail, the growth of the rice pathogen P. oryzae was inhibited by momilactones A and B, with the latter showing a greater efficacy against spore germination and germ tube growth of the fungus [68]. Momilactone A and B activities were also investigated on other fungal pathogens, giving encouraging results in the inhibition of B. cinerea, Fusarium solani and Colletrotrichum gloeosporioides [69].
Triterpenoids derive from the condensation of six isoprene units. In liverworts, the most common triterpenoids are hopanoids, including diploptene, diplopterol and α-zeorin [5][42][5,42]. In comparison to mono-, sesqui- and diterpenoids, mosses are richer in triterpenes, including ursane, fernane, friedelane, hopane, lupane, taraxane, cycloartane, obtusifolane, dammarane, polypodane and serratane [5][42][5,42]. No data are available about triterpenoids in hornworts so far.
References
- Kenrick, ; Crane, P.R. The Origin and Early Evolution of Plants on Land. Nature 1997, 389, 33–39, doi:10.1038/37918.
- Veeresham, Natural Products Derived from Plants as a Source of Drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201, doi:10.4103/2231-4040.104709.
- Rimington, R.; Pressel, S.; Duckett, J.G.; Field, K.J.; Read, D.J.; Bidartondo, M.I. Ancient Plants with Ancient Fungi: Liverworts Associate with Early-Diverging Arbuscular Mycorrhizal Fungi. Proc. R. Soc. B: Biol. Sci. 2018, 285, 20181600, doi:10.1098/rspb.2018.1600.
- Dixon, A. Natural Products and Plant Disease Resistance. Nature 2001, 411, 843–847, doi:10.1038/35081178.
- Chen, ; Ludwiczuk, A.; Wei, G.; Chen, X.; Crandall-Stotler, B.; Bowman, J.L. Terpenoid Secondary Metabolites in Bryophytes: Chemical Diversity, Biosynthesis and Biological Functions. Crit. Rev. Plant Sci. 2018, 37, 210–231, doi:10.1080/07352689.2018.1482397.
- Asakawa, Y. ChemInform Abstract: Chemical Constituents of the Bryophytes. Chemin- 2010, 27, 1–562,, doi:10.1002/chin.199618293.
- Asakawa, Recent Advances in Phytochemistry of Bryophytes-Acetogenins, Terpenoids and Bis(Bibenzyl)s from Selected Japanese, Taiwanese, New Zealand, Argentinean and European Liverworts. Phytochemistry 2001, 56, 297–312, doi:10.1016/S0031-9422(00)00454-4.
- Xie, -F.; Lou, H.-X. Secondary Metabolites in Bryophytes: An Ecological Aspect. Chem. Biodivers. 2009, 6, 303–312, doi:10.1002/cbdv.200700450.
- Asakawa, Terpenoids and Aromatic Compounds with Pharmacological Activity from Bryophytes. Bryophyt. Chem. Chem. Taxon. 1990, 369–410.
- Asakawa, Biologically Active Compounds from Bryophytes. Pure Appl. Chem. 2007, 79, 557–580, doi:10.1351/pac200779040557.
- Deora, S.; Deepti, S.; Gunjan, V. Antifungal Potential of Philonotis Revoluta-a Moss against Certain Phytopathogenic Fungi. J. Pure Appl. Microbiol. 2010, 4, 425–428.
- Perry, B.; Foster, L.M.; Lorimer, S.D.; May, B.C.H.; Weavers, R.T.; Toyota, M.; Nakaishi, E.; Asakawa, Y. Isoprenyl Phenyl Ethers from Liverworts of the Genus Trichocolea: Cytotoxic Activity, Structural Corrections, and Synthesis. J. Nat. Prod. 1996, 59, 729–733, doi:10.1021/np9603378.
- Asakawa, ; Toyota, M.; Nagashima, F.; Hashimoto, T. Chemical Constituents of Selected Japanese and New Zealand Liverworts. Nat. Prod. Commun. 2008, 3, 1934578X0800300238, doi:10.1177/1934578X0800300238.
