Convection enhanced delivery (CED), first described by Bobo et al. in 1994 [10], remains a promising technique for circumventing the Blood Brain Barrier (BBB) and delivering therapy in a non-diffusion dependent manner, thereby facilitating high local concentrations of infusate.
- convection enhanced delivery,glioma
1. Convection Enhanced Delivery—Rationale and Mechanisms
Convection relies on a constant hydraulic force that distributes infusate based on a pressure gradient causing bulk flow through the interstitial space. Utilizing this pressure gradient gives CED two key benefits: A larger volume of distribution and a constant concentration of infusate within the volume of distribution. This is in contrast to non-convective methods with systemic delivery, which are diffusion-dependent and rely on a passive concentration gradient for infusate distribution. This passive flow limits infusate distribution to just a few millimeters from the drug source and requires steep concentration gradients for adequate treatment [16][1].
1.1. Biophysical Properties
CED is achieved using a catheter attached to a hydraulic pump. The catheter is inserted directly into the interstitium of the brain. Infusate is distributed through pressure equalization of the interstitial fluid. Although the different tissue densities of gray or white matter can alter the volume of distribution, the infusate is not bound by these anatomical boundaries. However, the volume of distribution is bound by the pial surface. Unlike non-convective methods where a gradual concentration gradient will occur, infusate delivery is relatively constant across the volume of distribution with a steep drop off outside this volume, which then distributes further by diffusion [10,16][1][2].
Mechanical factors important to CED are viscosity of infusate, infusion flow rate, and volume of infusion. In general, the volume of distribution is proportional to the volume of infusion [10,17,18][2][3][4]. Intrinsic molecular properties including size, polarity, binding to extracellular matrix proteins and or enzymes and export through efflux pumps all contribute to the convection volume and persistence of therapy [10,17,18][2][3][4]. Effective flow rates range between 1 and 10 µL/min as higher flow rates are susceptible to backflow and poor convection.
1.2. CED Logistics
Prior to CED, advanced neuroimaging including computed tomography (CT) and magnetic resonance imaging (MRI) are necessary. MR imaging should at minimum include T1, T2, and T1 post-contrast images, which is especially true if gadolinium or another contrast agent is to be injected with the infusate. Additional methods including susceptibility weighted imaging, diffusion weighted imaging, or T2-gradient echo and BOLD-fMRI are helpful in better characterization of necrosis, edema, and functional neuroanatomic structures. In addition, all can help with catheter trajectory planning [16,19][2][5]. Tumor volume, areas of necrosis, potential flow voids, proximity to ventricles, peritumoral edema, and vasculature or breaches into the ventricular system are all critical variables for determining the catheter tip placement and optimal convection volume [16][2].
Historically, the CED infusion time has spanned a few hours to at most a few days. Most studies have had infusions lasting at maximum 96 h. These infusions have used an externalized catheter connected to an infusion pump. Patients generally remain as inpatients in order to help minimize the risk for infection and to facilitate catheter removal. Recent studies have used catheters attached to transcutaneous ports to allow for the infusion which occurs to outpatients, as no externalized hardware is present [20][6]. Our group has also attempted to address the chronicity but through the use of a subcutaneous implantable pump, as described below [21][7].
1.3. Pitfalls of CED
Two key considerations that impact the efficacy of CED are backflow or reflux and the presence of pathology near the catheter tip. Reflux or backflow of the infusate can alter this relationship and dramatically diminish the volume of distribution [10,16,22][1][2][8]. Backflow occurs when a pocket forms around the catheter due to mechanical shearing during catheter placement or pressure spikes due to the flow rate. Mechanical shearing can be minimized through the use of soft and thin catheters [23][8]. Stepped profile cannulas have been shown to limit the reflux even with high flow rate infusions. This design combines a wide bore cannula with a narrow tip attached by a sharp transition or step [24,25][9][10] . The presence of pressure spikes can be minimized by the use of porous catheters with multiple infusate outlets preventing occlusions [23][8].
