Periodontal regeneration is a technique that aims to regenerate the damaged tissue around periodontally compromised teeth. The regenerative process aims to use scaffolds, cells, and growth factors to enhance biological activity.
- periodontal regeneration
- GTR
- biomaterials
- growth factors
- biologics
- periodontitis
- Introduction and History
1. Introduction and History
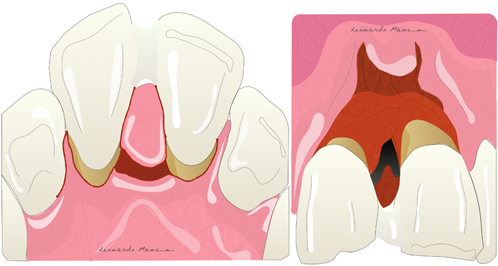
Periodontitis is a multifactorial disease characterized by microbially-associated, host-mediated inflammation that results in loss of periodontal attachment, eventually leading to tooth loss [1]. Periodontitis is the sixth most prevalent disease for mankind [2] and is a public health problem since it is so widely prevalent, causes disability [3], and numerous clinical and experimental studies have shown the presence of an association between periodontitis and some systemic diseases, in particular cardiovascular diseases, diabetes, lung diseases, and pregnancy complications [4][5]. The goal of periodontal therapy is to arrest progressive attachment loss, through the control of infection, to prevent tooth loss [6]. Probing pocket depth reduction as a surrogate outcome variable is validated by data demonstrating lower risk for disease progression and tooth loss [7][8] associated with the absence of bleeding on probing [9][10]. Periodontal pockets related to intraosseous defects often remain after nonsurgical treatment and could increase risk of progressive periodontitis [11][12] and, as such, are often considered to require surgical intervention. Based on the studies of Melcher (1976) [13], who developed the concept of using barrier membranes to “guide” the biological process of wound healing, in the mid-1980s clinical reports showed that intraosseous defects have potential for healing through regeneration using barrier membranes [14][15]. Today we know which bio-clinical principles regulate periodontal regeneration: wound stability, space provision, and primary intention healing [16]. Many randomized controlled trials and systematic reviews have shown that periodontal regenerative therapies can achieve better treatment outcomes compared to open flap debridement in the treatment of angular defects [17][18][19]. Several techniques and biomaterials have been studied for periodontal regeneration of intraosseous defects, but from a histological and clinical point of view, guided tissue regeneration (GTR), enamel matrix derivatives (EMD), and decalcified freeze-dried bone allograft (DFDBA) are the most effective approaches to periodontal regeneration [20][21][22][23][24]. A recent consensus report of the American Academy of Periodontology recommended surgical intervention as the treatment of choice for intraosseous defects [25].
Periodontitis is a multifactorial disease characterized by microbially-associated, host-mediated inflammation that results in loss of periodontal attachment, eventually leading to tooth loss [1]. Periodontitis is the sixth most prevalent disease for mankind [2] and is a public health problem since it is so widely prevalent, causes disability [3], and numerous clinical and experimental studies have shown the presence of an association between periodontitis and some systemic diseases, in particular cardiovascular diseases, diabetes, lung diseases, and pregnancy complications [4,5]. The goal of periodontal therapy is to arrest progressive attachment loss, through the control of infection, to prevent tooth loss [6]. Probing pocket depth reduction as a surrogate outcome variable is validated by data demonstrating lower risk for disease progression and tooth loss [7,8] associated with the absence of bleeding on probing [9,10]. Periodontal pockets related to intraosseous defects often remain after nonsurgical treatment and could increase risk of progressive periodontitis [11,12] and, as such, are often considered to require surgical intervention. Based on the studies of Melcher (1976) [13], who developed the concept of using barrier membranes to “guide” the biological process of wound healing, in the mid-1980s clinical reports showed that intraosseous defects have potential for healing through regeneration using barrier membranes [14,15]. Today we know which bio-clinical principles regulate periodontal regeneration: wound stability, space provision, and primary intention healing [16]. Many randomized controlled trials and systematic reviews have shown that periodontal regenerative therapies can achieve better treatment outcomes compared to open flap debridement in the treatment of angular defects [17–19]. Several techniques and biomaterials have been studied for periodontal regeneration of intraosseous defects, but from a histological and clinical point of view, guided tissue regeneration (GTR), enamel matrix derivatives (EMD), and decalcified freeze-dried bone allograft (DFDBA) are the most effective approaches to periodontal regeneration [20–24]. A recent consensus report of the American Academy of Periodontology recommended surgical intervention as the treatment of choice for intraosseous defects [25].
- Techniques
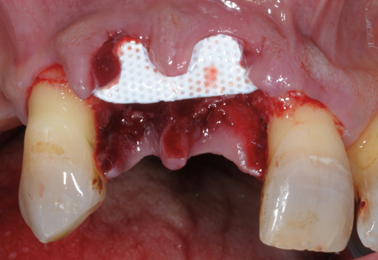
Membrane exposure resulting in bacterial contamination during healing has been the major complication of regenerative procedures in the past, with a prevalence in the range of 50–100% [26,27]. Cortellini et al. [28] reported that the prevalence of membrane exposure could be significantly reduced with the use of access flaps specifically designed to preserve interdental tissues (the modified papilla preservation technique). The first papilla preservation technique was described by Takey et al. 1985 [29] and is used to prevent soft tissue collapse and to maintain stability during the regeneration phase (Figure 1).
2. Techniques
Membrane exposure resulting in bacterial contamination during healing has been the major complication of regenerative procedures in the past, with a prevalence in the range of 50–100% [26][27]. Cortellini et al. [28] reported that the prevalence of membrane exposure could be significantly reduced with the use of access flaps specifically designed to preserve interdental tissues (the modified papilla preservation technique). The first papilla preservation technique was described by Takey et al. 1985 [29] and is used to prevent soft tissue collapse and to maintain stability during the regeneration phase (Figure 1).
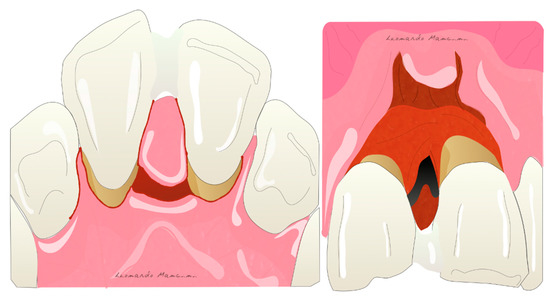
Figure 1. Illustration of the papilla preservation flap according to Takey et al 1985.
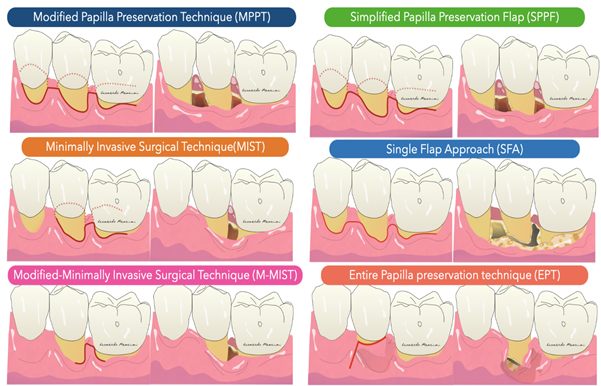
In general, the development of new procedures has been aimed at complete preservation of the marginal tissue in order to obtain and maintain a primary closure above the applied regenerative material during the critical phases of healing. Specifically, the flap designs sought to achieve passive primary closure of the flap combined with excellent wound stability. Today, papilla-preserving flap designs and flap closure techniques are the standard approach for regenerative periodontal surgery (Figure 2).
