Mast cells (MCs) are well-known as key effector cells of type I allergic reactions, commonly named anaphylactic responses.
- mast cell
- adaptive immunity
1. Introduction
Mast cells (MCs) are well-known as key effector cells of type I allergic reactions, commonly named anaphylactic responses. In this case, MCs are activated by the crosslinking of cell-surface-bound FcεRI-IgE complexes by a specific antigen, which results in a three-step-response: (a) The immediate degranulation of MC secretory granules; (b) the release of lipid mediators (including thromboxanes, prostaglandins, and leukotrienes); and (c) the secretion of a wide spectrum of de novo synthesized mediators (including cytokines, chemokines, and growth factors) [1][2][3][1,2,3]. However, MCs are also equipped with a spectrum of surface receptors allowing the sensing of various pathogen-associated patterns (PAMPS), danger-associated molecular patterns (DAMPS), cytokines, chemokines, neuropeptides, and others [1][3][4][5][6][7][1,3,4,5,6,7]. Moreover, the mode of ligand-receptor-based MC activation and downstream signaling determines the mode of MC action, which can consist of the full three-step-response, but can also solely involve de novo synthesized mediator release, without degranulation [6][8][6,8]. Based on this spectrum of sensing capacities, and the ability to deploy specific responses, there is increasing evidence that MCs critically contribute to innate host defense against pathogens. Additionally, MCs influence the induction, amplitude, and function of the adaptive arm of the immune defense, either by direct effects on T cells or indirectly, by modifying the properties of antigen-presenting cells (APCs) [6][9][6,9]. Importantly, MCs even modulate lymph node-borne adaptive responses remotely from the periphery. In this review, we provide a summary of recent findings that explain how MCs act as a link between the innate and adaptive immune response, all the way from sensing invading pathogens, dangerous situations, and allergens to orchestrating the final outcome of the immune reaction.
2. Innate MC Functions in Peripheral Tissues Fostering Adaptive Responses
MCs are tissue-resident myeloid cells, populating, at a high density, tissues lining the interface to the environment, such as skin, lung, and intestinal epithelium, and are also found in lower cell numbers in organ-defining barriers of the lymph nodes (LN), spleen, kidney, bone marrow (BM), and brain [3][10][3,10]. Due to their strategic positioning, MCs critically contribute to the first line of host defense against invading pathogens [4][11][12][13][4,11,12,13]. MCs are equipped with a wide array of pattern recognition receptors to identify invading pathogens, including Toll-like receptors (TLR), Fc receptors, and complement receptors [4][5][6][7][11][12][4,5,6,7,11,12]. Importantly, MCs have also been reported as sensors of cell stress and tissue damage, through alarmin and purinergic receptors [4][13][14][15][16][4,13,14,15,16]. Finally, MCs can be activated or modulated by binding cytokines, growth factors like stem cell factor (SCF), chemokines, and neuropeptides [14][15][16][14,15,16].
A unique characteristic of MCs is the high number of intracellular secretory granules, which in turn each contain a plethora of preformed mediators, such as histamine, proteases, cytokines, and chemokines [8]. An MC granule consists of a proteoglycan scaffold, in which the mediators are embedded based on electrostatic interactions [8][17][8,17]. Connective tissue-type murine MCs utilize heparin as the dominant proteoglycan, which allows for the detection of MC granules by metachromatic staining with Giemsa or Toluidine-blue, as well as by fluorochrome-conjugated avidin. In comparison, mucosal-type MCs in the lung and intestinal epithelial layers contain chondroitin sulfate-based granules [8][17][8,17]. Upon IgE/FcεRI crosslinking by a specific antigen or other stimuli, MCs release these secretory granules within only seconds to minutes, in a process called degranulation [1][4][1,4].