While systemic toxicity is greatly reduced, CED can be associated with certain complications. A risk of infection (i.e., bacterial meningitis, subdural empyema, abscess at the catheter tip) is rare but has been reported [26][11]. More common dose limiting toxicities include chemical meningitis or seizure highlighting the need for studies identifying the maximum tolerated dose [27][12].
Factors related to brain pathology also impact distribution patterns with CED. Areas of necrosis with a lack of interstitial architecture can lead to infusate pooling. Highly vascular regions—common in high-grade glioma—can lead to infusate leaking into systemic circulation [28][13]. In a phase I trial of CED of the Delta-24-RGD adenovirus in recurrent GBM patients, the treatment infusion was preceded by gadolinium infusion. These studies demonstrated that the leakage of gadolinium into the CSF was associated with the decreased volume of distribution to volume of infusion ratios. Furthermore, they also showed that intratumoral catheter placement led to the decreased volume of distributions likely due to tumor vasculature and necrosis [29][14]. These considerations are important when combining catheter placement and surgical resection for high grade glioma patients.
2. Convection Enhanced Delivery—The Columbia University Medical Center Experience
2.1. Preclinical Data—Small Animal Models
At our institution, we have repeatedly demonstrated the efficacy of CED in treating orthotopic models of glioblastoma. Our investigational therapies have focused on topoisomerase inhibitors, specifically topotecan (TPT). TPT has a long clinical history demonstrating safety in local and systemic delivery [21,30,31,32,33][15][16][17][18]. The presence of dose limiting systemic toxicities make it an optimal candidate for local delivery. Moreover, as a topoisomerase 1 inhibitor, it is toxic to glioma cells and relatively non-toxic to the normal brain tissue [34][19].
The first TPT CED study involved treating rats with orthotopically implanted C6 glioma cells. These mice were treated with intracerebral infusion of topotecan (TPT) through a CED microcatheter. Both a high dose (160 µg/kg/day) and low dose (32 µg/kg/day) CED of topotecan led to complete tumor regression and cure. Notably one mouse in the high-dose group did die from neurological toxicity [34][19]. This preliminary study showed that TPT CED was safe as animals tolerated the infusion without decreases in weight. More importantly it showed that TPT CED was effective as 11/12 mice became long-term survivors [34][19]. These results were replicated in an orthotopic syngeneic rat model that relies on retroviral infection with a PDGF-IRES-GFP construct. Infected cells overexpress the platelet derived growth factor (PDGF) and reliably form tumors with characteristic features of glioblastoma including endothelial proliferation, pseudopalisading necrosis, and hypercellularity [35][20]. Topotecan at a dose of 136 uM was infused for 1, 4, or 7 days. Compared to the control, all topotecan infusion groups had improved survival. Importantly, rats treated with 7 days of topotecan CED had a 1.7× increase in the median survival over rats treated with 4 days of topotecan CED (Figure 1) [35][20]. The correlation between the prolonged treatment duration and treatment efficacy likely relates to the TPTs mechanism of action. Topotecan is a topoisomerase I inhibitor and thus acts during the S-phase of the cell cycle. The greater duration of treatment ensures that more cells will enter the S-phase and experience the cytotoxic effects of TPT [36][21].
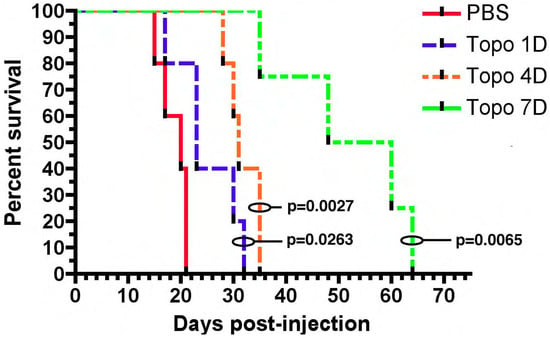
Figure 1. Chronic convection enhanced delivery (CED) of topotecan provides a significant survival advantage. Rats with orthotopic virally-induced, platelet derived growth factor (PDGF) driven tumors were treated with CED of topotecan for various days (1, 4, or 7 days). p-values show a comparison to the PBS control. 1, 4, and 7 days CED survival were each significantly greater than PBS (p < 0.05). The median survival for 7 days CED was significantly greater than the other groups, (median survival: PBS—20 dpi, 1 day—23 dpi, 4 day—31 dpi, 7 day—54 dpi; p < 0.05). The figure is reproduced with permission from [35][20], Cancer Res. 2011.