Illustration of the papilla preservation flap according to Takey et al. 1985.
In general, the development of new procedures has been aimed at complete preservation of the marginal tissue in order to obtain and maintain a primary closure above the applied regenerative material during the critical phases of healing. Specifically, the flap designs sought to achieve passive primary closure of the flap combined with excellent wound stability. Today, papilla-preserving flap designs and flap closure techniques are the standard approach for regenerative periodontal surgery (Figure 2).
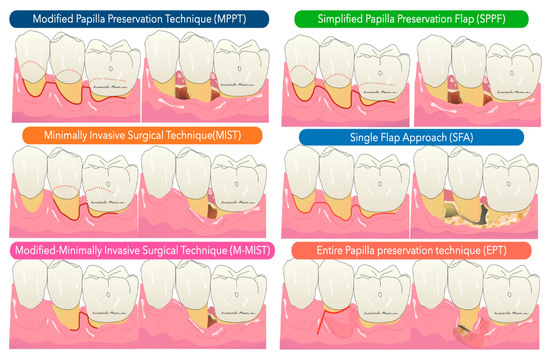
Figure 2. Illustration of flap design for periodontal regeneration.
Illustration of flap design for periodontal regeneration.
The Modified Papilla Preservation (MPPT) technique was developed to increase the space for regeneration and to achieve and maintain primary flap closure in the interdental space [30]. MPPT allows primary closure of the interdental space, resulting in better protection of the membrane from the oral environment [28]. MMPT can be successfully applied at sites where the interdental space width is at least 2 mm in the most coronal portion of the papilla. When the interdental sites are narrower, a different papilla preservation procedure (the simplified papilla preservation flap, SPPF) has been proposed [31]. In the latter study, it was possible to close 100% of the narrow interdental papillae over resorbable membranes and 67% maintained primary closure over time, resulting in clinical attachment gains of 4.9 ± 1.8 mm. In order to further preserve wound stability and further limit morbidity, a papilla-preserving flap can be used in the context of a minimally invasive surgical technique associated with high-powered magnification systems [32]. The minimally invasive approaches (MIST, M-MIST) are particularly suitable in the case of therapies that involve the use of biologically active agents such as EMD or growth factors. The interdental papilla associated with the defect is made accessible using the Simplified Papilla Preserving Flap (SPPF) or Modified Papilla Preservation (MPPT) technique. All surgical procedures are performed with the aid of a microscope or a magnification system (4X–16X) and microsurgical instruments [33]. A further development in the microsurgical field was the single flap approach [34][35]. This therapeutic strategy, which is limited to reconstructive surgery of intraosseous defects, involves the dissection of a mucoperiosteum flap from a single side (exclusively vestibular or exclusively lingual/palatal). The application of the SFA is indicated only when the extension of the intraosseous defect is prevalent from the buccal or lingual/palatal side and access from one side only allows adequate surgical cleansing of the intraosseous lesion and the radiological surface affected by the defect. Aslan et al. proposed a novel surgical approach, the ‘‘entire papilla preservation (EPP)’’ technique, for regenerative treatment of isolated deep intraosseous defects [36]. This novel concept provides an intact gingival space over the intraosseous defect, with a completely preserved interdental papilla. A one-year prospective cohort study [37] with 12 isolated deep non-contained intraosseous defects treated with a combination of EMD and deproteinized bovine bone mineral showed 100% primary closure during the healing period and resulted in 6.83 mm of average clinical attachment gain.
The Modified Papilla Preservation (MPPT) technique was developed to increase the space for regeneration and to achieve and maintain primary flap closure in the interdental space [30]. MPPT allows primary closure of the interdental space, resulting in better protection of the membrane from the oral environment [28]. MMPT can be successfully applied at sites where the interdental space width is at least 2 mm in the most coronal portion of the papilla. When the interdental sites are narrower, a different papilla preservation procedure (the simplified papilla preservation flap, SPPF) has been proposed [31]. In the latter study, it was possible to close 100% of the narrow interdental papillae over resorbable membranes and 67% maintained primary closure over time, resulting in clinical attachment gains of 4.9 ± 1.8 mm. In order to further preserve wound stability and further limit morbidity, a papilla-preserving flap can be used in the context of a minimally invasive surgical technique associated with high-powered magnification systems [32]. The minimally invasive approaches (MIST, M-MIST) are particularly suitable in the case of therapies that involve the use of biologically active agents such as EMD or growth factors. The interdental papilla associated with the defect is made accessible using the Simplified Papilla Preserving Flap (SPPF) or Modified Papilla Preservation (MPPT) technique. All surgical procedures are performed with the aid of a microscope or a magnification system (4X–16X) and microsurgical instruments [33]. A further development in the microsurgical field was the single flap approach [34,35]. This therapeutic strategy, which is limited to reconstructive surgery of intraosseous defects, involves the dissection of a mucoperiosteum flap from a single side (exclusively vestibular or exclusively lingual/palatal). The application of the SFA is indicated only when the extension of the intraosseous defect is prevalent from the buccal or lingual/palatal side and access from one side only allows adequate surgical cleansing of the intraosseous lesion and the radiological surface affected by the defect. Aslan et al. proposed a novel surgical approach, the ‘‘entire papilla preservation (EPP)’’ technique, for regenerative treatment of isolated deep intraosseous defects [36]. This novel concept provides an intact gingival space over the intraosseous defect, with a completely preserved interdental papilla. A one-year prospective cohort study [37] with 12 isolated deep non-contained intraosseous defects treated with a combination of EMD and deproteinized bovine bone mineral showed 100% primary closure during the healing period and resulted in 6.83 mm of average clinical attachment gain.
- Materials
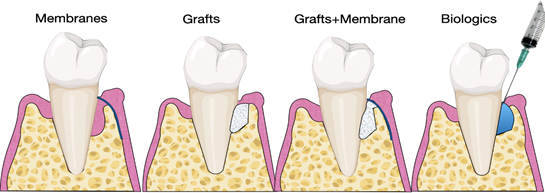
Several studies have investigated the use of biomaterials and biologics in periodontal regeneration [38–40]. Various products are currently available on the market, including bone grafts, bone fillers, scaffolds, membranes, and growth factors (Figure 3).
3. Materials
Several studies have investigated the use of biomaterials and biologics in periodontal regeneration [38][39][40]. Various products are currently available on the market, including bone grafts, bone fillers, scaffolds, membranes, and growth factors (Figure 3).
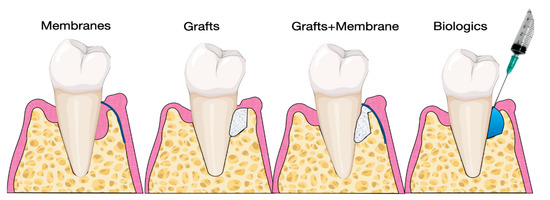
Figure 3. Illustration of materials for periodontal regeneration.