- Graham, E.; Wilcox, L.W.; Cook, M.E.; Gensel, P.G. Resistant Tissues of Modern Marchantioid Liverworts Resemble Enigmatic Early Paleozoic Microfossils. Proc. Natl. Acad. Sci. USA 2004, 101, 11025–11029, doi:10.1073/pnas.0400484101.
- Niu, ; Qu, J.-B.; Lou, H.-X. Antifungal Bis[Bibenzyls] from the Chinese Liverwort Marchantia polymorpha L. Chem. Biodivers. 2006, 3, 34–40, doi:10.1002/cbdv.200690004.
- Guo, X.-L.; Leng, P.; Yang, Y.; Yu, L.-G.; Lou, H.-X. Plagiochin E, a Botanic-Derived Phenolic Compound, Reverses Fungal Resistance to Fluconazole Relating to the Efflux Pump. Appl. Microbiol. 2008, 104, 831–838, doi:https://doi.org/10.1111/j.1365-2672.2007.03617.x.
- Sun, ; Lou, H.; Gao, Y.; Fan, P.; Ma, B.; Ge, W.; Wang, X. Liquid Chromatography–Tandem Mass Spectrometric Method for the Analysis of Fluconazole and Evaluation of the Impact of Phenolic Compounds on the Concentration of Fluconazole in Candida Albicans. J. Pharm. Biomed. Anal. 2004, 34, 1117–1124, doi:10.1016/j.jpba.2003.11.013.
- Sun, ; Gao, Y.; Ling, X.; Lou, H. The Combination Effects of Phenolic Compounds and Fluconazole on the Formation of Ergosterol in Candida Albicans Determined by High-Performance Liquid Chromatography/Tandem Mass Spectrometry. Anal. Biochem. 2005, 336, 39–45, doi:10.1016/j.ab.2004.06.038.
- Elibo, ; Ezer, T.; Kara, R.; Çelik, G.Y.; Çolak, E. Antifungal and Antibacterial Effects of Some Acrocarpic Mosses. Afr. J. Biotechnol. 2011, 10, 986–989, doi:10.4314/ajb.v10i6.
- Deora, S.; Guhil, N. Phytochemical Analysis and Antifungal Activity of Moss Bryum Cellulare against some Phytopathogenic Fungi. Int. J. Pharm. Sci. Res. 2015, 6, 688.
- Deora, S.; Guhil, N. Antifungal Potential of Bryum Cellulare against Some Common Diseases of Maize. Int. J. Res. Appl. Nat. Soc. Sci. 2014, 2, 21–28.
- Deora, ; Guhil, N. Studies on Antifungal Potential of Bryum Cellulare (a Moss) Crude Extracts against Spore Germination of Fungus Curvularia Lunata. Int. J. Pharm. Sci. Res. (IJPSR) 2016, 7, 353–357.
- Bodade, G.; Borkar, P.S.; Arfeen, S.; Khobragade, C.N. In Vitro Screening of Bryophytes for Antimicrobial Activity. J. Med. Plants 2008, 7, 6.
- Sabovljevic, S.; Sabovljević, A.D.; Ikram, N.K.K.; Peramuna, A.V.; Bae, H.; Simonsen, H.T. Bryophytes—An Emerging Source for Herbal Remedies and Chemical Production. Plant Genet. Resour. Characterisation Util. 2016, 14, 314–327, doi:10.1017/S1479262116000320.
- Martínez-Abaigar, ; Núñez-Olivera, E. Chapter 11—Novel biotechnological substances from bryophytes. In Natural Bioactive Compounds; Sinha, R.P., Häder, D.-P., Eds.; Academic Press (Cambridge, MA, USA) 2021; pp. 233–248, ISBN 978-0-12-820655-3.