We have also tested another topoisomerase inhibitor, etoposide, in an orthotopic syngeneic murine model of glioma. This model, which is also driven by PDGF overexpression, shows close transcriptional similarity with human proneural glioma [37,38][22][23]. CED with a high dose (80 µM) etoposide for 7 days led to a significant survival benefit with half of the animals becoming long-term survivors; a low dose treatment (4 µM) led to no survival benefit [37][22]. These findings point to two key factors that drive tumor response: High local concentrations and prolonged duration of treatment.
2.2. Pre-Clinical Data—Large Animal Models
Based on our murine and rodent studies, we hypothesized that prolonged or chronic TPT CED would enhance the therapeutic effect in glioma patients. To this end, we developed a mechanism for an implantable subcutaneous pump (Synchromed II, Medtronic; Minneapolis, MN). We first used a large animal pig model to demonstrate that the subcutaneous pump was safe, and allowed drug distribution for periods ranging from 3–10 days (Figure 2) [21][7]. We were further able to broaden this pig model by expanding the limits of chronic convection enhanced delivery using an implanted pump. We utilized a total of 12 pigs in which we performed CED using an implantable subcutaneous pump with varying convection durations ranging from 4 to 32 days. In both experiments, TPT concentrations of 136 µM were used. Neurobehavioral side effects were measured showing minimal measurable side effects of prolonged treatment.
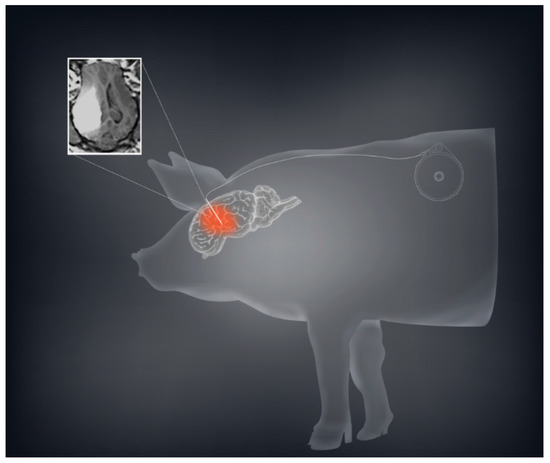
Figure 2. CED using a subcutaneous implantable pump is safe and feasible in a large animal model. A schematic demonstration with the sample MRI of convection volume with co-infusion of topotecan and gadolinium is shown demonstrating a sizeable volume of distribution.
2.3. Gadolinium as a Surrogate for Convection Volumes
The noninvasive methodology for monitoring drug distribution is critical for a successful application of CED in the clinic. To model this in our pig experiments gadodiamide was added to the TPT infusate. For an accurate evaluation of the convection treatment volume, the subcutaneous pump was loaded with a mixture of TPT and gadodiamide. Gadodiamide has a molecular weight of 591.7 g/mol and is freely water soluble, while TPT has a molecular weight of 421.4 g/mol and a solubility in water of 1 mg/mL. Topotecan is a quinolone alkaloid derivative with numerous hexacyclic rings [39,40][24][25]. Even with these different physical properties, our data suggest that TPT and gadodiamide distribute in similar patterns when co-infused using CED. We showed that co-infusion of a contrast agent with topotecan would allow gadolinium signal intensity to act as an accurate surrogate for topotecan concentration (Figure 3) [41][26]. This model also helped determine the time course associated with convection volumes. During the long-term infusions, a maximum contrast enhancement was reached by day 3 or 4. The greatest increase in volume of distribution occurred during the first 48 h. Over the 32 day infusion course, the volume of distribution would decline from the maximum volume until a steady state was achieved. Local anticipated signal hypodensities at the catheter tip were also observed with both changes likely attributable to the local tissue and tumor response to infusion. Again, such radiographic changes were not associated with the neurological deficit or evidence of toxicity [41][26].