3.1. Grafts and Fillers
Autogenous, allogeneic, xenogeneic bone substitutes, and synthetic materials are referred to as bone fillers, due to their capacity to narrow the gap of the periodontal defect. Osteoconduction, osteogenesis, and osteoinduction are the characteristics that enable cells to move in the defect, and to generate and induce the chemotaxis of other cells [41]. Not all of these properties are related to each biomaterial; however, osteoconduction is the most common property for bone fillers. According to Bosshardt and Sculean 2009 [42], the evaluation of biomaterials in periodontal regeneration should be histological rather than clinical, as the re-entry radiographs in several cases demonstrate an impressive volume gain. Indeed, a histological analysis might reveal the possible presence of fibrous encapsulation around bone grafts and a small portion of the newly formed bone in the proximity of pre-existing bone without any actual regeneration. Bovine xenografts, as opposed to biphasic calcium phosphate and bioactive glass, show unusual fibrous encapsulation [40,43,44]. Periodontal regeneration consists of the restoration of connective tissue attachment (CTA), cementum periodontal ligament, and proper bone. Evidence for new CTA remains limited and autogenous bone showed incomplete regeneration with a long junctional epithelium and little new CTA [45]; other considerations are the presence of a second surgical site and patient morbidity, which may hinder clinical procedures. The data on bone allografts are controversial; indeed, donor age and the site of origin might affect the regenerative potential [46]. A histological evaluation revealed the presence of a long junctional epithelium [47]. Regarding the demineralized freeze-dried allografts at the contrary to the freeze-dried allografts showed an evident and histologic periodontal regeneration [20]. However, in several countries in the European Union, regulations do not allow the use of these types of material. Xenografts and allogenic grafts showed a moderate periodontal regeneration with some new CTA; the CTA gain might be related to the use of membranes as barriers; indeed, encapsulation is recurrent for these types of filler (Figure 4) [48]. The disadvantage of these materials is the inability to regenerate and restore the CTA. Thus, using these materials is more suitable for a bone regeneration, and they are therefore commonly used in guided bone regeneration (GBR) [49].
Illustration of materials for periodontal regeneration.
3.1. Grafts and Fillers
Autogenous, allogeneic, xenogeneic bone substitutes, and synthetic materials are referred to as bone fillers, due to their capacity to narrow the gap of the periodontal defect. Osteoconduction, osteogenesis, and osteoinduction are the characteristics that enable cells to move in the defect, and to generate and induce the chemotaxis of other cells [41]. Not all of these properties are related to each biomaterial; however, osteoconduction is the most common property for bone fillers. According to Bosshardt and Sculean 2009 [42], the evaluation of biomaterials in periodontal regeneration should be histological rather than clinical, as the re-entry radiographs in several cases demonstrate an impressive volume gain. Indeed, a histological analysis might reveal the possible presence of fibrous encapsulation around bone grafts and a small portion of the newly formed bone in the proximity of pre-existing bone without any actual regeneration. Bovine xenografts, as opposed to biphasic calcium phosphate and bioactive glass, show unusual fibrous encapsulation [40][43][44]. Periodontal regeneration consists of the restoration of connective tissue attachment (CTA), cementum periodontal ligament, and proper bone. Evidence for new CTA remains limited and autogenous bone showed incomplete regeneration with a long junctional epithelium and little new CTA [45]; other considerations are the presence of a second surgical site and patient morbidity, which may hinder clinical procedures. The data on bone allografts are controversial; indeed, donor age and the site of origin might affect the regenerative potential [46]. A histological evaluation revealed the presence of a long junctional epithelium [47]. Regarding the demineralized freeze-dried allografts at the contrary to the freeze-dried allografts showed an evident and histologic periodontal regeneration [20]. However, in several countries in the European Union, regulations do not allow the use of these types of material. Xenografts and allogenic grafts showed a moderate periodontal regeneration with some new CTA; the CTA gain might be related to the use of membranes as barriers; indeed, encapsulation is recurrent for these types of filler (Figure 4) [48]. The disadvantage of these materials is the inability to regenerate and restore the CTA. Thus, using these materials is more suitable for a bone regeneration, and they are therefore commonly used in guided bone regeneration (GBR) [49].

Figure 4. (
(
A
) Xenogeneic collagenated bone graft; (
B) xenogeneic graft in particles.
3.2. Membranes
Membranes have been used since the introduction of the GTR concept [14,15]. As mentioned earlier, the principal aim is to select and isolate cells that are able to restore the periodontal ligament, cementum, and connective tissue. Guiding cells in the periodontal defect is the primary target and this is possible through the exclusion of epithelial cells, which have a fast turnover compared to osteoblasts and fibroblasts [50]. The ideal membrane should respect several principles:
| Collagen with intermingled elastin | |||
| Type I, III, IV, VI and other proteins | |||
| CollaTape | ® | ; Tutodent | ® BioGide®; botiss Jason® Creos xenoprotect DynaMatrix® |
Non-resorbable membranes are generally indicated in guided bone regeneration (GBR) or in situations of bone deficiency. They are no longer used in periodontal regeneration mainly because the introduction of minimal flaps does not allow the insertion of these unwieldy barriers. Titanium reinforced membranes and polytetrafluorethylene are the most common (
Figure 5 and Figure 6). Nowadays, new technologies and the introduction of tissue engineering mean that the regenerative process is oriented towards the use of resorbable materials, avoiding the need for a second surgical phase [51][52].
-
Resorbable membranes are made from collagen, a natural substance that can be resorbed (Figure 7). Several types are available, and these differ in resorption time. As reported in Table 1, the fabrication of these membranes may or may not involve cross-linking [53]. The cross-linking process aims to reinforce the chemical bonds among the collagen fibers, and this results in a long resorption time. Collagen membranes have a low risk of exposure in the oral cavity, but due to their low mechanical stability, the use of bone substitutes or fillers is also required [42]. The use of membranes raises the potential for complications such as exposure, which could reduce the regenerative potential and allow the infiltration of bacteria and possible infection of the site [42].
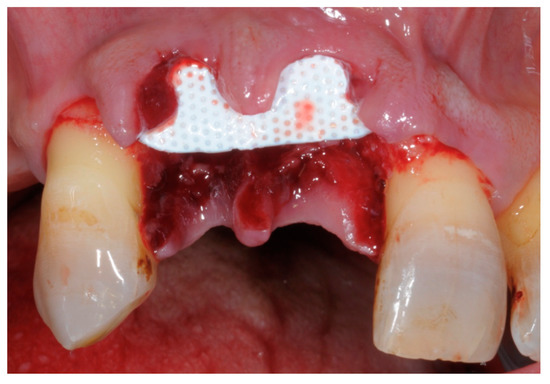
Figure 5. Example of a non-resorbable Polytetrafluorethylene (PTFE) membrane.
Example of a non-resorbable Polytetrafluorethylene (PTFE) membrane.

Figure 6. Example of old periodontal non resorbable PTFE membranes, (
Example of old periodontal non resorbable PTFE membranes, (
A
): wraparound shape for large defects; (
B): Interproximal shape for interproximal defects.