- Wilson, W. Observations on Concentric “Fairy Rings” in Arctic Moss Mat. J. Ecol. 1951, 39, 407–416, doi:10.2307/2257921.
- Racovitza, A. Étude Systématique et Biologique Des Champignons Bryophiles.[Hauptbd.]; Mus. Natl. Hist. Nat. Ser. B. Bot. 1959, 10: 1288.
- Felix, Fungi on Bryophytes, a Review. Bot. Helv. 1988, 98, 239–269, doi:10.5169/SEALS-68587.
- Kost, Moss inhabiting basidiomycetes. 3. interactions between basidiomycetes and bryophyta. Endocytobiosis Cell Res. 1988, 5, 287–308.
- Do¨bbeler, Biodiversity of Bryophilous Ascomycetes. Biodivers. Conserv. 1997, 6, 721–738, doi:10.1023/A:1018370304090.
- Thormann, N.; Currah, R.S.; Bayley, S.E. Microfungi Isolated from Sphagnum Fuscum from a Southern Boreal Bog in Alberta, Canada. Bryologist 2001, 104, 548–559.
- Davey, L.D.L.; Currah, R.S.C.S. Interactions between Mosses (Bryophyta) and Fungi. Botany 2006, doi:10.1139/b06-120.
- Redhead, A.; Spicer, K.W. Discinella Schimperi, a Circumpolar Parasite of Sphagnum Squarrosum, and Notes on Bryophytomyces Sphagni. Mycologia 1981, 73, 904–913, doi:10.1080/00275514.1981.12021420.
- During, J.; van Tooren, B.F. Bryophyte Interactions with Other Plants. Bot. J. Linn. Soc. 1990, 104, 79–98, doi:10.1111/j.1095-8339.1990.tb02212.x.
- Tsuneda, ; Chen, M.H.; Currah, R.S. Characteristics of a Disease of Sphagnum Fuscum Caused by Scleroconidioma Sphagnicola. Can. J. Bot. 2011, doi:10.1139/b01-102.
- Pressel, ; Bidartondo, M.I.; Ligrone, R.; Duckett, J.G. Fungal Symbioses in Bryophytes: New Insights in the Twenty First Century. Phytotaxa 2014, 9, 238–253, doi:10.11646/phytotaxa.9.1.13.
- Rimington, R.; Duckett, J.G.; Field, K.J.; Bidartondo, M.I.; Pressel, S. The Distribution and Evolution of Fungal Symbioses in Ancient Lineages of Land Plants. Mycorrhiza 2020, 30, 23–49, doi:10.1007/s00572-020-00938-y.
- Kowal, ; Pressel, S.; Duckett, J.G.; Bidartondo, M.I.; Field, K.J. From Rhizoids to Roots? Experimental Evidence of Mutualism between Liverworts and Ascomycete Fungi. Ann. Bot. 2018, 121, 221–227, doi:10.1093/aob/mcx126.
- Akita, ; Valkonen, J.P.T. A Novel Gene Family in Moss (Physcomitrella Patens) Shows Sequence Homology and a Phylogenetic Relationship with the TIR-NBS Class of Plant Disease Resistance Genes. J. Mol. Evol. 2002, 55, 595–605, doi:10.1007/s00239-002-2355-8.
- Mayaba, ; Minibayeva, F.; Beckett, R.P. An Oxidative Burst of Hydrogen Peroxide during Rehydration Following Desiccation in the Moss Atrichum Androgynum. New Phytol. 2002, 155, 275–283, doi:10.1046/j.1469-8137.2002.00454.x.
- Carella, ; Gogleva, A.; Hoey, D.J.; Bridgen, A.J.; Stolze, S.C.; Nakagami, H.; Schornack, S. Conserved Biochemical Defenses Underpin Host Responses to Oomycete Infection in an Early-Divergent Land Plant Lineage. Curr. Biol. 2019, 29, 2282–2294.e5, doi:10.1016/j.cub.2019.05.078.