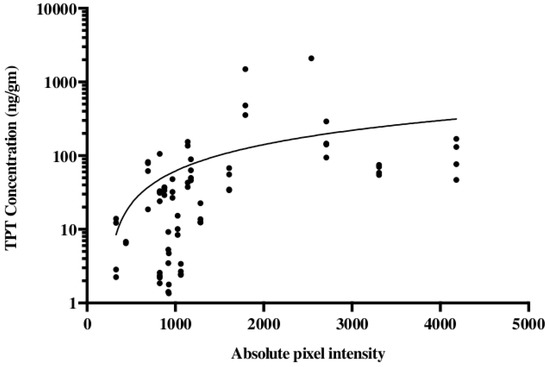
Figure 3. Topotecan concentration is correlated with the gadolinium absolute pixel intensity of T1 MRI. Studies were conducted in a porcine model using a subcutaneous pump that co-delivered topotecan and gadolinium. Liquid chromatography-mass spectrometry was used for the quantification of topotecan concentration and correlated with the absolute pixel intensity from T1-weighted MRI. The data are reproduced from D’Amico et al., 2019 [41][26].
2.4. Clinical Experience with Topotecan
Based on the success of TPT by CED in our animal models, we received FDA approval to conduct a clinical trial in patients with recurrent malignant gliomas. Our initial Phase IB clinical trial studied 10 glioblastoma and six anaplastic astrocytoma patients in a dose escalation study to determine the maximum tolerated dose. Infusions of topotecan continued for 100 h with a flow rate of 200 µL/h. Four dose levels were studied: 0.04, 0.0667, 0.1, and 0.133 mg/mL. We did observe two events of dose limiting toxicity that established the maximum tolerated dose. Eleven out of 16 patients demonstrated either early response or pseudoprogression showing that the infusion of topotecan could lead to tumor specific cell death with minimal adverse events [42][27]. Patients who had an early response or pseudoprogression on MRI had a significantly improved overall survival. GBM patients in this cohort had a 20%, 2-year survival following the treatment. Two patients became long-term survivors with a survival from treatment of over 10 years [42][27]. To date, one patient from the cohort remains alive (Figure 4). This finding coupled with the high percentage of patients with tumor response demonstrated that further optimization of treatment regimens may require chronic dosing of therapies through the CED catheter.
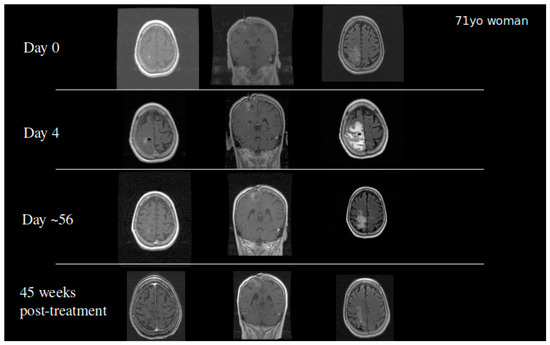
Figure 4. A 71-year-old woman who developed a parietal lobe syndrome on the fourth day of topotecan (TPT) by the CED treatment. Scans are shown at the time of treatment (day 0), at the time the dose limiting toxicity occurred (day 4), 8 weeks after treatment, and 45 weeks after treatment with approximately 90% return to her baseline neurological exam. She was progression free 3 years after the treatment and died just under 4 years post CED.
This patient cohort was evaluated using the HeadMinder Cognitive Stability Index (CSI) and the SF-36 Health Survey (SF-36) at baseline and 4, 8, 12, and 16 weeks post-treatment. These scales tested a host of neurocognitive functions including processing speed, spatial memory, and working memory. The quality of life assessments focused on physical pain, mental health, emotional health, and general vitality. Across both investigatory questionnaires, most patients reported stable or improved scores functioning across these areas at 4 weeks; over 75% of patients reported stable or improved scores at 8 and 16 weeks post-treatment [43][28].