- Resorbable membranes are made from collagen, a natural substance that can be resorbed (Figure 7). Several types are available, and these differ in resorption time. As reported in Table 1, the fabrication of these membranes may or may not involve cross-linking [53]. The cross-linking process aims to reinforce the chemical bonds among the collagen fibers, and this results in a long resorption time. Collagen membranes have a low risk of exposure in the oral cavity, but due to their low mechanical stability, the use of bone substitutes or fillers is also required [42]. The use of membranes raises the potential for complications such as exposure, which could reduce the regenerative potential and allow the infiltration of bacteria and possible infection of the site [42].
): Interproximal shape for interproximal defects.

Figure 7. (
(
A
) Soft tissue side; (
B) Internal tissue side of a collagen membrane.
3.3. Biologics
According to the United States Food and Drug Administration (FDA), biologics are a wide range of products, proteins, growth factors, or a complex combination of these substances used to treat various diseases or to enhance the regenerative process, as periodontal regeneration, via the activation and stimulation of periodontal cells [54]. Enamel matrix derivative (EMD), recombinant human platelet-derived growth factor-BB (PDGF—BB), and bone morphogenic proteins (BMP) are currently available on the market. EMD is extracted from pigs and is treated to make it biocompatible and thus to reduce adverse reactions. This material is unique because it is only sold as Emdogain
) Internal tissue side of a collagen membrane.
3.3. Biologics
According to the United States Food and Drug Administration (FDA), biologics are a wide range of products, proteins, growth factors, or a complex combination of these substances used to treat various diseases or to enhance the regenerative process, as periodontal regeneration, via the activation and stimulation of periodontal cells [54]. Enamel matrix derivative (EMD), recombinant human platelet-derived growth factor-BB (PDGF—BB), and bone morphogenic proteins (BMP) are currently available on the market. EMD is extracted from pigs and is treated to make it biocompatible and thus to reduce adverse reactions. This material is unique because it is only sold as Emdogain
® (Straumann AG, Basel, Switzerland). Also, according to the literature, it is the only biomaterial that led to the complete reformation of the periodontal ligament in a histological evaluation [55]. Several studies suggest the use of EMD alone or in combination with bone fillers [56]. The first study to show the efficacy of this material was conducted by Heiji et al. in 1997, in which EMD was compared to open flap debridement of intraosseous defects [57]. EMD was associated with significant CAL gains and pocket depth reduction [57]. Another series of studies compared EMD to GTR with comparable results [58]. Interesting data came from a study by Cortellini et al. in which the use of EMD as an adjunct to minimally invasive techniques such as MIST improved stability, minimizing the post-surgical phase [59]. In terms of CAL gains, no relevant differences were found between EMD + MIST and MIST alone [59]. In summary, EMD showed comparable results to GTR; moreover, the potential complications of GTR might indicate EMD as a more reliable material with fewer complications and easy handling [60]. Nevertheless, the defect’s anatomy plays a crucial role in the regenerative potential of this molecule. PDGF-BB is a growth factor that is active in hard and soft tissue healing, enhancing cell proliferation, angiogenesis and migration [61]. The disadvantage is in its handling, which requires the use of scaffold and fillers [62]. Several studies have evaluated the use of this molecule as an adjunct to Beta-tricalcium phosphate (B-TCP), EMD, and bone allograft, with positive results for EMD and allograft [62]. BMP are proteins found in bone and showed bone regeneration in an animal model [63]. The most studied forms of this type of molecule are BMP-2, BMP-6, and BMP-12. Interesting data came from a study by Wikesjo et al. in a canine model, where the use of BMP-12 showed a regenerated and well-oriented periodontal ligament with newly formed bone and cementum [64]. On the other hand, complications associated with the use of these molecules include possible ankylosis or root resorption [63]. BMP and PDGF-BB are available in the United States but have not been approved for use in Europe. Other biologics that have been used in periodontal regeneration are the blood derivates, platelet rich fibrin (PRF) and its surrogates L-PRF and A-PRF, which showed promising results in the regeneration of periodontal defects and furcations [65]. Several in vitro studies analyzed the biocompatibility and the behavior of these materials in contact with the fibroblast of the periodontal ligament and showed the activation of cytoplasmatic extensions and an increase in cell volume [66]. Moreover, these biologics are natural and enriched with growth factors such as platelet-derived growth factors (PDGF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), and insulin-like growth factor-1 (ILGF-1) that influence cellular differentiation and proliferation [66]. On the other hand, histological evidence regarding these types of biologics is still lacking.
3.4. Futures Biologics
Future potential biologics include several growth factors that have a specific function and are in experimental phase II and III of randomized clinical trials in human and canine studies. Protein 15 (P-15), osteogenic protein 1 (OP-1), parathormone (PTH), and anti-sclerostin antibodies (SOST) are under investigation. As shown in Table 2, several growth factors are already under investigation, but their use is always associated with a scaffold or bone filler.
(Straumann AG, Basel, Switzerland). Also, according to the literature, it is the only biomaterial that led to the complete reformation of the periodontal ligament in a histological evaluation [55]. Several studies suggest the use of EMD alone or in combination with bone fillers [56]. The first study to show the efficacy of this material was conducted by Heiji et al. in 1997, in which EMD was compared to open flap debridement of intraosseous defects [57]. EMD was associated with significant CAL gains and pocket depth reduction [57]. Another series of studies compared EMD to GTR with comparable results [58]. Interesting data came from a study by Cortellini et al. in which the use of EMD as an adjunct to minimally invasive techniques such as MIST improved stability, minimizing the post-surgical phase [59]. In terms of CAL gains, no relevant differences were found between EMD + MIST and MIST alone [59]. In summary, EMD showed comparable results to GTR; moreover, the potential complications of GTR might indicate EMD as a more reliable material with fewer complications and easy handling [60]. Nevertheless, the defect’s anatomy plays a crucial role in the regenerative potential of this molecule. PDGF-BB is a growth factor that is active in hard and soft tissue healing, enhancing cell proliferation, angiogenesis and migration [61]. The disadvantage is in its handling, which requires the use of scaffold and fillers [62]. Several studies have evaluated the use of this molecule as an adjunct to Beta-tricalcium phosphate (B-TCP), EMD, and bone allograft, with positive results for EMD and allograft [62]. BMP are proteins found in bone and showed bone regeneration in an animal model [63]. The most studied forms of this type of molecule are BMP-2, BMP-6, and BMP-12. Interesting data came from a study by Wikesjo et al. in a canine model, where the use of BMP-12 showed a regenerated and well-oriented periodontal ligament with newly formed bone and cementum [64]. On the other hand, complications associated with the use of these molecules include possible ankylosis or root resorption [63]. BMP and PDGF-BB are available in the United States but have not been approved for use in Europe. Other biologics that have been used in periodontal regeneration are the blood derivates, platelet rich fibrin (PRF) and its surrogates L-PRF and A-PRF, which showed promising results in the regeneration of periodontal defects and furcations [65]. Several in vitro studies analyzed the biocompatibility and the behavior of these materials in contact with the fibroblast of the periodontal ligament and showed the activation of cytoplasmatic extensions and an increase in cell volume [66]. Moreover, these biologics are natural and enriched with growth factors such as platelet-derived growth factors (PDGF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), and insulin-like growth factor-1 (ILGF-1) that influence cellular differentiation and proliferation [66]. On the other hand, histological evidence regarding these types of biologics is still lacking.