- Asakawa, ; Ludwiczuk, A.; Nagashima, F. Phytochemical and Biological Studies of Bryophytes. Phytochemistry 2013, 91, 52–80, doi:10.1016/j.phytochem.2012.04.012.
- Asakawa, ; Ludwiczuk, A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2018, 81, 641–660, doi:10.1021/acs.jnatprod.6b01046.
- Ludwiczuk, ; Asakawa, Y. Bryophytes as a Source of Bioactive Volatile Terpenoids—A Review. Food Chem. Toxicol. 2019, 132, 110649, doi:10.1016/j.fct.2019.110649.
- Peters, ; Gorzolka, K.; Bruelheide, H.; Neumann, S. Seasonal Variation of Secondary Metabolites in Nine Different Bryophytes. Ecol. Evol. 2018, 8, 9105–9117, doi:10.1002/ece3.4361.
- Opelt, ; Berg, G. Diversity and Antagonistic Potential of Bacteria Associated with Bryophytes from Nutrient-Poor Habitats of the Baltic Sea Coast. Appl. Environ. Microbiol. 2004, 70, 6569–6579, doi:10.1128/AEM.70.11.6569-6579.2004.
- Opelt, ; Berg, C.; Berg, G. The Bryophyte Genus Sphagnum Is a Reservoir for Powerful and Extraordinary Antagonists and Potentially Facultative Human Pathogens. FEMS Microbiol. Ecol. 2007, 61, 38–53, doi:10.1111/j.1574-6941.2007.00323.x.
- Asakawa, ; Nagashima, F.; Ludwiczuk, A. Distribution of Bibenzyls, Prenyl Bibenzyls, Bis-Bibenzyls, and Terpenoids in the Liverwort Genus Radula. J. Nat. Prod. 2020, 83, 756–769, doi:10.1021/acs.jnatprod.9b01132.
- Hajji, ; Bnejdi, F.; Saadoun, M.; Ben Salem, I.; Nehdi, I.; Sbihi, H.; Alharthi, F.A.; El Bok, S.; Boughalleb-M’Hamdi, N. High Reserve in δ-Tocopherol of Peganum Harmala Seeds Oil and Antifungal Activity of Oil against Ten Plant Pathogenic Fungi. Molecules 2020, 25, 4569, doi:10.3390/molecules25194569.
- Lange, M.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid Biosynthesis: The Evolution of Two Ancient and Distinct Pathways across Genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177, doi:10.1073/pnas.240454797.
- Rao, S.A. Structural information of natural product metabolites in bryophytes. In Evolutionary Diversity as a Source for Anticancer Molecules; Elsevier BV, 2021; pp. 209–231.
- He, ; Sun, Y.; Zhu, R.-L. The Oil Bodies of Liverworts: Unique and Important Organelles in Land Plants. Crit. Rev. Plant Sci. 2013, 32, 293–302, doi:10.1080/07352689.2013.765765.
- Tanaka, ; Esaki, T.; Kenmoku, H.; Koeduka, T.; Kiyoyama, Y.; Masujima, T.; Asakawa, Y.; Matsui, K. Direct Evidence of Specific Localization of Sesquiterpenes and Marchantin A in Oil Body Cells of Marchantia polymorpha L. Phytochemistry 2016, 130, 77–84, doi:10.1016/j.phytochem.2016.06.008.
- Boncan, A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382, doi:10.3390/ijms21197382.
- Ludwiczuk, ; Asakawa, Y. Chemotaxonomic Value of Essential Oil Components in Liverwort Species. A Review. Flavour Fragr. J. 2015, 30, 189–196, doi:10.1002/ffj.3236.
- Hammer, A.; Carson, C.F.; Riley, T.V. Antifungal Activity of the Components of Melaleuca Alternifolia (Tea Tree) Oil. J. Appl. Microbiol. 2003, 95, 853–860, doi:10.1046/j.1365-2672.2003.02059.x.
