The diarylurea is a scaffold of great importance in medicinal chemistry as it is present in numerous heterocyclic compounds with antithrombotic, antimalarial, antibacterial, and anti-inflammatory properties.
- diarylureas
- antitumor agents
1. Introduction
Ureas (R-NHCONH-R’) are known organic compounds that possess biological activities and serve as templates for numerous medicinal chemistry researches [1]. Barbital is a diethylmalonyl urea discovered at the beginning of 1900, used as sleep aid and hypnotic [2]. In the following century, the urea scaffold has represented the pharmacophore the backbone motif for entire classes of therapeutic agents [3].
Diarylureas are found in numerous heterocyclic compounds with various biological activities [4], such as antithrombotic, antimalarial, antibacterial, antinflammatory, and anticancer [5,6][5][6]. In particular, diarylurea is a prominent pharmacophore in anticancer drugs. This activity is due to its near-perfect binding with certain acceptors. The NH moiety behaves as hydrogen bond donor and the urea oxygen atom acts as acceptor (Figure 1) [7].
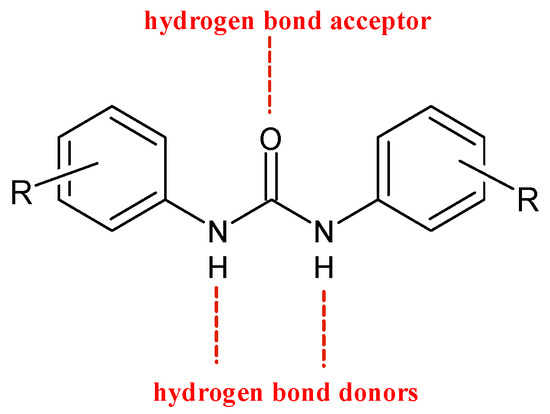
Figure 1. H-bonds in diarylureas.
This structure provides urea derivatives endowed with capability of binding several enzymes and receptors [8,9,10][8][9][10]. Moreover, it may link different pharmacophore fragments of new biological active compounds. A urea linker has been used to overcome the poor solubility of some phenyl N-mustards [11]. In this way, the authors obtained water soluble N-mustards, some of which showing high anticancer activity against various human tumor xenograft models and were able to introduce cross-linking within the DNA double strand. Diarylurea-based compounds present strong inhibitory activity against kinases, including RAF kinases [12], platelet derived growth factor receptor (PDGF) [13], vascular endothelial growth factor receptor 2 (VEGFR-2) [14], receptor tyrosine kinase (RTKs) [15], and Aurora kinases [16]. The diarylurea moiety is, in fact, widespread in type II kinase inhibitors. These compounds circumvent kinases in an inactive state, the so-called DFG-out, and occupy a hydrophobic pocket next to the ATP-binding site. The diarylurea fragment is able to link the hinge-binding moiety with the portion that occupies the hydrophobic pocket that is in the inactive conformation of kinases [17] [17] (Figure 2).
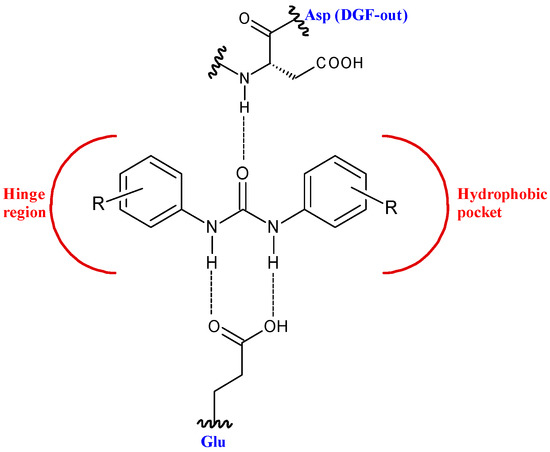
Figure 2. Diarylureas in the type II kinase inhibitor.
Diarylureas represent the skeleton of the main systemic therapies for several cancers, as advanced, metastatic hepatocellular carcinoma (HCC) [18], advanced renal cell carcinoma (RCC) [19], gastrointestinal stromal tumors (GISTs) [20], metastatic colorectal cancer (mCRC) [21]. Sorafenib is a multi-targeted small molecule tyrosine protein kinase that improves median survival over placebo for unresectable HCC patients [22]. In 2008 it obtained Food and Drug Administration (FDA) and European Medicinal Agency (EMEA) approval for the treatment of RCC and HCC [23]. Basing on sorafenib as the lead compound, several other diarylurea derivatives, such as regorafenib, linifanib, tivozanib, and ripretinib have been synthesized and evaluated as kinase inhibitors. Regorafenib was approved by the FDA in the United States in February 2013 in patients with advanced GISTs for those who had failed on imatinib and sunitinib [24,25][24][25]. Linifanib is currently being studied in HCC clinical trials [26]. Unlike the other inhibitors of VEGFR and PDGFR, linifanib seems to be also involved in adipocyte browning, thus being considered for the treatment of obesity [27]. Tivozanib is a diarylurea which is to be considered as third and fourth line therapy in patients with metastatic RCC in phase 3 study [28]. By end of 2019, the Chinese and European regulatory authorities have marketed five small-molecule protein kinase inhibitors (PKIs), including tivozanib [29]. Ripretinib has been suggested as a promising treatment for advanced GISTs [30].
2. Diarylureas in Therapy or in Clinical Studies as Anticancer Agents
2.1. Sorafenib
Sorafenib (BAY-43-9006, Nexavar®, Figure 3) is an oral receptor TKI that determines the inhibition of Raf serine/threonine kinases and receptor tyrosine kinases (VEGF 1, 2, 3 and PDGF-β, FMS-like tyrosine kinase-3 (FLT-3), and c-KIT) that are components of signaling pathways controlling tumor growth and angiogenesis [31].
