Accurate staging of prostate cancer (PCa) at initial diagnosis and at biochemical recurrence is important to determine prognosis and the optimal treatment strategy. To date, treatment of metastatic PCa has mostly been based on the results of conventional imaging with abdominopelvic computed tomography (CT) and bone scintigraphy. However, these investigations have limited sensitivity and specificity which impairs their ability to accurately identify and quantify the true extent of active disease. Modern imaging modalities, such as those based on the detection of radioactively labeled tracers with combined positron emission tomography/computed tomography (PET/CT) scanning have been developed specifically for the detection of PCa. Novel radiotracers include
18
F-sodium fluoride (NaF),
11
C-/
18
F-fluorocholine (FCH),
18
F-fluordihydrotestosterone (FDHT),
68
Gallium and
18
F-radiolabeled prostate-specific membrane antigen (e.g.,
68
Ga-PSMA-11,
18
F-DCFPyL). PET/CT with these tracers outperforms conventional imaging. As a result of this, although their impact on outcome needs to be better defined in appropriate clinical trials, techniques like prostate-specific membrane antigen (PSMA) PET/CT have been rapidly adopted into clinical practice for (re)staging PCa.
- rostate cancer
- bone metastases
- bone scintigraphy
- conventional imaging
- PET/CT
1. Introduction
Prostate cancer (PCa) is the second-most commonly diagnosed cancer in men worldwide, and has the highest incidence of all cancers among men in the Western world. In 2018, there were an estimated 1.3 million new cases and 359,000 deaths from PCa globally [1][2][3]. The behavior of PCa varies widely, from indolent to highly aggressive. In routine practice, initial clinical suspicion of PCa is usually triggered by an elevated prostate-specific antigen (PSA) and/or an abnormal digital rectal examination (DRE). For a definitive diagnosis, histopathological confirmation is required, and typically obtained by transrectal ultrasound (TRUS) guided needle biopsies [4]. PCa has been classified into five prognostically distinct Grade Groups (GGs) by the International Society of Urological Pathology (ISUP), based on the Gleason Score (GS) [5].
Prostate cancer (PCa) is the second-most commonly diagnosed cancer in men worldwide, and has the highest incidence of all cancers among men in the Western world. In 2018, there were an estimated 1.3 million new cases and 359,000 deaths from PCa globally [1,2,3]. The behavior of PCa varies widely, from indolent to highly aggressive. In routine practice, initial clinical suspicion of PCa is usually triggered by an elevated prostate-specific antigen (PSA) and/or an abnormal digital rectal examination (DRE). For a definitive diagnosis, histopathological confirmation is required, and typically obtained by transrectal ultrasound (TRUS) guided needle biopsies [4]. PCa has been classified into five prognostically distinct Grade Groups (GGs) by the International Society of Urological Pathology (ISUP), based on the Gleason Score (GS) [5].
The European Association of Urology (EAU) risk classification [4] (based on the D’Amico classification including initial PSA-value, clinical T-stage and biopsy GG [6]) is commonly used as a prognostic parameter to predict the risk of recurrence, dividing patients into three categories (low, intermediate and high-risk). Patients with high-risk, locally-advanced PCa have an increased risk for the development of metastases, and disease recurrence [4]. The most frequent sites of distant metastases are lymph nodes outside the pelvis (M1a) and bone (M1b) with occasional metastases elsewhere (e.g., visceral organs) (M1c).
Accurate staging of PCa, both at initial diagnosis and at biochemical recurrence (BCR) after previous curative-intent therapy, is important to determine prognosis, and for selecting the optimal treatment strategy. According to the current EAU guidelines, metastatic screening by means of “at least” an abdominopelvic computed tomography (CT)-scan and bone scintigraphy (BS) (
99mTc-phosphonate), is recommended in patients with intermediate or high-risk PCa to evaluate the extent of extra-prostatic disease [4]. However, these conventional imaging modalities have limited sensitivity and specificity, affecting their ability to accurately quantify the true extent of disease, especially at low PSA-levels or in the setting of limited volume, oligometastatic disease. This has led to an ongoing search for better imaging tests. As a result of this, prostate-specific membrane antigen (PSMA) positron emission tomography (PET/CT), has been recently introduced. This modern imaging technique using a novel radiotracer has shown high-levels of diagnostic accuracy in the detection of metastatic disease [7][8][9][10], and outperforms conventional imaging in primary staging of PCa [8].