We also explored the use of TPT CED in the treatment of DIPG. Our experience with two pediatric patients showed that the catheter placement and TPT infusion is technically feasible. We also observed that high infusion rates and high infusion volumes were associated with functional decline. This finding highlights the need for chronic treatment schedules for minimization of potential harms [44][29].
2.5. Current Chronic CED Clinical Studies
Based on our preclinical data showing that the efficacy of TPT with CED in gliomas improves with the increased treatment duration, we designed a clinical protocol that utilizes the implanted pump strategy validated in our pig model. We received FDA approval to conduct a Phase IB clinical trial in five human GBM patients, using chronic convection enhanced delivery of topotecan through an implantable and programmable subcutaneous pump (Synchromed II, Medtronic; Minneapolis). There were multiple goals of this trial. Primarily, we wanted to prove that chronic convection enhanced delivery via an implantable pump is a safe technique for drug delivery in humans. Furthermore, we wanted to validate the use of gadolinium as a surrogate marker for topotecan drug concentration when co-infused through the convection enhanced delivery catheter. Finally, we wanted to utilize our expertise with MRI-localized biopsies to get both histologic and molecular based analyses of pre-treatment and post-treatment samples to understand the various effects drug infusion can have on the tumor and tumor microenvironment. The full schematic of the clinical trial can be found in Figure 5. The trial was recently concluded and the results are currently being analyzed.
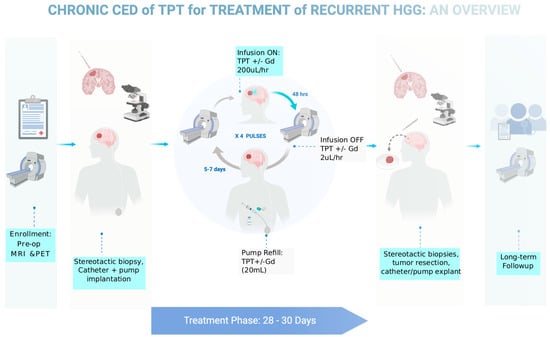
Figure 5. Diagram outlining the clinical trial parameters for a recently completed Phase IB trial for chronic CED of topotecan in patients with recurrent glioblastoma.
2.6. Best Practices
Our experience has led us to develop a set of best practices for CED clinical trials. Initially, compounds of interest need to be tested in a large animal model prior to initiation of the clinical trial. The most important reason for this is to validate the drug distribution parameters and their correlation with radiological findings such as gadolinium enhancement while proving safety. Newer techniques such as MR spectroscopy or PET imaging may also be important to validate for different classes of therapeutic molecules [45,46,47][30][31][32]. Neurobehavioral testing including performance outcomes are critical for determining dose limiting toxicities associated with CED. Finally, evaluation of the chronicity of treatment should be undertaken in relevant preclinical models to help guide the creation of optimal trial guidelines.