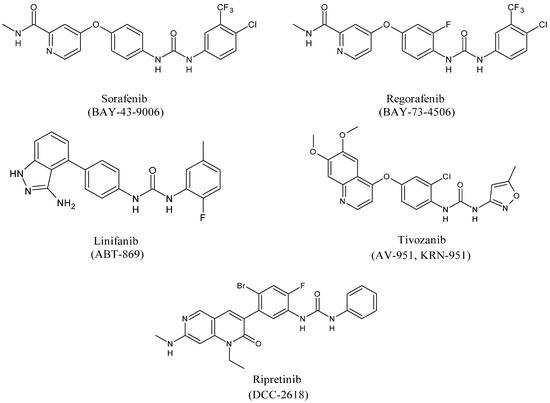
Figure 3. Structures of diarylureas.
Sorafenib inhibits the kinase activity of C-RAF and B-RAF (wild type and V600E mutant) showing IC50 = 6.22 and 38 nM, respectively. This compound is considered the most important drug in the late stage of injury for advanced stages of HCC [32] [32] which is the second cause of cancer-related mortality all over the world [33]. For more than ten years, sorafenib has been the sole systemic treatment for advanced HCC [34] [34]. However, some advanced HCC patients do not respond to therapy with sorafenib. Thus, combination studies of sorafenib with other drugs have been studied. Combined treatment with interferon-lambda 3 (IFN-λ3) and sorafenib show an effect of synergism in suppressing HCC cancer growth and in the promotion of cell apoptosis in vitro and in vivo [35]. A more recent study demonstrated that combination immunotherapy of sorafenib with atezolizumab, an immune checkpoint inhibitor (ICI) that target the programmed-cell death-1 receptor/ligand (PD-1/PD-L1) pathway, and bevacizumab, an anti-VEGF mAb, is superior to sorafenib alone as the first-line therapy of advanced HCC [36]. Moreover, given that liver function is essential for a correct prognosis, a precise rating for the safe prescription and clinical development of ICI in HCC is required. Recently, the albumin-bilirubin (ALBI) grade was used as an alternative biomarker for the prognosis [37]. The efficacy of sorafenib is limited by several factors as systematic tolerance and the poor solubility in water. Furthermore, the hydrophobicity of sorafenib is responsible of its low bioavailability as it decreases the absorption by the gastrointestinal tract. Nexavar® (Bayer Healthcare Pharmaceuticals–Onyx Pharmaceuticals) is used as tablets containing sorafenib tosylate to slightly improve the solubility. In order to increase the solubility and bioavailability of the drug, sorafenib-loaded lipid-based nanosuspensions were used [38]. The administration of sorafenib to the target cells could ameliorate patient survival and reduce the further proliferation of the tumor [39]. Thus, a drug delivery system for sorafenib has been recently studied in order to help the administration of therapies in malignant cells and raise its clinical efficacy [40]. Recently, the treatment of cancers of the gastrointestinal tract during the COVID-19 pandemic [41] has been studied: coronavirus-adapted institutional recommendations have been formulated. Sorafenib was recommended in the first-line setting only in patients with disease subtype Eastern Cooperative Oncology Group performance status (ECOG PS) 0 or 1 and Child-Pughscore A hepatocellular carcinoma [42]. In spite of its selectivity, sorafenib can determine adverse effects, such as severe respiratory and liver failure, fatigue, stomatitis, hand-foot syndrome, diarrhea, and myelosuppression, thus posing a challenge for oncologists [43]. The therapy with sorafenib might be done ad hoc to increase the therapeutic effects and reducing adverse effects [44]. Sorafenib is also studied for iodine-resistant advanced thyroid carcinoma [45]. Finally, it is important to note that sorafenib is also involved in cytoskeleton alteration that leads to cancer cells death by apoptosis. Wang et al. [46] [46] reported that the treatment of Hep3B and PLC/PRF/5 human hepatoma cells with sorafenib induces a drastic loss of actin fibers and the redistribution of F-actin around the cell nuclei. This effect is due to the regulation of protein kinases and phosphatases that ends with cofilin dephosphorylation, which is an actin-binding factor necessary for the reorganization of actin. Chen et al. [47] [47] demonstrated that the ability of sorafenib in inducing human prostate cancer cell line PC-3 apoptosis, through cytoskeleton destabilization, increased if combined with zinc exposure, suggesting that zinc may sensitize prostate cancer cells to sorafenib treatment. D’Alessandro et al. [48] [48] demonstrated a synergistic effect on HCC cells migration using combined doses of sorafenib and/or vitamin K1 with insulin like growth factor I receptor (IGF1-R) antagonists enhancing the reduction and reorganization of F-actin, probably through the modulation of MAPK cascade.
2.2. Regorafenib
Regorafenib (BAY 73-4506, Stivarga®, Figure 3) is the fluorinated analogue of sorafenib. It is an orally active diphenylurea multikinase inhibitor that targets stromal (PDGFR-β, FGFR-1), angiogenic (VEGFR1-3, TIE-2), and oncogenic receptor tyrosine kinases (c-KIT, RET, and RAF-1) [49]. It is the first multi-targeting kinase inhibitor which was approved by FDA in 2012 for the treatment of mCRC patients in refractory to standard chemotherapy [50]. In addition, regorafenib treatment determined an important amelioration in progression-free survival (PFS) in comparison with placebo in patients with metastatic GISTs after standard treatments; thus, it has also received FDA-approval for this indication since 2013. Then, in 2017, FDA approved regorafenib as a therapy for patients with advanced HCC [51]. Regorafenib showed a significant amelioration of PFS and overall survival (OS) in comparison with placebo. Regorafenib has been described in phase II clinical trials in different tumors, including RCC, soft-tissue sarcoma (STS), second- and third-line treatments for medullary thyroid cancer. However, several post-marketing observational studies, after the treatment of mCRC patients showed extensive data of toxicities (CORRELATE, REBECCA, RECORA, Japanese post-marketing study) [52,53,54][52][53][54]. It was found that adverse reactions due to regorafenib frequently occurred in the initial stages of treatment, mostly in the first cycle [55]. Thus, in patients with mCRC during the first cycle of regorafenib, the use of a dose-escalation strategy treatment was suggested [56] [56]. This strategy was then supported by a multicenter, open-label, phase II study [57]. Recently, during the COVID-19 outbreak, regorafenib has been considered as a therapy for gastrointestinal cancer [42]. A drug-delivery system has been studied for regorafenib, too [40]. The pivotal RESORCE (NCT01774344) phase III trial studied regorafenib therapy in patients with HCC who were tolerant to sorafenib, but who had progressed during sorafenib treatment [58]. Regorafenib has been demonstrated to inhibit glioblastoma multiforme (GBM) growth through PSAT1-mediated autophagy arrest [59], and to have beneficial effect in Alzheimer’s disease (AD) and formation of dendritic spine in vitro and in vivo [60].
