Long non-coding (lnc)RNAs have emerged as critical regulators of gene expression and are involved in almost every cellular process. They can bind to other molecules including DNA, proteins, or even other RNA types such messenger RNA or small RNAs. LncRNAs are typically expressed at much lower levels than mRNA, and their expression is often restricted to tissue- or time-specific developmental stages. They are also involved in several inter-species interactions, including vector–host–pathogen interactions, where they can be either vector/host-derived or encoded by pathogens. In these interactions, they function via multiple mechanisms including regulating pathogen growth and replication or via cell-autonomous antimicrobial defense mechanisms. Recent advances suggest that characterizing lncRNAs and their targets in different species may hold the key to understanding the role of this class of non-coding RNA in interspecies crosstalk.
- ncRNA
- lncRNA
1. Introduction
LncRNAs are therefore a diverse group of regulatory ncRNAs with various characteristics, localizations, and modes of action[1]. A comprehensive classification of lncRNAs is quite challenging, as most do not share structural, functional, or mechanistic features. Moreover, only a few lncRNAs have been thoroughly mechanistically characterized to date, with even fewer being functionally verified in vivo[2]. Overall, their functions depend on their subcellular localization, i.e., in the cytoplasm or nucleus. Here we describe the five archetypal molecular functions of lncRNAs: as signals, decoys, guides, scaffolds, and enhancers (
Figure 1).
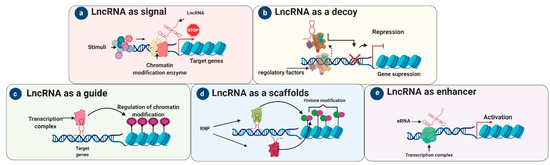

Figure 13.
a
b
c
d
e
2. LncRNAs as Signals
Figure 1) are known to interact with chromatin-modifying enzymes such as histone methyltransferases to silence their target genes by blocking their transcription or through heterochromatin formation [3].
3. LncRNA as Decoys
“Decoy” lncRNAs can act as molecular sinks by decreasing the accessibility of particular regulatory factors via decoy binding sites[3]. These lncRNAs repress transcription, in an indirect way, by sequestering regulatory factors (
Figure 1) including miRNAs, modifying complexes (chromatin subunits), catalytic proteins, and transcription factors, reducing their availability [3][4]. LncRNA decoys negatively modulated transcription by not allowing a particular effector to interact with its intrinsic target [3]. For example, the decoy lncRNA PANDA regulates apoptosis [3]: NF-YA is a transcription factor responsible for the activation of apoptosis-related genes, but when PANDA binds to NF-YA it is sequestered away from its target genes, resulting in the suppression of apoptosis-related genes[5][6]. Other decoy lncRNAs like
TUG1
MEG3 indirectly degrade and alter protein translation by isolating miRNA from mRNA and protein targets[3][7][8]. In addition, many other lncRNAs, such as highly up-regulated in liver cancer (
HULC
GAS5
HOTAIR
PTENP1
MALAT1,
Figure 1).
4. LncRNA as Guides
To regulate the genome, guide lncRNAs are necessary for the organization/localization of factors at specific genomic loci. As such, lncRNA guides direct chromatin modifiers and protein complexes (such as RNPs) or transcription factors to particular target gene(s) and help them localize appropriately at their transcriptional loci [9]. The exact targets of guide lncRNAs (
Figure 1) are stimulated by RNA–RNA, RNA–protein, and RNA–DNA interactions. However, the exact details of this mechanism are uncertain [10]. LncRNAs such as
HOTAIR
FIRRE
COLDAIR
KCNQ1
KCNQ1OT1
TUG1
HOTTIP
XIST
MEG3
ANRIL can act as guides by reprograming chromatin complexes and managing the recruitment of epigenetic modifiers to their definitive loci[11]. For instance,
KCNQ1OT1
HOTAIR bind to PRC2, a chromatin modifier, to regulate target genes in cis or trans and thereby inhibit gene expression [12][13][14].
5. LncRNA as Scaffolds
Scaffold lncRNAs play an important structural role in assembling multi-protein complexes such as short-lived ribonucleoprotein (RNP) complexes[15]. After the complete assembly of RNP complexes, they can suppress or activate transcription depending on the existence and nature of the involved RNAs and proteins[15]. For example, the well-studied lncRNA
TERC is an excellent example of a molecular scaffold, in which the telomerase complex combines associated proteins with reverse transcriptase action in a single RNP[16].
TERC
Figure 1). As substitutes, lncRNAs might have low-affinity interactions with protein-like mRNAs as they mature. In addition, lncRNAs like
MALAT1
ANRIL
TUG1 can serve as dynamic scaffolds by associating with PRC1 and PRC2 to stimulate target gene suppression or activation[4][17].
6. LncRNA as Enhancers
In the signal archetype, the lncRNA sends a molecular signal to initiate regulation at the transcriptional level in response to different stimuli [8][18]. For instance,
KCNQ1OT1 recruits PRC2 and chromatin-modifying enzymes (histone methyltransferases) by acting as a signal lncRNA [19]. In the enhancer archetype (
Figure 1), the “chromatin interaction” of DNA is influenced by the enhancer regions (ERs) as a result of enhancer RNAs (eRNAs). It is thought that these lncRNAs are not released from ERs but tether proteins to ERs [20].