Tc-phosphonate), is recommended in patients with intermediate or high-risk PCa to evaluate the extent of extra-prostatic disease [4]. However, these conventional imaging modalities have limited sensitivity and specificity, affecting their ability to accurately quantify the true extent of disease, especially at low PSA-levels or in the setting of limited volume, oligometastatic disease. This has led to an ongoing search for better imaging tests. As a result of this, prostate-specific membrane antigen (PSMA) positron emission tomography (PET/CT), has been recently introduced. This modern imaging technique using a novel radiotracer has shown high-levels of diagnostic accuracy in the detection of metastatic disease [7,8,9,10], and outperforms conventional imaging in primary staging of PCa [8].
2. Modern Nuclear Imaging in Prostate Cancer: New PET/CT Radiotracers
The three studies described above were all based on the use of conventional imaging (i.e., BS, CT and/or MRI), upon which the current treatment of metastatic PCa is mainly based. However, PCa imaging is evolving rapidly. Over the last few years, modern PET/CT imaging techniques, using radioactively labelled tracers have been introduced to the diagnostic armamentarium. In clinical practice it is preferable to use PET radiopharmaceuticals with high tumor-specific uptake and low background activity, capable of diagnosing bone, lymph node, and visceral metastases. Various tracers have been developed for metastatic PCa, based on osteoblastic activity (
18
F-sodium fluoride (NaF)), cellular phospholipid membrane proliferation (
11
C-/
18
F-fluorocholine (FCH)), androgen receptor expression (
18
F-fluordihydrotestosterone (FDHT)) and targeting the prostate-specific membrane antigen (
68
Gallium (
68
Ga) or
18
Flourine (
18F)) [11] (
F)) [31] (
).
Table 1.
Diagnostic performance of selected imaging methods for the detection of prostate cancer bone metastases.
| Sensitivity (%) | Specificity (%) | Reference | Type of Article |
|---|
| Bone scintigraphy | 79 | 82 | Shen [12] | Meta-analysis |
| CT | 8.8 | 98 | ||
| 8.8 | ||||
| Gabriele | ||||
| 98 | ||||
| [ | 13 | ] | ||
| Gabriele [ | ||||
| Retrospective cohort | ||||
| CT | 23] | Retrospective cohort | ||
| 18F-NaF PET/CT | 98 | 90 | Sheikhbahaei [14] | Meta-analysis |
| F-NaF PET/CT | 98 | 90 | Sheikhbahaei [11] | Meta-analysis |
| 18F-FDHT PET/CT | 63 | - | Dehdashti [15] | Prospective cohort |
| F-FDHT PET/CT | 63 | - | Dehdashti [37] | Prospective cohort |
| 18 | ||||
| F-FCH PET/CT | 87 | 97 | Shen [12] | Meta-analysis |
| 68 | ||||
| Ga-PSMA PET/CT | 77 | 97 | Perera [9] | Systematic review and meta-analysis |
| 18 | ||||
| F-DCFPyL PET/CT | - | - | - | - |
CT: computed tomography; PET: positron emission tomography; 18F: 18Flourine; NaF: sodium fluoride; FDHT: fluorodihydrotestosterone; FCH: fluorocholine; 68Ga-PSMA: 68Gallium prostate-specific membrane antigen; DCFPyL: (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid).