2.3. Linifanib
Linifanib (ABT-869, Abbott Laboratories, Abbott Park, IL, USA, Figure 3) is an orally available TKI which targets VEGFR and PDGFR with relevant specificity and low off-target inhibition. Linifanib can also inhibit FLT-3 [61]. It does not show significant activity against representative cytosolic tyrosine and serine/threonine kinases [62]. Linifanib is a colony-stimulating factor-1 receptor (CSF-1R) inhibitor through the inhibition of the phosphorylation of CSF-1R tyrosine kinase in transfected cells [63]. It is used as a therapy for non-small cell lung carcinoma (NSCLC), liver cancer, breast cancer, colorectal cancer [45]. Preclinical and early clinical trials showed interesting activity in various human neoplasms with a satisfactory profile of toxicity. Linifanib competes with ATP in the binding site domain of tyrosine kinase, thus it prevents downstream signaling [64]. Phase II trial studies show that linifanib is useful for the treatment of patients with advanced, refractory colorectal cancer that expresses k-Ras mutations [65]. In an open-label phase II trial linifanib showed interesting clinical activity, as monotherapy, in patients with advanced HCC [66]. Linifanib versus sorafenib was studied in terms of efficacy and tolerability. Linifanib and sorafenib showed similar OS in advanced HCC. Linifanib did not meet predefined superiority and non-inferiority OS boundaries; thus, the study did not reach the primary end point. Secondary end points, time to progression (TTP), and objective response rate (ORR), favored Linifanib; safety results favored sorafenib [67]. Although linifanib is currently examined in HCC clinical trials, it has not yet been studied in preclinical and clinical studies for gastric cancer. 5-Fluorouracil (5-FU) and cisplatin represent the first-line chemotherapy for patients with gastric cancer and the combined use with linifanib inhibits synergistically the viability of some gastric cancer cell lines and led to remarkable suppression of VEGF-induced angiogenesis in vitro and in vivo [68]. Linifanib has demonstrated to be also useful in the treatment of anaplastic thyroid cancer (ATC), that is considered the most aggressive form of thyroid cancer. The synergistic use of linifanib and irinotecan significantly increased the survival of ATC-affected mice. These observations have been made by using an orthotopic in vivo model that better recapitulates features of human tumors than the more simplistic subcutaneous xenograft models, suggesting a potential role of this co-treatment in ATC patient’s treatment [69]. Finally, linifanib was demonstrated to interfere with adipocyte browning. It suppresses STAT3 signaling pathway, thus leading to the enhancement of adipocyte browning and inhibition of adipogenesis. Linifanib’s blocking browning effect was demonstrated as the phosphorylation of STAT3 was reduced by linifanib and the STAT3 activator SD19, as well [27].
2.4. Tivozanib
Tivozanib (AV-951, KRN-951, FOTIVDA®, Figure 3), used as the hydrochloride monohydrate salt, is a bioavailable inhibitor of angiogenesis which targets VEGFR tyrosine kinases with high antitumor activity. It is a VEGF-TKI specific for VEGFR1–3, showing an inhibitor effect at nanomolar concentrations, with IC50 values of 30 nM, 6.5 nM, and 15 nM for VEGFR1, 2 and 3, respectively. The compound is unique in that it is highly specific for VEGFR1–3, and presents minimal residual effects on c-KIT and PDGFR-β [70]. It presents a long half-life, too [71] [71]. It has shown considerable efficacy for the treatment of advanced RCC over the past decade. In August 2017, tivozanib was approved by the EMEA as a first-line therapy for patients with advanced RCC and those who are VEGFR and mTOR pathway inhibitor-naïve following disease progression after previous therapy with cytokines for advanced RCC. Tivozanib was compared with sorafenib in a phase III trial for patients with metastatic RCC. Tivozanib improved PFS, but not OS, and showed a differentiated safety profile, in comparison with sorafenib, as initial targeted therapy for metastatic RCC [72]. Preclinical data and phase III trials of tivozanib in RCC, TIVO-1, and TIVO-3 have been recently summarized. Given the agent’s excellent tolerability profile it is appropriate for those patients with heavily pretreated disease that could exhibit clinical deterioration. Currently, the standard therapy is represented by nivolumab and ipilimumab, followed by cabozantinib. Tivozanib may represent a third-line treatment after failure of these agents [73]. The results of phase III TIVO-3 trial (American Society of Clinical Oncology Virtual Scientific Program, 2020) showed that tivozanib significantly improved PFS, compared with sorafenib, in patients with highly relapsed or refractory metastatic RCC [74,75,76,77][74][75][76][77]. Tivozanib activity has been also investigated in hepatocellular carcinoma in association with durvalumab [78] [78] and in recurrent, platinum-resistant ovarian cancer, fallopian tube cancer, and primary peritoneal cancer [79,80][79][80]. Tivozanib has been studied in phase I and II clinical trials as monotherapy and in combination with other drugs for the treatment of STS [81], glioblastoma [82], breast [83], and colorectal cancers, and other advanced gastrointestinal cancers [84,85][84][85].