References
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Souweidane, M.M. Editorial: Convection-enhanced delivery for diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2014, 13, 273–275. [Google Scholar] [CrossRef]
- Ung, T.H.; Malone, H.; Canoll, P.; Bruce, J.N. Convection-enhanced delivery for glioblastoma: Targeted delivery of antitumor therapeutics. CNS Oncol. 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Mohammed, W.; Hong, X.N.; Haibin, S.; Jingzhi, M. Clinical applications of susceptibility-weighted imaging in detecting and grading intracranial gliomas: A review. Cancer Imaging 2013, 13, 186–195. [Google Scholar] [CrossRef]
- Barua, N.U.; Hopkins, K.; Woolley, M.; O’Sullivan, S.; Harrison, R.; Edwards, R.J.; Bienemann, A.S.; Wyatt, M.J.; Arshad, A.; Gill, S.S. A novel implantable catheter system with transcutaneous port for intermittent convection-enhanced delivery of carboplatin for recurrent glioblastoma. Drug Deliv. 2014, 23, 167–173. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Stuart, R.M.; Yun, J.; Yanagihara, T.; Mohajed, H.; Dashnaw, S.; Bruce, S.S.; Brown, T.; Romanov, A.; Sebastian, M.; et al. Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro-Oncology 2011, 13, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-Enhanced Delivery for Diffuse Intrinsic Pontine Glioma Treatment. Curr. Neuropharmacol. 2016, 15, 116–128. [Google Scholar] [CrossRef]
- Sillay, K.A.; McClatchy, S.G.; Shepherd, B.A.; Venable, G.T.; Fuehrer, T.S. Image-guided Convection-enhanced Delivery into Agarose Gel Models of the Brain. J. Vis. Exp. 2014, e51466. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Forsayeth, J.; Bankiewicz, K.S. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J. Neurosci. Methods 2010, 187, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lewis, O.; Woolley, M.; Johnson, D.; Rosser, A.E.; Barua, N.U.; Bienemann, A.S.; Gill, S.S.; Evans, S.L. Chronic, intermittent convection-enhanced delivery devices. J. Neurosci. Methods 2016, 259, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A Phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sanche, L. Convection-Enhanced Delivery in Malignant Gliomas: A Review of Toxicity and Efficacy. J. Oncol. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Van Putten, E.H.; Wembacher-Schröder, E.; Smits, M.; Dirven, C.M. Magnetic Resonance Imaging–Based Assessment of Gadolinium-Conjugated Diethylenetriamine Penta-Acetic Acid Test-Infusion in Detecting Dysfunction of Convection-Enhanced Delivery Catheters. World Neurosurg. 2016, 89, 272–279. [Google Scholar] [CrossRef]
- Bruce, J.N.; Falavigna, A.; Johnson, J.P.; Hall, J.S.; Birch, B.D.; Yoon, J.T.; Wu, E.X.; Fine, R.L.; Parsa, A.T. Intracerebral clysis in a rat glioma model. Neurosurgery 2000, 46, 683–691. [Google Scholar] [CrossRef]
- Yamashita, Y.; Krauze, M.T.; Kawaguchi, T.; Noble, C.O.; Drummond, D.C.; Park, J.W.; Bankiewicz, K.S. Convection-enhanced delivery of a topoisomerase I inhibitor (nanoliposomal topotecan) and a topoisomerase II inhibitor (pegylated liposomal doxorubicin) in intracranial brain tumor xenografts1. Neuro-Oncology 2007, 9, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.B.; Vasilyeva, A.; Gajjar, A.; Santana, V.M.; Stewart, C.F. Determining success rates of the current pharmacokinetically guided dosing approach of topotecan in pediatric oncology patients. Pediatr. Blood Cancer 2018, 66, e27578. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.B.; Goldstein, S.C.; Dawson, J.L.; Kim, J.; Field, T.L.; Partyka, J.S.; Fields, K.K.; Maddox, B.L.; Simonelli, C.E.; Neuger, A.M.; et al. Phase I Study of Topotecan, Ifosfamide, and Etoposide (TIME) with Autologous Stem Cell Transplant in Refractory Cancer: Pharmacokinetic and Pharmacodynamic Correlates. Clin. Cancer Res. 2011, 17, 7743–7753. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.G.; Parsa, A.T.; Fine, R.L.; Hall, J.S.; Chakrabarti, I.; Bruce, J.N. Tissue distribution and antitumor activity of topotecan delivered by intracerebral clysis in a rat glioma model. Neurosurgery 2000, 47, 1391–1398; discussion 1398–1399. [Google Scholar] [CrossRef]