The
18
F-NaF PET/CT enables accurate detection of osseous metastases, but is nonspecific for lymph node metastatic disease and is therefore not suitable for comprehensive staging of metastatic PCa. Uptake of
18F-NaF is determined by osteoblastic activity as it attaches to sites of new bone formation [11]. A recent meta-analysis showed a pooled sensitivity and specificity of
F-NaF is determined by osteoblastic activity as it attaches to sites of new bone formation [31]. A recent meta-analysis showed a pooled sensitivity and specificity of
18
F-NaF PET/CT for the detection of bone metastases on a per patient basis of 98% (95%CI 95–99) and 90% (95%CI 86–93), and on per a lesion basis of 97% (95%CI 95–98) and 84% (95%CI 81–87), respectively. The diagnostic performance of
18F-NaF PET/CT is superior compared to BS [14]. However, in patients with newly diagnosed PCa scheduled for radical prostatectomy, no added value of
F-NaF PET/CT is superior compared to BS [11]. However, in patients with newly diagnosed PCa scheduled for radical prostatectomy, no added value of
18F-NaF PET/CT was found for the detection of bone metastases in case of a negative BS [16]. The advantages of
F-NaF PET/CT was found for the detection of bone metastases in case of a negative BS [32]. The advantages of
18
F-NaF PET/CT include: superior image quality, due to a higher bone uptake and faster blood clearance, and superior spatial resolution, with better definition of bone metastases, thus contributing to a higher diagnostic accuracy. However, BS has advantages over
18
F-NaF PET/CT in terms of cost-effectiveness and availability, and therefore it remains the preferred technique for generalized use.
The relatively new oncological tracer,
18F-FDHT, is a radiolabeled analogue of dihydrotestosterone, directly binding to the androgen receptor (AR). It allows in-vivo visualization and quantification of AR expression [17][18]. The AR is crucial for PCa growth, and essential for AR-directed therapies in metastatic CRPC.
F-FDHT, is a radiolabeled analogue of dihydrotestosterone, directly binding to the androgen receptor (AR). It allows in-vivo visualization and quantification of AR expression [33,34]. The AR is crucial for PCa growth, and essential for AR-directed therapies in metastatic CRPC.
18F-FDHT PET/CT was successfully used in early phase clinical trials to demonstrate AR specific drug binding [19][20]. Larson et al. studied
F-FDHT PET/CT was successfully used in early phase clinical trials to demonstrate AR specific drug binding [35,36]. Larson et al. studied
18
F-FDHT PET uptake in seven patients with progressive clinically metastatic PCa. Conventional imaging identified 59 lesions, and 78% of the lesions (46 of 59 lesions) were
18F-FDHT positive [21]. Dehdashti et al. [15] enrolled 19 patients with advanced PCa, with biopsy and/or radiologically proven metastatic disease, and found a sensitivity for
F-FDHT positive [18]. Dehdashti et al. [37] enrolled 19 patients with advanced PCa, with biopsy and/or radiologically proven metastatic disease, and found a sensitivity for
18
F-FDHT PET of 63%. This finding suggests that
18
F-FDHT PET/CT seems to be a promising predictive biomarker in the evaluation of AR status, and for treatment response assessment, rather than for the primary detection of PCa metastases. Further investigation is needed and it has not yet entered routine clinical use.
PCa cells are known for their increased proliferation and upregulation of choline kinase. Choline is a precursor for the biosynthesis of phosphatidylcholine which is a key component of cell membrane proliferation. This amino acid can be targeted with
11
C- or
18F, resulting in radio-labeled choline. These radiotracers are extensively used in PCa, particularly in the setting of BCR, and as potential biomarkers of response after chemotherapy [22]. Shen et al. [12] found, on a per patient analysis, a pooled sensitivity and specificity for the detection of bone metastases using choline PET/CT in patients with PCa of 87% and 97%, respectively. Radiolabeled choline PET/CT has been shown to have a pooled sensitivity and specificity in recurrent disease for all sites (prostate, lymph nodes, bone) of 85.6% and 92.6%, respectively [23]. A limitation of
F, resulting in radio-labeled choline. These radiotracers are extensively used in PCa, particularly in the setting of BCR, and as potential biomarkers of response after chemotherapy [38]. Shen et al. [12] found, on a per patient analysis, a pooled sensitivity and specificity for the detection of bone metastases using choline PET/CT in patients with PCa of 87% and 97%, respectively. Radiolabeled choline PET/CT has been shown to have a pooled sensitivity and specificity in recurrent disease for all sites (prostate, lymph nodes, bone) of 85.6% and 92.6%, respectively [39]. A limitation of
18F-Choline is the low sensitivity for the detection of PCa metastases (bone and lymph node) at low PSA-values, where it is clearly outperformed by radiolabeled-PSMA [24][25].
F-Choline is the low sensitivity for the detection of PCa metastases (bone and lymph node) at low PSA-values, where it is clearly outperformed by radiolabeled-PSMA [40,41].