2.5. Ripretinib
Ripretinib (DCC-2618, QINLOCKTM, Figure 3) is an oral inhibitor of tyrosine kinase that primarily inhibits KIT proto-oncogene receptor tyrosine kinase and platelet-derived growth factor receptor A (PDGFRA) kinase signaling. Ripretinib also inhibits other kinases, such as PDGFRB, TIE2, VEGFR2, and BRAF. It was designed for cancers and myeloproliferative neoplasms, especially GISTs. Ripretinib is a “switch-control” kinase inhibitor that forces the activation loop (or activation “switch”) into the inactive conformation. In preclinical cancer models it has shown efficacy, and preliminary clinical data show that Ripretinib inhibits a broad range of KIT mutants in patients with drug-resistant GISTs [86]. The INVICTUS study demonstrated the efficacy and safety of ripretinib as the fourth-line treatment versus placebo in patients with advanced GISTs [87]. In May 2020, ripretinib received approval from the US FDA for the treatment of patients with advanced GISTs who had received previous treatment with more than two kinase inhibitors [88]. Ripretinib is being evaluated in an ongoing phase III study (INTRIGUE) as a second-line therapy in comparison with sunitinib after progressing on imatinib [89]. Recently, ripretinib is being investigated in clinical trials for systemic mastocytosis (SM) [90], and has been also proposed for the treatment of STS [91].
2.6. Mechanisms of Inhibition of Diarylureas
The proposed inhibitory mechanisms of diarylureas depend on their structure. Garuti et al. [5] [5] reported the crystal structure of V600EB-RAF kinase domains in complex with sorafenib. The pyridyl ring is shown to occupy the ATP adenine-binding pocket and to interact with three amino acids residues. The trifluoromethyl phenyl, that is a lipophilic moiety, fits into a hydrophobic pocket. The urea moiety forms two hydrogen bonds with V600EB-RAF, one with the aspartate, and one with the glutamate residue. Recently, the 2D interaction of the co-crystallized sorafenib inside the active site of B-Raf has been reported [92]. Regorafenib differs from sorafenib only for a fluorine atom, thus its interactions are similar to those of sorafenib. Chen et al. (2017) proposed an alternative mechanism for colorectal cancer for regorafenib. It seems that it interacts with microRNA-21 (miR-21), an oncogenic miRNA which plays a crucial role in resisting programmed cell death in CRC cells. RNA–ligand docking, molecular dynamics simulation showed that regorafenib can directly bind to miR-21 pre-element [93]. Docking studies of linifanib with FLT3 were recently reported, evidencing that 3-amino-indazole interacts with the ATP-bind site [61]. Kajal et al. (2018) has reported the 2D co-crystal-binding conformation of VEGFR2-Tivozanib, in which tivozanib mimics the binding pattern of ATP [94]. Finally, studies on the mechanism of action of ripretinib have been recently reported [86].
References
- Ghosh, A.K.; Brindisi, M. Urea derivatives in modern drug discovery and medicinal chemistry. J. Med. Chem. 2019, 63, 2751–2788.
- López-Muñoz, F.; Ucha-Udabe, R.; Alamo, C. The history of barbiturates a century after their clinical introduction. Neuro-psychiatr. Dis. Treat. 2005, 1, 329–343.
- Jagtap, A.D.; Kondekar, N.B.; Sadani, AA.; Chern, J.W. Ureas: Applications in Drug Design. Curr. Med. Chem. 2017, 24, 622–651.
- Asif, M. Short Notes on Diaryl Ureas Derivatives. J. Adv. Res. BioChem. Pharmacol. 2018, 1, 38–41.
- Garuti, L.; Roberti, M.; Bottegoni, G.; Ferraro, M. Diaryl urea: A privileged structure in anticancer agents. Curr. Med. Chem. 2016, 23, 1528–1548.
- Ceramella, J.; Mariconda, A.; Rosano, C.; Iacopetta, D.; Caruso, A.; Longo, P.; Sinicropi, M.S.; Saturnino, C. α–ω Alkenyl-bis-S-guanidine thiourea dihydrobromide affects HeLa cell growth hampering tubulin polymerization. ChemMedChem 2020, doi:10.1002/cmdc.202000544.
- Wu, Y.C.; Ren, X.Y.; Rao, G.W. Research Progress of Diphenyl Urea Derivatives as Anticancer Agents and Synthetic Meth-odologies. Mini Rev. Org. Chem. 2019, 16, 617–630.
- Rizza, P.; Pellegrino, M.; Caruso, A.; Iacopetta, D.; Sinicropi, M.S.; Rault, S.; Lancelot, J.C.; El-Kashef, H.; Lesnard, A.; Ro-chais, C.; et al. 3-(Dipropylamino)-5-hydroxybenzofuro [2, 3-f] quinazolin-1 (2H)-one (DPA-HBFQ-1) plays an inhibitory role on breast cancer cell growth and progression. Eur. J. Med. Chem. 2016, 107, 275–287.
- Saturnino, C.; Barone, I.; Iacopetta, D.; Mariconda, A.; Sinicropi, M.S.; Rosano, C.; Campana, A.; Catalano, S.; Longo, P.; Andò, S. N-heterocyclic carbene complexes of silver and gold as novel tools against breast cancer progression. Fut. Med. Chem. 2016, 8, 2213–2229.
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Ceramella, J.; Miuà, N.; Saturnino, C.; Puo-ci, F.; et al. New insights for the use of quercetin analogs in cancer treatment. Fut. Med. Chem. 2017, 9, 2011–2028.
- Kapuriya, N.; Kakadiya, R.; Dong, H.; Kumar, A.; Lee, P.-C.; Zhang, X.; Chou, T.-C.; Lee, T.-C.; Chen, C.-H.; Lam, K.; et al. Design, synthesis, and biological evaluation of novel water-soluble N-mustards as potential anticancer agents. Bioorg. Med. Chem. 2011, 19, 471–485.