- Lopez, K.A.; Tannenbaum, A.M.; Assanah, M.C.; Linskey, K.; Yun, J.; Kangarlu, A.; Gil, O.D.; Canoll, P.; Bruce, J.N. Convection-Enhanced Delivery of Topotecan into a PDGF-Driven Model of Glioblastoma Prolongs Survival and Ablates Both Tumor-Initiating Cells and Recruited Glial Progenitors. Cancer Res. 2011, 71, 3963–3971. [Google Scholar] [CrossRef]
- Mei, C.; Lei, L.; Tan, L.-M.; Xu, X.-J.; He, B.-M.; Luo, C.; Yin, J.-Y.; Li, X.; Zhang, W.; Zhou, H.-H.; et al. The role of single strand break repair pathways in cellular responses to camptothecin induced DNA damage. Biomed. Pharmacother. 2020, 125, 109875. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Carminucci, A.S.; Amendolara, B.; Bansal, M.; Leung, R.; Lei, L.; Realubit, R.; Li, H.; Karan, C.; Yun, J.; et al. Convection-enhanced delivery of etoposide is effective against murine proneural glioblastoma. Neuro-Oncology 2014, 16, 1210–1219. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Bansal, M.; Guarnieri, P.; Lei, L.; Amendolara, B.; Soderquist, C.; Leung, R.; Yun, J.; Kennedy, B.; Sisti, J.; et al. The transcriptional regulatory network of proneural glioma determines the genetic alterations selected during tumor progression. Cancer Res. 2014, 74, 1440–1451. [Google Scholar] [CrossRef]
- PubChem. Gadodiamide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gadodiamide (accessed on 18 December 2020).
- PubChem. Topotecan. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/topotecan (accessed on 8 December 2020).
- D’Amico, R.S.; Neira, J.A.; Yun, J.; Alexiades, N.G.; Banu, M.; Englander, Z.K.; Kennedy, B.C.; Ung, T.H.; Rothrock, R.J.; Romanov, A.; et al. Validation of an effective implantable pump-infusion system for chronic convection-enhanced delivery of intracerebral topotecan in a large animal model. J. Neurosurg. 2020, 133, 614–623. [Google Scholar] [CrossRef]
- Bruce, J.N.; Fine, R.L.; Canoll, P.; Yun, J.; Kennedy, B.C.; Rosenfeld, S.S.; Sands, S.A.; Surapaneni, K.; Lai, R.; Yanes, C.L.; et al. Regression of Recurrent Malignant Gliomas with Convection-Enhanced Delivery of Topotecan. Neurosurgery 2011, 69, 1272–1279; discussion 1279–1280. [Google Scholar] [CrossRef]
- Oberg, J.A.; Dave, A.N.; Bruce, J.N.; Sands, S.A. Neurocognitive functioning and quality of life in patients with recurrent malignant gliomas treated on a phase Ib trial evaluating topotecan by convection-enhanced delivery. Neuro-Oncol. Pract. 2014, 1, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.E.; Kennedy, B.; Yanes, C.L.; Garvin, J.; Needle, M.; Canoll, P.; Feldstein, N.A.; Bruce, J.N. Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J. Neurosurg. Pediatr. 2013, 11, 289–295. [Google Scholar] [CrossRef]
- Pöpperl, G.; Goldbrunner, R.; Gildehaus, F.J.; Kreth, F.W.; Tanner, P.; Holtmannspötter, M.; Tonn, J.C.; Tatsch, K. O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zhou, Z.; Tisnado, J.; Haque, S.; Peck, K.K.; Young, R.J.; Tsiouris, A.J.; Thakur, S.B.; Souweidane, M.M. A novel magnetic resonance imaging segmentation technique for determining diffuse intrinsic pontine glioma tumor volume. J. Neurosurg. Pediatr. 2016, 18, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Guisado, D.I.; Singh, R.; Minkowitz, S.; Zhou, Z.; Haque, S.; Peck, K.K.; Young, R.J.; Tsiouris, A.J.; Souweidane, M.M.; Thakur, S.B. A Novel Methodology for Applying Multivoxel MR Spectroscopy to Evaluate Convection-Enhanced Drug Delivery in Diffuse Intrinsic Pontine Gliomas. Am. J. Neuroradiol. 2016, 37, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