PSMA-PET/CT is a novel imaging technique increasingly used in routine practice. PSMA is a class II cell-surface transmembrane protein overexpressed in malignant prostatic epithelial cells, making it an excellent target for imaging. The degree of PSMA-expression is correlated with higher tumor grades, and higher risk of disease progression, leading to it being described as a marker of disease aggressiveness [26][27]. In a recent study of 90 patients with biopsy proven primary PCa, PSA-value and GG correlated with the intensity of tracer expression on
PSMA-PET/CT is a novel imaging technique increasingly used in routine practice. PSMA is a class II cell-surface transmembrane protein overexpressed in malignant prostatic epithelial cells, making it an excellent target for imaging. The degree of PSMA-expression is correlated with higher tumor grades, and higher risk of disease progression, leading to it being described as a marker of disease aggressiveness [42,43]. In a recent study of 90 patients with biopsy proven primary PCa, PSA-value and GG correlated with the intensity of tracer expression on
68Ga-PSMA-11 PET/CT, with a significantly higher tumor-related tracer uptake seen in patients with either PSA ≥ 10 ng/mL or GG ≥ 4 [28].
Ga-PSMA-11 PET/CT, with a significantly higher tumor-related tracer uptake seen in patients with either PSA ≥ 10 ng/mL or GG ≥ 4 [44].
68Ga-labeled PSMA tracers are the most intensively studied, demonstrating high detection rates for both bone and lymph node metastases [7][9][29]. Perera et al. [9] evaluated the diagnostic accuracy of
Ga-labeled PSMA tracers are the most intensively studied, demonstrating high detection rates for both bone and lymph node metastases [7,9,45]. Perera et al. [9] evaluated the diagnostic accuracy of
68
Ga-PSMA-11 PET/CT for the detection of metastatic disease in both primary (high-risk and advanced prostate cancer) and secondary staging (at BCR) (
). The lesion-based analysis showed a pooled sensitivity of 75%, and specificity of 99% for primary staging, with corresponding figures of 77% and 97%, respectively, for a per patient analysis. The positivity rate of the
68
Ga-PSMA PET/CT in secondary staging increased in patients with higher PSA-levels: 33% (PSA < 0.2 ng/mL), 45% (0.2–0.49 ng/mL), 59% (0.5–0.99 ng/mL), 75% (1–1.99 ng/mL), and 95% (≥2 ng/mL). These percentages are substantially higher than those for conventional imaging techniques.
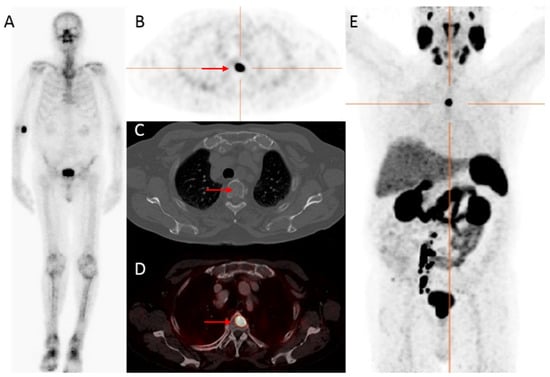
Figure 1.
An 83-year-old patient, with castration-resistant prostate cancer (CRPC) after initial treatment with hormonal therapy (2009), and secondary abiraterone (2019), showed improved detection of bone metastatic prostate cancer PCa with
68
Ga-PSMA PET/CT compared to bone scintigraphy. The prostate—specific antigen (PSA)—value at PET scanning was 25.9 ng/mL. On the bone scintigraphy, no suspect bone metastases were visualized (
A
). Transversal
68
Ga-PSMA PET (
B
), fused PET/CT (
D
) and maximum intensity projection (MIP) (
E
) revealed a lesion located in the thoracic spine with increased PSMA expression (red arrow), with no evident substrate on CT (
C
). Time interval between bone scintigraphy and
68
Ga-PSMA PET/CT was 5 weeks.