3.4. Futures Biologics
Future potential biologics include several growth factors that have a specific function and are in experimental phase II and III of randomized clinical trials in human and canine studies. Protein 15 (P-15), osteogenic protein 1 (OP-1), parathormone (PTH), and anti-sclerostin antibodies (SOST) are under investigation. As shown in Table 2, several growth factors are already under investigation, but their use is always associated with a scaffold or bone filler.
Table 2. Growth factors under investigation for periodontal regeneration.
P-15: Protein15; RhPDGF-BB: recombinant human platelet-derived growth factor; BMP-2: bone morphogenic protein-2; BMP-6: bone morphogenic protein-6; BMP-12: bone morphogenic protein-12; rhFGF2: recombinant human fibroblast growth factor-2; OP-1: osteogenic protein-1; SOST antibodies: anti-sclerostin antibodies; PTH: parathormone.
3.5. Future Therapies
Therapies that are currently under development are based on the use of cells and 3D printing scaffolds [73]. Several articles have suggested the use of cell cultures in periodontal regeneration but their high cost and the need to involve specialist laboratories remain a crucial problem for dentists. Nevertheless, according to Trovato et al., the use of a micrograft (a collagen membrane enriched with stem cells) has potential in the regenerative process [74]. A recent review by Mummolo et al. suggested that micrografts in periodontal and bone regeneration are a good alternative to GTR or to other biomaterials such as bone substitutes [75]. However, they have some limitations due to the availability of progenitor cells in the tissue sample used, and the selection of the right scaffold as a carrier. Another promising therapy is related to the 3D printing technology; indeed, it is starting to enter the oral regenerative field with tailored scaffolds that can be customized for each type of defect. Rasperini et al. showed for the first time the possibility of using a 3D printed resorbable scaffold for a large osseous defect. The material used was a synthetic polymer enriched with recombinant human platelet derived growth factor BB (rhPDGF-BB) [76]. The 3D printing process allows the customization of the biomaterial respect to the defect type achieving a better handling and a tailored treatment. The possible complications reported in this case report were several such as the wound dehiscence, exposure and afterwards microbial contaminations. The most recent technique is the connective tissue graft (CTG) wall technique, introduced to suggest the use of CTG as a barrier in adjunct to EMD reducing gingival recession and in some cases the possible soft tissue restoration [77]. The advantages of this technique are the use of connective tissue as barrier preventing the exposure, reducing the complications and improving the soft tissue response. Moreover, the CTG positioned coronally respect to the defect seems to prevent the soft tissue collapsing. This technique according to the authors is reliable in deep intrabony defects with the absence of buccal bone (Figure 8). A disadvantage is the presence of a second surgical site on the palatal aspect for the CTG sampling and patient discomfort during the healing period.
Growth factors under investigation for periodontal regeneration.
| Growth Factors | Biologic Function | Phase of Investigation | Evidence for Periodontal Regeneration? |
|---|---|---|---|
| P-15 | Improving cells adhesion | FDA approved | Yes [67] |
| RhPDGF-BB | Chemiotaxis of progenitor’s cells and angiogenesis stimulation | FDA approved | Yes [68] |
| BMP-2 | Osteogenic differentiation | FDA approved | Yes [69] |
| BMP-6 | Osteogenesis enhancer | Preclinical | On dog [70] |
| BMP-12 | Active on ligaments and tendons | Preclinical |
- Biocompatibility: the membrane should not activate an immune response or an acute inflammation, which may worsen the regenerative phase.
- Cell-exclusion: it should act as a barrier and exclude specific types of cells.
- Tissue integration: it should prevent the down-growth of epithelial cells and the encapsulation of the material.
) xenogeneic graft in particles.
3.2. Membranes
Membranes have been used since the introduction of the GTR concept [14][15]. As mentioned earlier, the principal aim is to select and isolate cells that are able to restore the periodontal ligament, cementum, and connective tissue. Guiding cells in the periodontal defect is the primary target and this is possible through the exclusion of epithelial cells, which have a fast turnover compared to osteoblasts and fibroblasts [50]. The ideal membrane should respect several principles:
| Non Resorbable | |||
| Titanium | Mesh with biological hole or completely covered | Regenplate; Ridge—Form Mesh®; Frios® bone shields |
|
| Non Resorbable | Polytetrafluorethylene (PTFE) | Dense PTFE. Expanded PTFE. Dual textured PTFE. Titanium reinforced PTFE. |
Cytoplast® TXT-200 Gore-tex® NeoGen® Gore-Tex® Ti; NeoGen® Ti. Reinforced and Cytoplast Ti-250® |
- Space-making
- :
- it should create and maintain space adjacent to the root surface, allowing the ingrowth of tissue from the periodontal ligament.
- Clinical handling: it should be easy to handle.
There are several membranes on the market (Table 1):
| Resorbable (animal origin) | |||
| Cross Linked | |||
| Cross linked type I collagen | Cross-linked type I and type III |
OsseoGuard® BioMend | |
| On dog | |||
| [ | 64 | ] | |
| rhFGF2 | Fibroblast and endothelial proliferation | FDA approved | Yes [71] |
| OP-1 | Increase mitogenesis and differentiation of osteoblast | Preclinical | On dog [63] |
| SOST antibodies | Antiresorption effect on bone | FDA approved | Yes [72] |
| PTH | Anabolic effect on bone | Clinical | N/A |
-
Biocompatibility: the membrane should not activate an immune response or an acute inflammation, which may worsen the regenerative phase.
-
Cell-exclusion: it should act as a barrier and exclude specific types of cells.
-
Tissue integration: it should prevent the down-growth of epithelial cells and the encapsulation of the material.
-
Space-making: it should create and maintain space adjacent to the root surface, allowing the ingrowth of tissue from the periodontal ligament.
-
Clinical handling: it should be easy to handle.
|
Class |
Material |
Description |
Commercial Name |
|
Non Resorbable |
Titanium |
Mesh with biological hole or completely covered |
Regenplate; Ridge—Form Mesh®; Frios® bone shields |
There are several membranes on the market (Table 1):
Table 1. Classification of membranes for guided tissue regeneration (GTR).
- Non-resorbable membranes are generally indicated in guided bone regeneration (GBR) or in situations of bone deficiency. They are no longer used in periodontal regeneration mainly because the introduction of minimal flaps does not allow the insertion of these unwieldy barriers. Titanium reinforced membranes and polytetrafluorethylene are the most common (Figure 5, Figure 6). Nowadays, new technologies and the introduction of tissue engineering mean that the regenerative process is oriented towards the use of resorbable materials, avoiding the need for a second surgical phase [51,52].