Because they can immediately degranulate, MCs respond to invading pathogens or cell stress faster than other tissue-resident immune cells and therefore, in many cases, are the initiators of immune responses. Whenever an inflammatory insult is causing MC degranulation, the immediate release of histamine triggers vascular responses, in particular vasodilatation and vessel permeabilization, within only minutes, finally leading to tissue edema [12][18][19][20][21][22][12,18,19,20,21,22]. The effect of histamine on endothelial cell activation and vascular barrier disintegration is potentiated by the MC release of tumor necrosis factor (TNF) [23][24][25] [23,24,25] and proteases [26][27][28][29][26,27,28,29], as well as the rapid production of lipid mediators [30][31][30,31]. Complementing the vascular effects, MCs are also critical initiators of neutrophil recruitment, for example, during sepsis and peritonitis [26][32][33][34][35][26,32,33,34,35], upon lipopolysaccharide (LPS)-induced lung inflammation [36], as well as at sites of skin inflammation [18][37][38][39][18,37,38,39] and bone fracture [40] [40] and in areas of arteriogenesis [41] [41] and atherosclerotic plaque progression [42][43][42,43]. More specifically, MCs contribute to early neutrophil recruitment by the release of the neutrophil chemoattractants CXCL-1 (KC) and CXCL-2 (MIP-2), in addition to vascular effects [34][38][44][45][34,38,44,45]. Importantly, we have recently demonstrated in a model of contact hypersensitivity (CHS) that MCs degranulate directionally into the blood stream and thereby infuse TNF that primes circulating neutrophils for efficient extravasation [46]. Beside their boosting effect on neutrophil influx, MCs were also reported to enhance neutrophil effector functions [47][48][47,48]. In addition, MCs are a potent source of eosinophil-attracting chemokines (eotaxins) and, via histamine, inducers of eotaxin release by endothelial cells. Thereby, MCs are key drivers of eosinophil recruitment and have been shown to interact with eosinophils [49][50][51][49,50,51].
The MC-mediated vessel permeability and subsequent edema formation further support the recruitment of adaptive immune effector cells to the site of infection or inflammation. Indeed, by blocking the activity of MC-released histamine on the vasculature, subsequent T-cell-driven adaptive immune responses were severely impaired [18]. Furthermore, the edema-related relaxation of connective tissue is important for dendritic cell (DC) motility at the site of infection/inflammation and their subsequent migration toward the draining LNs (DLNs), in order to induce antigen-specific immune responses. Moreover, Weber et al. showed that, in CHS, (MC-initiated) neutrophil influx is required for an efficient activation and migration of DCs and hapten-specific T-cell priming, and consequently, for sensitizing efficiency of the hapten [37]. Neutrophil-released mediators with alarmin activity promote DC recruitment to sites of inflammation/infection and their maturation, thereby augmenting innate and adaptive immunity (reviewed in [52]). In contrast, neutrophil extracellular traps (NETs) and cathelicidins can downregulate LPS-induced DC activation and the T-cell priming capacity, in part by neutralizing LPS [53][54][55][56][53,54,55,56].
3. MC Functions in LN Conditioning and Hypertrophy
Besides local vasoactivation and edema formation, peripheral MCs support APC and lymphocyte influx in DLNs by exerting remote effects (Figure 1). Increased TNF levels have been detected in prenodal lymph and DLNs [57][58][59][57,58,59], as early as one hour after peripheral MC activation. Indeed, McLachlan et al. showed that, upon intradermal bacterial challenge, peripheral MC-derived TNF is the main driver of DLN hypertrophy and the recruitment of circulating T cells. In addition, MCs play a pivotal role in TNF-independent, but complement-regulated, LN hypertrophy and Langerhans cell mobilization, following intradermal peptidoglycan injection [56]. Moreover, Anopheles mosquito bite-induced dermal MC degranulation was not only shown to lead to local inflammation and neutrophil influx, but also to be required for T-cell and DC recruitment to the DLN, which is a prerequisite for T- and B-cell priming [60]. The mechanisms that underlie peripheral MC long-distance effects on DLNs and facilitate LN hypertrophy and circulating lymphocyte influx have barely been examined, but might be related to MC mediator drainage. Gashev and colleagues showed that, in rats, MCs reside close to mesenteric lymphatic vessels (MLVs) and direct the recruitment of MHC class II-positive cells [61][62][61,62]. The histamine release of perilymphatic MCs impacts the lymphatic microenvironment in an NFκB-dependent manner [63][64][63,64]. Importantly, the perilymphatic mesenteric MCs directly regulate themselves via histamine receptors in an autocrine loop, which is essential for acute inflammation-induced trafficking of MHC class II-expressing leukocytes [65]. Given the significant distance between the inflamed peripheral site and the DLN, it is still unclear how peripheral MC-derived cytokines, such as TNF, can reach the LN without being degraded or diluted to ineffective concentrations, particularly considering the short half-life period of TNF in vivo [66]. The remote effect of MC-derived TNF may be explained by its storage in the proteoglycan-backbone of the secretory granules. Importantly, we and others were able to visualize in vivo that the secretory granules are released by peripheral MCs in an intact and stable form [8][67][68][8,67,68]. Mediators such as histamine that are not highly charged rapidly diffuse from the proteoglycan matrix upon MC granule secretion to the extracellular fluid. In contrast, other mediators, such as MC proteases and TNF, are released slowly and sequentially from the secreted granules, which may enhance their activity and prolong their presence in the extracellular tissue [68][69][70][68,69,70]. Kunder et al. reported that, upon the topical application of phorbol-acetate-myristate (PMA), resulting in peripheral MC degranulation, some of the MC granules can enter the lymphatics and drain to local LNs, while no degranulation of LN-resident MCs was detected [68]. Furthermore, the authors demonstrated that the drained granules, carrying TNF, could efficiently elicit profound LN hypertrophy (Figure 1). Due to this adjuvant effect of MC granules, the same group modeled synthetic carbohydrate-backbone particles with encapsulated inflammatory mediators and showed their efficiency in enhancing adaptive immune responses upon influenza virus hemagglutinin vaccination [71].