PSMA PET/CT has shown high detection rates (98–100%) for the primary prostate tumor [30][31][32], and provides more sensitive screening for metastatic disease at initial staging than conventional imaging modalities [8]. A recent meta-analysis confirmed the higher diagnostic accuracy of
PSMA PET/CT has shown high detection rates (98–100%) for the primary prostate tumor [46,47,48], and provides more sensitive screening for metastatic disease at initial staging than conventional imaging modalities [8]. A recent meta-analysis confirmed the higher diagnostic accuracy of
68Ga-PSMA PET/CT compared to BS with higher sensitivity (0.97 versus 0.86) and specificity (1.00 versus 0.95) for detecting bone metastases [10]. Hofman et al. [8] prospectively compared
Ga-PSMA PET/CT compared to BS with higher sensitivity (0.97 versus 0.86) and specificity (1.00 versus 0.95) for detecting bone metastases [10]. Hofman et al. [8] prospectively compared
68
Ga-PSMA PET/CT with conventional imaging in patients with high-risk PCa (
n
= 302).
68
Ga-PSMA PET/CT showed an enhanced diagnostic accuracy for identifying either pelvic nodal or distant metastases compared to conventional imaging (
p
< 0.0001). In subgroup analysis,
68
Ga-PSMA PET/CT was superior in detecting pelvic lymph node metastases (91% versus 59%), and distant metastases (95% versus 74%). First-line
68
Ga-PSMA PET/CT (
n
= 148) found abdominal lymph node metastases in 13 patients (9%), bone metastases in 15 patients (10.1%), and visceral metastases in one (1%). In the primary staging of PCa, conventional imaging and
68
Ga-PSMA PET/CT led to a change in treatment approach in 23 (15%) and 41 patients (28%), respectively (
p
= 0.008).
Next to the intensively studied
68
Ga-labeled PSMA tracers,
18
F-labeled tracers, such as
18F-DCFPyL [33] and
F-DCFPyL [49] and
18F-PSMA-1007 [34], appear promising.
F-PSMA-1007 [50], appear promising.
18
F-labeled tracers are attractive due to a shorter positron range and higher positron yield compared to
68Ga, providing higher resolution PET-images which may improve early detection of small metastases [29], and
Ga, providing higher resolution PET-images which may improve early detection of small metastases [45], and
18
F-DCFPyL has shown higher tumor to background ratios compared to
68Ga-PSMA [35]. For
Ga-PSMA [51]. For
18
F-DCFPyL, initial experience with the detection of bone metastases in primary prostate cancer has been published (
), but the diagnostic accuracy of
18F-DCFPyL PET/CT for detecting pelvic lymph nodes metastases in initial staging is less well described. Wondergem et al. [36] enrolled 160 patients with high-risk PCa who underwent an
F-DCFPyL PET/CT for detecting pelvic lymph nodes metastases in initial staging is less well described. Wondergem et al. [52] enrolled 160 patients with high-risk PCa who underwent an
18
F-DCFPyL PET/CT for primary staging. PSMA-positive bone metastases were detected in 49 patients (31%) and lymph nodes were found in 81 patients (51%) of which 52% (
n
= 42) were enlarged on CT-scan. The treatment plan was adjusted after
18
F-DCFPyL in 17% (
n = 27) of the patients. Jansen et al. [37] prospectively analyzed 117 patients who underwent imaging prior to robot-assisted radical prostatectomy and extended pelvic lymph node dissection, in order to determine the diagnostic accuracy of
= 27) of the patients. Jansen et al. [53] prospectively analyzed 117 patients who underwent imaging prior to robot-assisted radical prostatectomy and extended pelvic lymph node dissection, in order to determine the diagnostic accuracy of
18
F-DCFPyL PET/CT for pelvic lymph node staging in intermediate- (36.8%) and high-risk (63.3%) PCa. Histological lymph node metastases were found in 17/117 (14.5%) patients, of whom seven had a suspicious PET/CT, resulting in a limited sensitivity (41.2%), but high specificity (94.0%). The low sensitivity is explained by the median tumor size of 1.5 mm for PET/CT undetected lymph node metastases and illustrates the “resolution” challenges faced by the newly developed imaging techniques.