Classification of membranes for guided tissue regeneration (GTR).
| Class | Material | Description | Commercial Name |
|---|---|---|---|
| ® | |||
| ; OSSIX | ® | PLUS | MatrixDerm™; Osseo Guard Flex®; EZCure™ |
| Resorbable (animal origin) | Non-Cross Linked | Type I collagen Type I and III collagen | |
Non Resorbable |
Polytetrafluorethylene (PTFE) |
Dense PTFE. Expanded PTFE. Dual textured PTFE. Titanium reinforced PTFE. |
Cytoplast® TXT-200 Gore-tex® NeoGen® Gore-Tex® Ti; NeoGen® Ti. Reinforced and Cytoplast Ti-250® |
|
Resorbable (animal origin) |
Cross Linked |
Cross linked type I collagen Cross-linked type I and type III |
OsseoGuard® BioMend®; OSSIX®PLUS MatrixDerm™; Osseo Guard Flex®; EZCure™ |
|
Resorbable (animal origin) |
Non-Cross Linked |
Type I collagen Type I and III collagen Collagen with intermingled elastin Type I, III, IV, VI and other proteins |
CollaTape®; Tutodent® BioGide®; botiss Jason® Creos xenoprotect DynaMatrix® |
|
Growth Factors |
Biologic Function |
Phase of Investigation |
Evidence for Periodontal Regeneration? |
|
P-15 |
Improving cells adhesion |
FDA approved |
Yes [67] |
|
RhPDGF-BB |
Chemiotaxis of progenitor’s cells and angiogenesis stimulation |
FDA approved |
Yes [68] |
|
BMP-2 |
Osteogenic differentiation |
FDA approved |
Yes [69] |
|
BMP-6 |
Osteogenesis enhancer |
Preclinical |
On dog [70] |
|
BMP-12 |
Active on ligaments and tendons |
Preclinical |
On dog [64] |
|
rhFGF2 |
Fibroblast and endothelial proliferation |
FDA approved |
Yes [71] |
|
OP-1 |
Increase mitogenesis and differentiation of osteoblast |
Preclinical |
On dog [63] |
|
SOST antibodies |
Antiresorption effect on bone |
FDA approved |
Yes [72] |
|
PTH |
Anabolic effect on bone |
Clinical |
N/A |
P-15: Protein15; RhPDGF-BB: recombinant human platelet-derived growth factor; BMP-2: bone morphogenic protein-2; BMP-6: bone morphogenic protein-6; BMP-12: bone morphogenic protein-12; rhFGF2: recombinant human fibroblast growth factor-2; OP-1: osteogenic protein-1; SOST antibodies: anti-sclerostin antibodies; PTH: parathormone.
3.5. Future Therapies
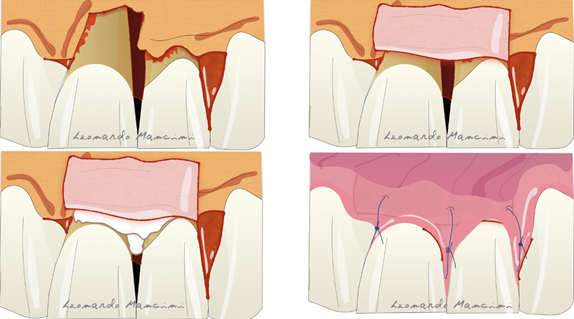
Therapies that are currently under development are based on the use of cells and 3D printing scaffolds [73]. Several articles have suggested the use of cell cultures in periodontal regeneration but their high cost and the need to involve specialist laboratories remain a crucial problem for dentists. Nevertheless, according to Trovato et al., the use of a micrograft (a collagen membrane enriched with stem cells) has potential in the regenerative process [74]. A recent review by Mummolo et al. suggested that micrografts in periodontal and bone regeneration are a good alternative to GTR or to other biomaterials such as bone substitutes [75]. However, they have some limitations due to the availability of progenitor cells in the tissue sample used, and the selection of the right scaffold as a carrier. Another promising therapy is related to the 3D printing technology; indeed, it is starting to enter the oral regenerative field with tailored scaffolds that can be customized for each type of defect. Rasperini et al. showed for the first time the possibility of using a 3D printed resorbable scaffold for a large osseous defect. The material used was a synthetic polymer enriched with recombinant human platelet derived growth factor BB (rhPDGF-BB) [76]. The 3D printing process allows the customization of the biomaterial respect to the defect type achieving a better handling and a tailored treatment. The possible complications reported in this case report were several such as the wound dehiscence, exposure and afterwards microbial contaminations. The most recent technique is the connective tissue graft (CTG) wall technique, introduced to suggest the use of CTG as a barrier in adjunct to EMD reducing gingival recession and in some cases the possible soft tissue restoration [77]. The advantages of this technique are the use of connective tissue as barrier preventing the exposure, reducing the complications and improving the soft tissue response. Moreover, the CTG positioned coronally respect to the defect seems to prevent the soft tissue collapsing. This technique according to the authors is reliable in deep intrabony defects with the absence of buccal bone (Figure 8). A disadvantage is the presence of a second surgical site on the palatal aspect for the CTG sampling and patient discomfort during the healing period.
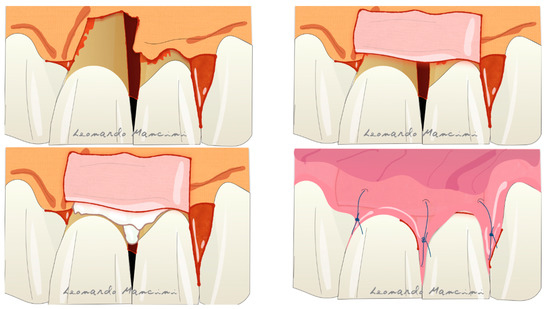
Figure 8. Illustration of the connective tissue graft (CTG) wall technique with the use of connective tissue as barrier and enamel matrix derivatives (EMD) as biologics.
Illustration of the connective tissue graft (CTG) wall technique with the use of connective tissue as barrier and enamel matrix derivatives (EMD) as biologics.
- Conclusions
Periodontal regeneration is an approach involving constant evolution, techniques, and biomaterials that are constantly being improved to achieve better performance. In conclusion, it is a promising approach for periodontally compromised patients.
4. Conclusions
Periodontal regeneration is an approach involving constant evolution, techniques, and biomaterials that are constantly being improved to achieve better performance. In conclusion, it is a promising approach for periodontally compromised patients.
References
- Tonetti, M.S.; Eickholz, P.; Loos, B.G.; Papapanou, P.; van der Velden, U.; Armitage, G.; Bouchard, P.; Deinzer, R.; Dietrich, T.; Hughes, F.; et al. Principles in prevention of periodontal diseases: Consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J. Clin. Periodontol. 2015, 42, S5–S11.
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053.
- Baehni, P.; Tonetti, M.S.; Group 1 of the European Workshop on Periodontology. Conclusions and consensus statements on periodontal health, policy and education in Europe: A call for action--consensus view 1. Consensus report of the 1st European Workshop on Periodontal Education. Eur. J. Dent. Educ. Off. J. Assoc. Dent. Educ. Eur. 2010, 14, 2–3.
- Kinane, D.; Bouchard, P.; On Behalf of Group E of the European Workshop on Periodontology. Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 333–337.
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-analyses. J. Dent. Res. 2021, 100, 37–49.