- El-Nassan, H.B. Recent progress in the identification of BRAF inhibitors as anticancer agents. Eur. J. Med. Chem. 2014, 72, 170–205.
- Ravez, S.; Barczyk, A.; Six, P.; Cagnon, A.; Garofalo, A.; Goossens, L.; Depreux, P. Inhibition of tumor cell growth and angi-ogenesis by 7-aminoalkoxy-4-aryloxy-quinazoline ureas, a novel series of multi-tyrosine kinase inhibitors. Eur. J. Med. Chem. 2014, 79, 360–381.
- Wang, C.; Gao, H.; Dong, J.; Zhang, Y.; Su, P.; Shi, Y.; Zhang, J. Biphenyl derivatives incorporating urea unit as novel VEGFR-2 inhibitors: Design, synthesis and biological evaluation. Bioorg. Med. Chem. 2014, 22, 277–284.
- Lu, Y.Y.; Zhao, C.R.; Wang, R.Q.; Li, W.-B.; Qu,X.-J. A novel anticancer diarylurea derivative HL-40 as a multi-kinases inhib-itor with good pharmacokinetics in Wistar rats. Biomed. Pharmacother. 2015, 69, 255–259.
- Curtin, M.L.; Frey, R.R.; Heyman, H.R.; Soni, N.B.; Marcotte, P.A.; Pease, L.J.; Glaser, K.B.; Magoc, T.J.; Tapang, P.; Albert, D.H.; et al. Thienopyridine ureas as dual inhibitors of the VEGF and Aurora kinase families. Bioorg. Med. Chem. Lett. 2012, 22, 3208–3212.
- Ceramella, J.; Iacopetta, D.; Barbarossa, A.; Caruso, A.; Fedora, G.; Bonomo, M.G.; Mariconda, A.; Longo, P.; Saturnino, C.; Sinicropi, M.S. Carbazole derivatives as kinase-targeting inhibitors for cancer treatment. Mini Rev. Med. Chem. 2020, 20, 444–465.
- Tella, S.H.; Kommalapati, A.; Mahipal, A. Systemic therapy for advanced hepatocellular carcinoma: Targeted therapies. Chin. Clin. Oncol. 2020, doi:10.21037/cco-20-117.
- Escudier, B.; Worden, F.; Kudo, M. Sorafenib: Key lessons from over 10 years of experience. Exp. Rev. Anticanc. Ther. 2019, 19, 177–189.
- Mazzocca, A.; Napolitano, A.; Silletta, M.; Spalato Ceruso, M.; Santini, D.; Tonini, G.; Vincenzi, B. New frontiers in the med-ical management of gastrointestinal stromal tumours. Ther. Adv. Med. Oncol. 2019, 11, doi:10.1177/1758835919841946.
- Strumberg, D.; Scheulen, M.E.; Schultheis, B.; Richly, H.; Frost, A.; Büchert, M.; Christensen, O.; Jeffers, M.; Heinig, R.; Boix, O.; et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: A phase I study. Br. J. Canc. 2012, 106, 1722–1727.
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Nagai, H.; Mukozu, T.; Ogino, Y.U.; Matsui, D.; Matsui, T.; Wakui, N.; Momiyama, K.; Igarashi, Y.; Sumino, Y.; Higai, K. So-rafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor throm-bus. Anticancer Res. 2015, 35, 2269–2277.
- Sirohi, B.; Philip, D.S.; Shrikhande, S.V. Regorafenib in gastrointestinal stromal tumors. Future Oncol. 2014, 10, 1581–1587.
- Scrivano, L.; Parisi, O.I.; Iacopetta, D.; Ruffo, M.; Ceramella, J.; Sinicropi, M.S.; Puoci, F. Molecularly imprinted hydrogels for sustained release of sunitinib in breast cancer therapy. Pol. Adv. Technol. 2019, 30, 743–748.
- Mossenta, M.; Busato, D.; Baboci, L.; Di Cintio, F.; Toffoli, G.; Dal Bo, M. New insight into therapies targeting angiogenesis in hepatocellular carcinoma. Cancers 2019, 11, 1086, doi:10.3390/cancers11081086.
- Zhao, S.; Chu, Y.; Zhang, Y.; Zhou, Y.; Jiang, Z.; Wang, Z.; Mao, L.; Li, K.; Sun, W.; Li, P.; et al. Linifanib exerts dual an-ti-obesity effect by regulating adipocyte browning and formation. Life Sci. 2019, 222, 117–124.
- Jacob, A.; Shook, J.; Hutson, T.E. Tivozanib, a highly potent and selective inhibitor of VEGF receptor tyrosine kinases, for the treatment of metastatic renal cell carcinoma. Future Oncol. 2020, 16, 2147–2164.
- Bournez, C.; Carles, F.; Peyrat, G.; Aci-Sèche, S.; Bourg, S.; Meyer, C.; Bonnet, P. Comparative assessment of protein kinase inhibitors in public databases and in PKIDB. Molecules 2020, 25, 3226.
- Nemunaitis, J.; Bauer, S.; Blay, J.Y.; Choucair, K.; Gelderblom, H.; George, S.; Schöffski, P.; von Mehren, M.; Zalcberg, J.; Achour, H.; et al. Intrigue: Phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Future Oncol. 2020, 16, 4251–4264.
- Iyer, R.; Fetterly, G.; Lugade, A.; Thanavala, Y. Sorafenib: A clinical and pharmacologic review. Exp. Opin. Pharmacother. 2010, 11, 1943–1955.
- Raoul, J.L.; Kudo, M.; Finn, R.S.; Edeline, J.; Reig, M.; Galle, P.R. Systemic therapy for intermediate and advanced hepatocel-lular carcinoma: Sorafenib and beyond. Canc. Treat. Rev. 2018, 68, 16–24.