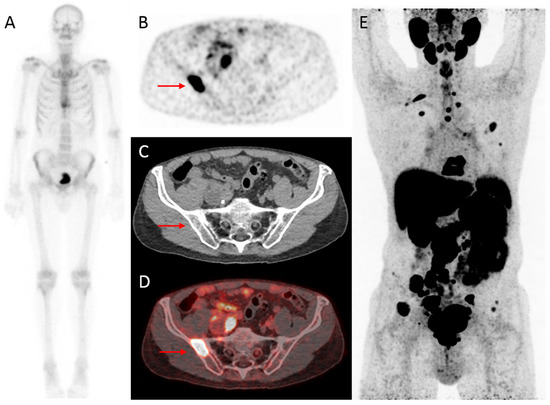
Figure 2.
Initial assessment of a 75-year old patient, newly diagnosed with PCa (Grade Group (GG) 5), with an initial PSA-value of 1396 ng/mL. On bone scintigraphy, the increased uptake in the thoracic spine was attributed to an (osteoporotic) collapsed vertebra, and the faint uptake in the left third rib to a post-traumatic origin. Despite the high PSA-value, no abnormal uptake consistent with osseous metastases was visualized (
A
). However, extensive metastatic disease was found on
18
F-DCFPyL PET/CT (
B
–
E
). For example, transversal
18
F-DCFPyL PET (
B
) and fused PET/CT (
D
) showed highly increased PSMA-expression in the right iliac bone (red arrow, maximum standardized uptake value (SUV
max
) 8.15), compatible with a lytic lesion on CT (
C
). The time interval between bone scintigraphy and
18
F-DCFPyL PET/CT was 5 days.
With the better performance of novel imaging modalities, patients with BCR can be diagnosed earlier with metastatic disease. Consequently, the number of patients diagnosed with oligometastatic PCa (usually considered to be a maximum of 3–5 metastases) has increased. The true oligometastatic state is considered to be one of limited metastatic potential in which the local treatment of all visible metastases has the potential to bring about long-term survival in some patients [38]. The treatment of oligometastatic disease (in practice mostly patients with 1–2 lesions) now attracts considerable interest [39]. The most studied treatment approach in patients with oligometastatic recurrent PCa, is metastasis-directed local therapy (MDT; for example, stereotactic radiotherapy or surgery), with the potential goals of delaying the start of ADT and influencing prognosis [40]. A recent randomized phase II study of patients with oligometastatic recurrent PCa showed improved ADT-free survival in patients who underwent MDT compared to surveillance [41]. The effect of MDT on OS needs to be addressed in further clinical trials.
With the better performance of novel imaging modalities, patients with BCR can be diagnosed earlier with metastatic disease. Consequently, the number of patients diagnosed with oligometastatic PCa (usually considered to be a maximum of 3–5 metastases) has increased. The true oligometastatic state is considered to be one of limited metastatic potential in which the local treatment of all visible metastases has the potential to bring about long-term survival in some patients [54]. The treatment of oligometastatic disease (in practice mostly patients with 1–2 lesions) now attracts considerable interest [55]. The most studied treatment approach in patients with oligometastatic recurrent PCa, is metastasis-directed local therapy (MDT; for example, stereotactic radiotherapy or surgery), with the potential goals of delaying the start of ADT and influencing prognosis [56]. A recent randomized phase II study of patients with oligometastatic recurrent PCa showed improved ADT-free survival in patients who underwent MDT compared to surveillance [57]. The effect of MDT on OS needs to be addressed in further clinical trials.
In summary, PSMA PET/CT has the potential for more accurate metastasis detection and (re)staging at initial diagnosis and at BCR than standard conventional imaging modalities (in practice BS and CT). This could potentially lead to changes in treatment and selection of more optimal strategies. However, the clinical benefit of (even) earlier detection of metastases has yet to be shown in well-powered randomized studies. As a result, current EAU guidelines do not recommend the routine use of PSMA (PET/CT) in their imaging algorithms [4]. Currently, at Amsterdam UMC, location VUmc, we are conducting two prospective, clinical trials looking at the diagnostic accuracy of
18
F-DCFPyL PET/CT compared to conventional imaging for the detection of metastases in patients with newly diagnosed high risk PCa who have a negative BS (trial 1, VUmc IRB number: 2019.051) or a positive BS with low volume disease (trial 2, VUmc IRB number: 2019.054). The change in treatment approach will be evaluated as a secondary outcome.