- Hujoel, P.P. Endpoints in periodontal trials: The need for an evidence-based research approach. Periodontol. 2000 2004, 36, 196–204.
- Claffey, N.; Egelberg, J. Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. J. Clin. Periodontol. 1995, 22, 690–696.
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Brägger, U.; Zwahlen, M.; Lang, N.P. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J. Clin. Periodontol. 2008, 35, 685–695.
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing. An indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721.
- Schätzle, M.; Löe, H.; Lang, N.P.; Bürgin, W.; Anerud, A.; Boysen, H. The clinical course of chronic periodontitis. J. Clin. Periodontol. 2004, 31, 1122–1127.
- Papapanou, P.N.; Wennström, J. The angular bony defect as indicator of further alveolar bone loss. J. Clin. Periodontol. 1991, 18, 317–322.
- Rams, T.E.; Listgarten, M.A.; Slots, J. Radiographic alveolar bone morphology and progressive periodontitis. J. Clin. Periodontol. 2018, 89, 424–430.
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260.
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296.
- Gottlow, J.; Nyman, S.; Lindhe, J.; Karring, T.; Wennström, J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J. Clin. Periodontol. 1986, 13, 604–616.
- Susin, C.; Fiorini, T.; Lee, J.; De Stefano, J.A.; Dickinson, D.P.; Wikesjö, U.M. Wound healing following surgical and regenerative periodontal therapy. Periodontology 2000 2015, 68, 83–98.
- Cortellini, P.; Tonetti, M.S. Clinical and radiographic outcomes of the modified minimally invasive surgical technique with and without regenerative materials: A randomized-controlled trial in intra-bony defects. J. Clin. Periodontol. 2011, 38, 365–373.
- Pagliaro, U.; Cortellini, P.; Nieri, M.; Rotundo, R.; Cairo, F.; Pini-Prato, G.; Carnevale, G.; Esposito, M. Author’s Response: Re: Finkelman RD. Letter to the Editor: Re: “Clinical Guidelines of the Italian Society of Periodontology for the Reconstructive Surgical Treatment of Angular Bony Defects in Periodontal Patients”. J. Clin. Periodontol. 2009, 80, 722.
- Tu, Y.K.; Woolston, A.; Faggion, C.M. Do bone grafts or barrier membranes provide additional treatment effects for infrabony lesions treated with enamel matrix derivatives? A network meta-analysis of randomized-controlled trials. J. Clin. Periodontol. 2010, 37, 59–79.
- Bowers, G.M.; Chadroff, B.; Carnevale, R.; Mellonig, J.; Corio, R.; Emerson, J.; Stevens, M.; Romberg, E. Histologic evaluation of new attachment apparatus formation in humans. Part I. J. Periodontol. 1989, 60, 664–674.
- Cortellini, P.; Pini Prato, G.; Tonetti, M.S. Periodontal regeneration of human infrabony defects. II. Re-entry procedures and bone measures. J. Periodontol. 1993, 64, 261–268.
- Sculean, A.; Donos, N.; Blaes, A.; Lauermann, M.; Reich, E.; Brecx, M. Comparison of enamel matrix proteins and bioabsorbable membranes in the treatment of intrabony periodontal defects. A split-mouth study. J. Periodontol. 1999, 70, 255–262.
- Sanz, M.; Tonetti, M.S.; Zabalegui, I.; Sicilia, A.; Blanco, J.; Rebelo, H.; Rasperini, G.; Merli, M.; Cortellini, P.; Suvan, J.E. Treatment of intrabony defects with enamel matrix proteins or barrier membranes: Results from a multicenter practice-based clinical trial. J. Periodontol. 2004, 75, 726–733.
- Needleman, I.G.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R.J. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst. Rev. 2006, CD001724.
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal regeneration—Intrabony defects: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S77–S104.
- Selvig, K.A.; Kersten, B.G.; Wikesjö, U.M. Surgical treatment of intrabony periodontal defects using expanded polytetrafluoroethylene barrier membranes: Influence of defect configuration on healing response. J. Periodontol. 1993, 64, 730–733.
- De Sanctis, M.; Zucchelli, G.; Clauser, C. Bacterial colonization of bioabsorbable barrier material and periodontal regeneration. J. Periodontol. 1996, 67, 1193–1200.
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The modified papilla preservation technique. A new surgical approach for interproximal regenerative procedures. J. Periodontol. 1995, 66, 261–266.
- Takei, H.H.; Han, T.J.; Carranza, F.A., Jr.; Kenney, E.B.; Lekovic, V. Flap technique for periodontal bone implants. Papilla preservation technique. J. Periodontol. 1985, 56, 204–210.
- Cortellini, P.; Pini Prato, G.; Tonetti, M.S. Periodontal regeneration of human intrabony defects with titanium reinforced membranes. A controlled clinical trial. J. Periodontol. 1995, 66, 797–803.
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int. J. Periodontics Restor. Dent. 1999, 19, 589–599.
- Cortellini, P.; Tonetti, M.S. A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: A novel approach to limit morbidity. J. Clin. Periodontol. 2007, 34, 87–93.
- Cortellini, P.; Tonetti, M.S. Microsurgical approach to periodontal regeneration. Initial evaluation in a case cohort. J. Periodontol. 2001, 72, 559–569.
- Trombelli, L.; Farina, R.; Franceschetti, G. Use of the single flap approach in periodontal reconstructive surgery. Dent. Cadmos. 2007, 8, 15–25.
- Cortellini, P.; Tonetti, M.S. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J. Clin. Periodontol. 2009, 36, 157–163.
- Aslan, S.; Buduneli, N.; Cortellini, P. Entire Papilla Preservation Technique: A Novel Surgical Approach for Regenerative Treatment of Deep and Wide Intrabony Defects. Int. J. Periodontics Restor. Dent. 2017, 37, 227–233.
- Aslan, S.; Buduneli, N.; Cortellini, P. Entire papilla preservation technique in the regenerative treatment of deep intrabony defects: 1-Year results. J. Clin. Periodontol. 2017, 44, 926–932.
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration--a materials perspective. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2012, 28, 703–721.
- Sculean, A.; Stavropoulos, A.; Windisch, P.; Keglevich, T.; Karring, T.; Gera, I. Healing of human intrabony defects following regenerative periodontal therapy with a bovine-derived xenograft and guided tissue regeneration. Clin. Oral Investig. 2004, 8, 70–74.
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontology 2015, 68, 182–216.
- Bosshardt, D.D.; Hjørting-Hansen, E.; Buser, D. The fate of the autogenous bone graft. Forum Implantol. 2009, 5, 4–11.
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontol. 2000 2009, 51, 208–219.
- Windisch, P.; Szendroi-Kiss, D.; Horváth, A.; Suba, Z.; Gera, I.; Sculean, A. Reconstructive periodontal therapy with simultaneous ridge augmentation. A clinical and histological case series report. Clin. Oral Investig. 2008, 12, 257–264.
- Sculean, A.; Windisch, P.; Keglevich, T.; Gera, I. Clinical and histologic evaluation of an enamel matrix protein derivative combined with a bioactive glass for the treatment of intrabony periodontal defects in humans. Int. J. Periodontics Restor. Dent. 2005, 25, 139–147.