- Qiu, X.; Li, M.; Wu, L.; Xin, Y.; Mu, S.; Li, T.; Song, K. Severe Fatigue is an Important Factor in the Prognosis of Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib. Cancer Manag. Res. 2020, 12, 7983.
- Rich, N.E.; Yopp, A.C.; Singal, A.G. Medical Management of Hepatocellular Carcinoma. J. Oncol. Pract. 2017, 13, 356–364.
- Yan, Y.; Wang, L.; He, J.; Liu, P.; Lv. X.; Zhang. Y.; Xu, X.; Zhang, L.; Zhang, Y. Synergy with interferon-lambda 3 and soraf-enib suppresses hepatocellular carcinoma proliferation. Biomed. Pharmacother. 2017, 88, 395–402.
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.; Ducreux, M.; Zhu, A.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; et al. LBA3IMbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann. Oncol. 2019, 30, ix186–ix187.
- Pinato, D.J.; Kaneko, T.; Saeed, A.; Pressiani, T.; Kaseb, A.; Wang, Y.; Szafron, D.; Jun, T.; Dharmapuri, S., Naqash, A.R.; et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: Adjunctive role of the ALBI grade. Cancers 2020, 12, 1862.
- Zhang, N.; Zhang, B.; Gong, X.; Wang, T.; Liu, Y.; Yang, S. In vivo biodistribution, biocompatibility, and efficacy of soraf-enib-loaded lipid-based nanosuspensions evaluated experimentally in cancer. Int. J. Nanomed. 2016, 11, 2329–2343.
- Guo, Y.; Zhong, T.; Duan, X.-C.; Zhang, S.; Yao, X.; Yin, Y.-F.; Huang, D.; Ren, W.; Zhang, Q.; Zhang, X. Improving an-ti-tumor activity of sorafenib tosylate by lipid- and polymer-coated nanomatrix. Drug Deliv. 2017, 24, 270–277.
- Srimathi, U.; Nagarajan, V.; Chandiramouli, R. Investigation on graphdiyne nanosheet in adsorption of sorafenib and regorafenib drugs: A DFT approach. J. Mol. Liq. 2019, 277, 776–785.
- Catalano, A. COVID-19: Could Irisin Become the Handyman Myokine of the 21st Century? Coronaviruses 2020, 1, 32–41.
- Pietrantonio, F.; Morano, F.; Niger, M.; Corallo, S.; Antista, M.; Raimondi, A.; Prisciandaro, M.; Pagani, F.; Prinzi, N.; Ni-chetti, F.; et al. Systemic treatment of patients with gastrointestinal cancers during the COVID-19 outbreak: COVID-19-adapted recommendations of the national cancer institute of Milan. Clin. Colorect. Canc. 2020, 19, 156–164.
- Granito, A.; Marinelli, S.; Negrini, G.; Menetti, S.; Benevento, F.; Bolondi, L. Prognostic significance of adverse events in pa-tients with hepatocellular carcinoma treated with sorafenib. Ther. Adv. Gastroenterol. 2016, 9, 240–249.
- Tovoli, F.; Ielasi, L.; Casadei-Gardini, A.; Granito, A.; Foschi, F.G.; Rovesti, G.; Negrini, G.; Orsi, G.; Renzulli, M.; Piscaglia, F. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J. Hepatol. 2019, 71, 1175–1183.
- Borriello, A.; Caldarelli, I.; Bencivenga, D.; Stampone, E.; Perrotta, S.; Oliva, A.; Della Ragione, F. Tyrosine kinase inhibitors and mesenchymal stromal cells: Effects on self-renewal, commitment and functions. Oncotarget 2017, 8, 5540, doi:10.18632/oncotarget.12649.
- Wang, Z.; Wang, M.; Carr, B.I. Involvement of receptor tyrosine phosphatase DEP‐1 mediated PI3K‐cofilin signaling path-way in Sorafenib‐induced cytoskeletal rearrangement in hepatoma cells. J. Cell. Physiol. 2010, 224, 559–565.
- Chen, X.; Che, X.; Wang, J.; Chen, F.; Wang, X.; Zhang, Z.; Fan, B.; Yang, D.; Song, X. Zinc sensitizes prostate cancer cells to sorafenib and regulates the expression of Livin. Acta Biochim Biophys Sin. 2013, 45, 353–358.
- D’Alessandro, R.; Refolo, M.G.; Lippolis, C.; Carella, N.; Messa, C.; Cavallini, A.; Carr, B.I. Strong enhancement by IGF1-R antagonists of hepatocellular carcinoma cell migration inhibition by Sorafenib and/or vitamin K1. Cell. Oncol. 2018, 41, 283–296.
- Ettrich, T.J.; Seufferlein, T. Regorafenib. In Small Molecules in Oncology; Springer: Cham, Springer, Berlin, Heidel-berg,Switzerland, 2018; pp. 45–56.
- Arai, H.; Battaglin, F.; Wang, J.; Lo, J.H.; Soni, S.; Zhang, W.; Lenz, H.J. Molecular insight of regorafenib treatment for colo-rectal cancer. Canc. Treat. Rev. 2019, 81, 101912.
- Pelosof, L.; Lemery, S.; Casak, S.; Jiang, X.; Rodriguez, L.; Pierre, V.; Bi, Y.; Liu, J.; Zirkelbach, J.F.; Patel, A.; et al. Benefit‐risk summary of regorafenib for the treatment of patients with advanced hepatocellular carcinoma that has progressed on so-rafenib. Oncologist 2018, 23, 496–500.
- O’Connor, J.M.; Öhler, L.; Scheithauer, W.; Metges, J.P.; Dourthe, L.M.; de Groot, J.W.; Thaler, J.; Yeh, K.H.; Lin, J.K.; Falcone, A.; et al. Real-world dosing of regorafenib in metastatic colorectal cancer (mCRC): Interim analysis from the prospective, observational CORRELATE study. Liver 2017, 260, 52.