- Sonwa, M.; König, W.A. Chemical Constituents of the Essential Oil of the Hornwort Anthoceros Caucasicus. Flavour Fragr. J. 2003, 18, 286–289, doi:10.1002/ffj.1201.
- Melching, ; Bülow, N.; Wihstutz, K.; Jung, S.; König, W.A. Natural Occurrence of Both Enantiomers of Cadina-3,5-Diene and δ-Amorphene. Phytochemistry 1997, 44, 1291–1296, doi:10.1016/S0031-9422(96)00749-2.
- Knoche, ; Ourisson, G.; Perold, G.W.; Foussereau, J.; Maleville, J. Allergenic Component of a Liverwort: A Sesquiterpene Lactone. Science 1969, 166, 239–240, doi:10.1126/science.166.3902.239.
- Asakawa, Chemical Constituents of the Hepaticae. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Asakawa, Y., Heidelberger, M., Herz, W., Grisebach, H., Kirby, G.W., Eds.; Springer: Vienna, Austria, 1982; pp. 1–285.
- Fricke, ; Rieck, A.; Hardt, I.H.; König, W.A.; Muhle, H. Identification of (+)-β-Caryophyllene in Essential Oils of Liverworts by Enantioselective Gas Chromatography. Phytochemistry 1995, 39, 1119–1121, doi:10.1016/0031-9422(95)00184-9.
- von Reuß, S.H.; Wu, C.L.; Muhle, H.; König, W.A. Sesquiterpene Constituents from the Essential Oils of the Liverworts Mylia Taylorii and Mylia Phytochemistry 2004, 65, 2277–2291, doi:10.1016/j.phytochem.2004.04.039.
- Ludwiczuk, ; Asakawa, Y. Fingerprinting of Secondary Metabolites of Liverworts: Chemosystematic Approach. J. Aoac Int. 2014, 97, 1234–1243, doi:10.5740/jaoacint.SGELudwiczuk.
- Neves, ; Morais, R.; Gafner, S.; Stoeckli-Evans, H.; Hostettmann, K. New Sesquiterpene Lactones from the Portuguese Liverwort Targionia Lorbeeriana. Phytochemistry 1999, 50, 967–972.
- Scher, M.; Speakman, J.-B.; Zapp, J.; Becker, H. Bioactivity Guided Isolation of Antifungal Compounds from the Liverwort Bazzania trilobata (L.) S.F. Gray. Phytochemistry 2004, 65, 2583–2588, doi:10.1016/j.phytochem.2004.05.013.
- Nozaki, ; Hayashi, K.; Nishimura, N.; Kawaide, H.; Matsuo, A.; Takaoka, D. Momilactone A and B as Allelochemicals from Moss Hypnum Plumaeforme: First Occurrence in Bryophytes. Biosci. Biotechnol. Biochem. 2007, 71, 3127–3130, doi:10.1271/bbb.70625.
- Li, -L.; Wie, L.-L.; Chen, C.; Liu, D.; Gu, Y.-Q.; Duan-Mu, J.-X.; Chen, G.-T.; Song, Y. Bioactive Constituents from the Bryophyta Hypnum Plumaeforme. Chem. Biodivers. 2020, 17, e2000552, doi:10.1002/cbdv.202000552.
- Zhao, ; Cheng, J.; Guo, B.; Duan, J.; Che, C.-T. Momilactone and Related Diterpenoids as Potential Agricultural Chemicals. J. Agric. Food Chem. 2018, 66, 7859–7872, doi:10.1021/acs.jafc.8b02602.
- Fukuta, ; Xuan, T.D.; Deba, F.; Tawata, P.S.; Khanh, T.D.; Chung, I.M. Comparative Efficacies in Vitro of Antibacterial, Fungicidal, Antioxidant, and Herbicidal Activities of Momilatones A and B. J. Plant Interact. 2007, 2, 245–251, doi:10.1080/17429140701713811.