- Wang, H.L.; Greenwell, H.; Fiorellini, J.; Giannobile, W.; Offenbacher, S.; Salkin, L.; Townsend, C.; Sheridan, P.; Genco, R.J.; Research, Science and Therapy Committee. Periodontal regeneration. J. Periodontol. 2005, 76, 1601–1622.
- Committee on Research, Science and Therapy of the American Academy of Periodontology. Tissue banking of bone allografts used in periodontal regeneration. J. Periodontol. 2001, 72, 834–838.
- Dragoo, M.R.; Kaldahl, W.B. Clinical and histological evaluation of alloplasts and allografts in regenerative periodontal surgery in humans. Int. J. Periodontics Restor. Dent. 1983, 3, 8–29.
- Karring, T.; Nyman, S.; Gottlow, J.; Laurell, L. Development of the biological concept of guided tissue regeneration--animal and human studies. Periodontology 2000, 1, 26–35.
- Urban, I.A.; Monje, A. Guided Bone Regeneration in Alveolar Bone Reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 331–338.
- Bosshardt, D.D.; Stadlinger, B.; Terheyden, H. Cell-to-cell communication--periodontal regeneration. Clin. Oral Implant. Res. 2015, 26, 229–239.
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25.
- Eickholz, P.; Kim, T.S.; Holle, R.; Hausmann, E. Long-term results of guided tissue regeneration therapy with non-resorbable and bioabsorbable barriers. I. Class II furcations. J. Periodontol. 2001, 72, 35–42.
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11.
- US FDA. What Are “Biologics” Questions and Answers. Available online: www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers (accessed on 31 December 2020).
- Cambini, A.F.T.; Ordesi, P.; Arcara, C.; Caccianiga, G. Rigenerazione tissutale guidata in difetti infraosseimediante innesto di amelogenine. Dent. Clin. N. Am. 2012, 3, 19–26.
- Giannobile, W.V.; Somerman, M.J. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann. Periodontol. 2003, 8, 193–204.
- Heijl, L.; Heden, G.; Svärdström, G.; Ostgren, A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J. Clin. Periodontol. 1997, 24, 705–714.
- Pontoriero, R.; Wennström, J.; Lindhe, J. The use of barrier membranes and enamel matrix proteins in the treatment of angular bone defects. A prospective controlled clinical study. J. Clin. Periodontol. 1999, 26, 833–840.
- Cortellini, P.; Nieri, M.; Prato, G.P.; Tonetti, M.S. Single minimally invasive surgical technique with an enamel matrix derivative to treat multiple adjacent intra-bony defects: Clinical outcomes and patient morbidity. J. Clin. Periodontol. 2008, 35, 605–613.
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683.
- Kaigler, D.; Avila, G.; Wisner-Lynch, L.; Nevins, M.L.; Nevins, M.; Rasperini, G.; Lynch, S.E.; Giannobile, W.V. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin. Biol. Ther. 2011, 11, 375–385.
- Nevins, M.; Giannobile, W.V.; McGuire, M.K.; Kao, R.T.; Mellonig, J.T.; Hinrichs, J.E.; McAllister, B.S.; Murphy, K.S.; McClain, P.K.; Nevins, M.L.; et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J. Periodontol. 2005, 76, 2205–2215.
- Giannobile, W.V.; Ryan, S.; Shih, M.S.; Su, D.L.; Kaplan, P.L.; Chan, T.C. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J. Periodontol. 1998, 69, 129–137.
- Wikesjö, U.M.; Guglielmoni, P.; Promsudthi, A.; Cho, K.S.; Trombelli, L.; Selvig, K.A.; Jin, L.; Wozney, J.M. Periodontal repair in dogs: Effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J. Clin. Periodontol. 1999, 26, 392–400.
- Tarallo, F.; Mancini, L.; Pitzurra, L.; Bizzarro, S.; Tepedino, M.; Marchetti, E. Use of platelet-rich fibrin in the treatment of grade 2 furcation defects: Systematic review and meta-analysis. J. Clin. Med. 2020, 9, 2104.
- Marchetti, E.; Mancini, L.; Bernardi, S.; Bianchi, S.; Cristiano, L.; Torge, D.; Marzo, G.; Macchiarelli, G. Evaluation of different autologous platelet concentrate biomaterials: Morphological and biological comparisons and considerations. Materials 2020, 13, 2282.
- Yukna, R.; Salinas, T.J.; Carr, R.F. Periodontal regeneration following use of ABM/P-1 5: A case report. Int. J. Periodontics Restor. Dent. 2002, 22, 146–155.
- Camelo, M.; Nevins, M.L.; Schenk, R.K.; Lynch, S.E.; Nevins, M. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int. J. Periodontics Restor. Dent. 2003, 23, 213–225.
- Thoma, D.S.; Payer, M.; Jakse, N.; Bienz, S.P.; Hüsler, J.; Schmidlin, P.R.; Jung, U.W.; Hämmerle, C.; Jung, R.E. Randomized, controlled clinical two-centre study using xenogeneic block grafts loaded with recombinant human bone morphogenetic protein-2 or autogenous bone blocks for lateral ridge augmentation. J. Clin. Periodontol. 2018, 45, 265–276.
- Chiu, H.C.; Chiang, C.Y.; Tu, H.P.; Wikesjö, U.M.; Susin, C.; Fu, E. Effects of bone morphogenetic protein-6 on periodontal wound healing/regeneration in supraalveolar periodontal defects in dogs. J. Clin. Periodontol. 2013, 40, 624–630.
- de Santana, R.B.; de Santana, C.M. Human intrabony defect regeneration with rhFGF-2 and hyaluronic acid—A randomized controlled clinical trial. J. Clin. Periodontol. 2015, 42, 658–665.
- Yao, Y.; Kauffmann, F.; Maekawa, S.; Sarment, L.V.; Sugai, J.V.; Schmiedeler, C.A.; Doherty, E.J.; Holdsworth, G.; Kostenuik, P.J.; Giannobile, W.V. Sclerostin antibody stimulates periodontal regeneration in large alveolar bone defects. Sci. Rep. 2020, 10, 16217.
- McGuire, M.K.; Tavelli, L.; Feinberg, S.E.; Rasperini, G.; Zucchelli, G.; Wang, H.L.; Giannobile, W.V. Living cell-based regenerative medicine technologies for periodontal soft tissue augmentation. J. Periodontol. 2020, 91, 155–164.
- Trovato, L.; Naro, F.; D’Aiuto, F.; Moreno, F. Promoting tissue repair by micrograft stem cells delivery. Stem Cells Int. 2020, 2195318.
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® autologous micrografts in oral regeneration: Clinical, histological, and radiographical evaluations. Appl. Sci. 2020, 10, 5084.
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed bioresorbable scaffold for periodontal repair. J. Dent. Res. 2015, 94, 153S–157S.
- Zucchelli, G.; Mounssif, I.; Marzadori, M.; Mazzotti, C.; Felice, P.; Stefanini, M. Connective Tissue Graft Wall Technique and Enamel Matrix Derivative for the Treatment of Infrabony Defects: Case Reports. Int. J. Periodontics Restor. Dent. 2017, 37, 673–681.