- Komatsu, Y.; Muro, K.; Yamaguchi, K.; Satoh, T.; Uetake, H.; Yoshino, T.; Nishida, T.; Takikawa, H.; Kato, T.; Chosa, M.; et al. Safety and efficacy of regorafenib post-marketing surveillance (PMS) in Japanese patients with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2017, 35, 721. DOI: 10.1200/JCO.2017.35.4_suppl.721
- Adenis, A.; de la Fouchardiere, C.; Paule, B.; Burtin, P.; Tougeron, D.; Wallet, J.; Dourthe, L.M.; Etienne, P.L.; Mineur, L.; Cli-sant, S.; et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: Results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016, 16, 1–8.
- Grothey, A.; George, S.; Van Cutsem, E.; Blay, J.Y.; Sobrero, A.; Demetri, G.D. Optimizing treatment outcomes with regorafenib: Personalized dosing and other strategies to support patient care. Oncologist 2014, 19, 669–680.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer. Version 4.2018 National Comprehen-sive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 12 November 2020).
- Bekaii-Saab, T.S.; Ou, F.S.; Ahn, D.H.; et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): A randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019, 20, 1070–1082.
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66.
- Jiang, J.; Zhang, L.; Chen, H.; Lei, Y.; Zhang, T.; Wang, Y.; Jin, P.; Lan, J.; Zhou, L.; Huang, Z.; et al. Regorafenib induces le-thal autophagy arrest by stabilizing PSAT1 in glioblastoma. Autophagy 2020, 16, 106–122.
- Han, K.M.; Kang, R.J.; Jeon, H.; Lee, H.; Lee, J.S.; Park, H.H.; Jeon, S.G.; Suk, K.; Seo, J.; Hoe, H.S. Regorafenib regulates AD pathology, neuroinflammation, and dendritic spinogenesis in cells and a mouse model of AD. Cells 2020, 9, 1655, doi:10.3390/cells9071655.
- Shi, Z.H.; Liu, F.T.; Tian, H.Z.; Zhang, Y.M.; Li, N.G.; LU, T. Design, synthesis and structure-activity relationship of di-aryl-ureas with novel isoxazol [3,4-b]pyridine-3-amino-structure as multi-target inhibitors against receptor tyrosine kinase. Bioorg. Med. Chem. 2018, 26, 4735–4744.
- Zhou, J.; Goh, B.C.; Albert, D.H.; Chen, C.S. ABT-869, a promising multi-targeted tyrosine kinase inhibitor: From bench to bedside. J. Hematol. Oncol. 2009, 2, 33, doi:10.1186/1756-8722-2-33.
- Guo, J.; Marcotte, P.A.; McCall, J.O.; Dai, Y.; Pease, L.J.; Michaelides, M.R.; Davidsen, S.K.; Glaser, K.B. Inhibition of phos-phorylation of the colonystimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other ty-rosine kinase inhibitors. Mol. Cancer Ther. 2006, 5, 1007–1013.
- Aversa, C.; Leone, F.; Zucchini, G.; Serini, G.; Geuna, E.; Milani, A.; Valdembri, D.; Martinello, R.; Montemurro, F. Linifanib: Current status and future potential in cancer therapy. Exp. Rev. Anticancer Ther. 2015, 15, 677–687.
- Lin, W.H.; Hsu, J.T.A.; Hsieh, S.Y.; Chen, C.T.; Song, J.S.; Yen, S.C.; Hsu, T.; Chen, C.H.; Chou, L.H.; Yang, Y.N.; et al. Discov-ery of 3-phenyl-1H-5-pyrazolylamine derivatives containing a urea pharmacophore as potent and efficacious inhibitors of FMS-like tyrosine kinase-3 (FLT3). Bioorg. Med. Chem. 2013, 21, 2856–2867.
- Toh, H.C.; Chen, P.J.; Carr, B.I.; Knox, J.J.; Gill, S.; Ansell, P.; McKeegan, E.M.; Dowell, B.; Pedersen, M.; Qin, Q.; et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 2013, 119, 380–387.
- Cainap, C.; Qin, S.; Huang, W.T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.K.; Chen, P.J.; Toh, H.C.; et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015, 33, 172–180.
- Chen, J.; Guo, J.; Chen, Z.; Wang, J.; Liu, M.; Pang, X. Linifanib (ABT-869) potentiates the efficacy of chemotherapeutic agents through the suppression of receptor tyrosine kinase-mediated akt/mtor signaling pathways in gastric cancer. Sci. Rep. 2016, 6, 1–11.
- Banchi, M.; Orlandi, P.; Gentile, D. Synergistic activity of linifanib and irinotecan increases the survival of mice bearing or-thotopically implanted human anaplastic thyroid cancer. Am. J. Cancer Res. 2020, 10, 2120–2127.
- Nakamura, K.; Taguchi, E.; Miura, T.; Yamamoto, A.; Takahashi, K.; Bichat, F.; Guilbaud, N.; Hasegawa, K.; Kubo, K.; Fujiwara, Y.; et al. KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res. 2006, 66, 9134–9142.
- Momeny, M.; Moghaddaskho, F.; Gortany, N.K.; Yousefi, H.; Sabourinejad, Z.; Zarrinrad, G.; Mirshahvaladi, S.; Eyvani, H.; Barghi, F.; Ahmadinia, L. Blockade of vascular endothelial growth factor receptors by tivozanib has potential anti-tumour effects on human glioblastoma cells. Sci. Rep. 2017, 7, 44075, doi:10.1038/srep44075.
- Motzer, R.J.; Nosov, D.; Eisen, T.; Bondarenko, I.; Lesovoy, V.; Lipatov, O.; Tomczak, P.; Lyulko, O.; Alyasova, A.; Harza, M.; et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. J. Clin. Oncol. 2013, 31, 3791.
- Salgia, N.J.; Zengin, Z.B.; Pal, S.K. Tivozanib in renal cell carcinoma: A new approach to previously treated disease. Ther. Adv. Med. Oncol. 2020, 12, doi:10.1177/1758835920923818.