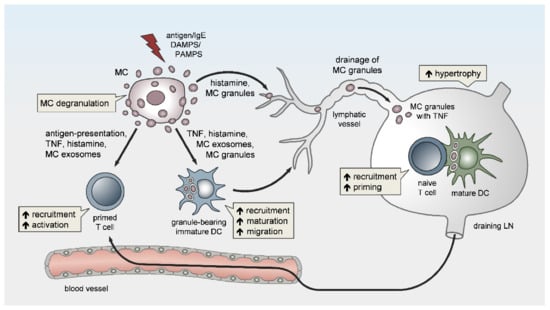
Figure 1. Peripheral mast cells (MCs) orchestrate the induction and amplitude of local innate responses and distant lymph node-borne adaptive immunity. The sensing of pathogens or danger-associated patterns by MCs or MC activation by IgE crosslinking in the periphery may result in MC degranulation and/or the de novo synthesis of pro-inflammatory mediators. Peripheral MCs exert remote effects on lymph node (LN) hypertrophy via histamine, TNF, and the drainage of intact MC secretory granules. The migration, maturation, and antigen-presenting capacity of dendritic cells (DCs) is promoted by MC soluble mediators, secretory granules, and exosomes, thereby facilitating T-cell expansion in draining LNs (DLNs). Finally, MCs enhance the homing of effector T cells to peripheral sites of inflammation/infection and may contribute to effector T-cell activation.
4. MCs Affect Adaptive Immunity via the Modulation of Dendritic Cells
Beside the effect on LN conditioning and hypertrophy, MCs are indirectly implicated in LN-borne adaptive immune responses via the modulation of DC functions (Figure 1). In peripheral tissues, and particularly those lining the interface to the environment such as the skin, MCs reside in a dense network of tissue-resident innate immune cells and are involved in a variety of intercellular interactions [72][73][72,73]. We have previously shown that MCs and macrophages (Mph) cooperate in initiating the recruitment of neutrophils in a model of LPS-induced peritonitis [34]. However, despite their close proximity, the interaction between MCs and Mph and its impact on the recruitment and activity of effector T cells remains elusive. Several studies have reported intense communication between MCs and DCs and the MC-driven modulation of DC migration, maturation, and function, thereby linking MCs to adaptive responses [73][74][73,74]. On one hand, peripheral MC activation is critical for the recruitment of additional DCs to sites of bacterial infection and protective immunity [75]. On the other hand, MCs promote DC migration from the skin to the DLN after IgE-mediated activation [76][77][76,77], and in response to bacteria [75] or bacterial products [59][78][59,78].
In terms of CHS, in a mouse model of T-cell-driven disease allergic contact dermatitis, we found that, upon hapten sensitization, MCs promote DC migration to skin-DLNs and DC maturation, and thereby critically enhance T-cell expansion [18]. Consequently, the expansion of both CD4+ and CD8+ T cells in skin-DLNs, and the T-cell-triggered adaptive skin inflammation upon hapten challenge, were markedly reduced in the absence of MCs [18]. In particular, peripheral TNF release by MCs is required for the efficient initiation of skin and airway DC migration to DLNs [78][79][80][78,79,80] (Figure 2A). Using MC-specific TNF knockout, we could show in vivo that MC-derived TNF predominantly targets cDC1 migration and priming capacity upon hapten sensitization, thereby promoting CD8+ effector T-cell responses [80].
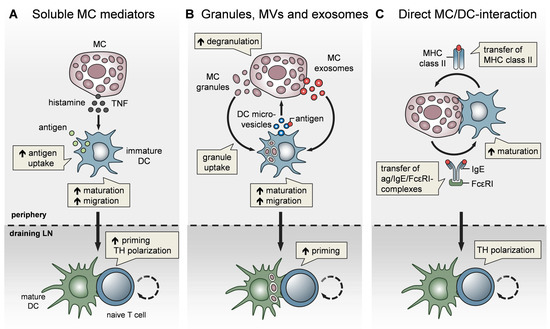
Figure 2. MCs impact T-cell activation by modulating DC functionality. MCs communicate with DCs in three different modes. (A) Soluble MC mediators, in particular histamine and TNF, promote the migration, maturation, and antigen-presenting capacity of DCs, thereby enhancing T-cell priming and fine-tuning TH cell polarization. (B) MC exosomes and intact MC secretory granules, engulfed by DCs upon MC degranulation, facilitate DC migration and maturation, and consequently, boost T-cell priming. In turn, DCs relay antigen to MCs via extracellular microvesicles and thereby induce MC degranulation (C) MCs and DCs undergo dynamic physical interactions and synapse formation allowing bidirectional exchange. MCs transfer endocytosed antigen-IgE-FcεRI complexes to DCs, facilitating the activation of allergen-specific T cells. In turn, MCs are “cross-dressed” by DCs with MHCII complexes, thereby enabling the activation of effector T cells by MCs with antigen processed by DCs.