- Wright, K.M. Final data analysis supports tivozanib as superior treatment for patients with RCC. Oncology (Williston Park, NY) 2020, 34, 257.
- Rini, B.I.; Pal, S.K.; Escudier, B.J.; Atkins, M.B.; Hutson, T.E.; Porta, C.; Verzoni, E.; Needle, M.N.; McDermott, D.F. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): A phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020, 21, 95–104.
- Pal, S.K.; Escudier, B.J.; Atkins, M.B. Final overall survival results from a phase 3 study to compare tivozanib to sorafenib as third-or fourth-line therapy in subjects with metastatic renal cell carcinoma. Eur. Urol. 2020, 78, 783–785.
- Westerman, M.E.; Wood, C.G. Editorial Commentary: Tivozanib versus sorafenib in patients with advanced renal cell car-cinoma (TIVO-3): A phase 3, multicentre, randomised, controlled, open-label study. Ann. Transl. Med. 2020, 8, 1037.
- Iyer, R.V.; Li, D.; Dayyani, F.; Needle, M.N.; Abrams, T.A. A phase Ib/II, open-label study of tivozanib in combination with durvalumab in subjects with untreated advanced hepatocellular carcinoma. J. Clin. Oncol. 2020, 38 (Suppl. 16599), doi:10.1200/jco.2020.38.15_suppl.e16599.
- Swetzig, W.M.; Lurain, J.R.; Berry, E.; Pineda, M.J.; Shahabi, S.; Perry, L.; Neubauer, N.L.; Nieves-Neira, W.; Schink, J.C.; Schiller, A.; et al. Efficacy and safety of tivozanib in recurrent, platinum resistant ovarian, fallopian tube or primary peri-toneal cancer. J. Clin. Oncol. 2019, 37 (Suppl. 5538).
- Momeny, M.; Sabourinejad, Z.; Zuzzinrad, G.; Maghaddaskho, F.; Eyvani, H.; Yousefi, H.; Mirshahvaladi, S.; Poursani, E.M.; Barghi, F.; Poursheikhani, A.; et al. Anti-tumour activity of tivozanib, a pan-inhibitor of VEGF receptors, in thera-py-resistant ovarian carcinoma cells. Sci. Rep. 2017, 7, 1–13.
- Martin-Liberal, J.; Pérez, E.; García Del Muro, X. Investigational therapies in phase II clinical trials for the treatment of soft tissue sarcoma. Exp. Opin. Invest. Drugs 2019, 28, 39–50.
- Kalpathy-Cramer, J.; Chandra, V.; Da, X.; Ou, Y.; Emblem, K.E.; Muzikansky, A.; Cai, X.; Douw, L.; Evans, J.G.; Dietrich, J.; et al. Phase II study of tivozanib, an oral VEGFR inhibitor, in patients with recurrent glioblastoma. J. Neurooncol. 2017, 131, 603–610.
- Jamil, M.O.; Hathaway, A.; Mehta, A. Tivozanib: Status of development. Curr. Oncol. Rep. 2015, 17, 24.
- Oldenhuis, C.N.; Loos, W.J.; Esteves, B.; van Doorn, L.; Cotreau, M.M.; Strahs, A.L.; den Hollander, M.W.; Gietema, J.A.; de Vries, E.G.E.; Eskens, F.A.L.M. A phase Ib study of the VEGF receptor tyrosine kinase inhibitor tivozanib and modified FOLFOX-6 in patients with advanced gastrointestinal malignancies. Clin. Colorectal Cancer 2015, 14, 18–24.e1.
- Benson, A.B.; Kiss, I.; Bridgewater, J.; Eskens, F.A.; Sasse, C.; Vossen, S.; Chen, J.; Van Sant, C.; Ball, H.A.; Keating, A.; et al. BATON-CRC: A phase II randomized trial comparing tivozanib plus mFOLFOX6 with bevacizumab plus mFOLFOX6 in stage IV metastatic colorectal cancer. Clin. Cancer Res. 2016, 22, 5058–5067.
- Smith, B.D.; Kaufman, M.D.; Lu, W.P.; et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell 2019, 35, 738–751.
- Blay, J.Y.; Serrano, C.; Heinrich, M.C.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, e415, doi:10.1016/s1470-2045(20)30168-6.
- US Food and Drug Administration. QINLOCK Prescribing Information. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213973s000lbl.pdf (accessed on 12 November 2020).
- Khoshnood, A. Gastrointestinal stromal tumor—A review of clinical studies. J. Oncol. Pharm. Pract. 2019, 25, 1473–1485.
- Jawhar, M.; Gotlib, J.; Reiter, A. Tyrosine kinase inhibitors in systemic mastocytosis. In Mastocytosis; Springer: Cham, Swit-zerland, 2020; pp. 257–265.
- Haddox, C.L.; Riedel, R.F. Individualizing systemic therapy for advanced soft tissue sarcomas based on tumor histology and biology. Exp. Rev. Anticancer Ther. 2020, 20, 5–8.
- Thabit, M.G.; Mostafa, A.S.; Selim, K.B.; et al. Design, synthesis and molecular modeling of phenyl dihydropyridazinone derivatives as B-Raf inhibitors with anticancer activity. Bioorg. Chem. 2020, 103, 104148, doi:10.1016/j.bioorg.2020.104148.
- Chen, X.; Xie, B.; Cao, L.; et al. Direct binding of microRNA-21 pre-element with Regorafenib: An alternative mechanism for anti-colorectal cancer chemotherapy? J. Mol. Graph. Model. 2017, 73, 48–53.
- Kajal, K.; Panda, A.K.; Bhat, J.; et al. Andrographolide binds to ATP-binding pocket of VEGFR2 to impede VEGFA-mediated tumor-angiogenesis. Sci. Rep. 2019, 9, 1–10.