In addition to their effect on DC migration, MCs have been reported to enhance DC maturation, antigen processing, and T-cell priming capacities. Specifically, histamine promotes DC maturation [81], antigen uptake, and cross-presentation [82] [82] and regulates the DC cytokine response, thereby polarizing T cells toward a Th2 phenotype [83]. In line with this, IgE-stimulated MCs control the Th1/Th2 balance by promoting Th2-generating DCs [84][85] [84,85] (Figure 2A). Importantly, MCs exert effects on DC functionality not only by soluble mediators, but also via the secretory MC granules, MC-derived exosomes, and physical contact (Figure 2). By means of MC granule staining in vivo, directly inside the MCs, and intravital 2-photon-microscopy, we could monitor MC degranulation and track the fate of MC granules after their exocytosis. We found that, upon skin inflammation, dermal DCs accumulate at the site of MC degranulation, engulf the intact MC granules, and actively shuttle them to skin-DLNs [67]. This MC granule uptake facilitates DC migration and maturation, and boosts their T-cell priming capacity (Figure 2B). The cDC1 subpopulation was the most efficient in MC granule uptake in a partially TNF-dependent manner. Importantly, the intradermal (i.d.) injection of MC granules into MC-deficient mice was able to induce a profound expansion of T cells, indicating their adjuvant effect. Extending the finding of Kunder et al. [68], we provided evidence that MC degranulation in the periphery may exert long-distance effects on LN-borne adaptive T-cell responses in two ways: (a) The trafficking of MC granules via lymphatic vessels towards the DLNs, and (b) the active shuttling of MC granules by DCs along with DC-modulating effects [67].
In addition to MC granules, MC-derived exosomes offer an additional mechanism for intercellular communication, by having a greater stability in the interstitial space compared to soluble mediators [72][86] [72,86] and being able to promote DC maturation and the antigen-presenting capacity [87][88] [87,88] (Figure 2B). Importantly, DCs may also communicate with MCs and vice versa, via extracellular microvesicles. Choi et al. recently demonstrated that CD301b+ perivascular DCs sample the blood and relay blood antigens to neighboring perivascular MCs, which can subsequently result in antigen/IgE-crosslinking-induced MC degranulation and an anaphylactic response [89].
Confirming our findings on CHS, Otsuka et al. showed impaired skin DC maturation and migration in the absence of MCs and highlighted the relevance of a direct interaction between DCs and MCs, leading to an upregulation of membrane-bound TNF by MCs [90]. Given the close proximity of MCs and DCs in peripheral tissues, especially in the skin, a physical cell-to-cell interaction was considered likely and studied in several in vitro studies (Figure 2C). Non-activated peritoneal MCs, resembling connective tissue-type skin MCs, underwent direct crosstalk with immature DCs, inducing DC maturation and CD4+ T-cell polarization toward Th1 and Th17 responses [91]. Upon FcεRI crosslinking, MCs have been shown to form immunological synapses with DCs, enabling the transfer of endocytosed antigens from MCs to DCs to activate T cells [92] [92] (Figure 2C). However, there is still little in vivo evidence for a functional relevance of the MC/DC cell-to-cell interaction. In a recent study, using intravital 2-photon-microscopy of MC/DC double reporter mice, we demonstrated in vivo, for the first time, that upon skin inflammation, MCs and DCs rapidly undergo a highly dynamic interaction, which evolves to long-term synapse formation [93]. This MC/DC communication culminates in a protein exchange from DCs to MCs, including MHC class II complexes. Intriguingly, the DC “cross-dressing” of MCs with functionally active MHC class II complexes equipped the MCs with an antigen-presenting capacity, which subsequently enhanced T-cell-driven skin inflammation (Figure 2C). This MC bestowal with antigen-presenting capacity, particularly antigens that have been engulfed and processed by DCs before leaving the peripheral tissue towards the DLN, suggests a role for MCs in activating effector T cells that enter the peripheral tissue [93].
