HematogenouThis tumor metastasis begins with the invasion and spread of primary tumor cells in the local tissue leading to intravasationwork addresses how the migration of cancer cell line MDA-MB-231 cells is regulated. Directional migration of primary cancer cells toward intratumor blood/lymphatic vessels should elevate the probability for intravasation and ultimate hematogenous metastasis. Many presume, on the analogy of chemotaxis by specific chemoattractants, that concentration gradients of energy substrates/metabolites in tumor tissue would be a guiding cue for directional cell migration, whereas strong experimental evidence is scarce at present. Here In this study, using a novel microfluidic device, we clearly demonstrated that the gradient of extracellular pH is a cue for directional migration of MDA-MB-231 cells in vitro. Much smaller pH gradien ts compared to those found in Na+/H+ exchanger-driven cell mitrogration were sufficient to guide the cell. This study answers a longstanding and important question regarding the regulation of cancer cell migration and therefore relevant from the standpoint of not only cell physiology but also clinical sciences, particularly in cancer biology.
- metastasis
- cell migration
- pH gradient
- oxygen gradient
- MDA-MB-231 cells
- pH gradients
- oxygen gradients
1. Introduction
Extracellular Gradients of Oxygen Concentration under GCG
We successfully visualized the oxygen concentration gradient under GCG. As shown in Figure 1A, immediately after placing the GCG, the oxygen concentration along the oxygen diffusion path was constant up to 1200 µm inside the GCG. Subsequently, the oxygen concentration gradients developed slowly and, at six hours after placement of the GCG, a −6.5% O2/mm linear gradient was established under GCG. The oxygen concentration gradient abolished after treatment with a mitochondrial respiratory complex III inhibitor (antimycin A, 2 µM), indicating that the oxygen concentration gradient depends upon mitochondrial respiration (Figure 1B). Magnitude of the oxygen concentration gradient increased in proportion to the number of cells per unit volume (Figure 1C). In our culture conditions, 100% confluence corresponded to the cell density of ~5.5 × 106 cells/mL and, therefore, the largest magnitude of the oxygen concentration gradient attainable in our GCG system was ~6% O2/mm. It is notable that the oxygen concentration at the entrance of the GCG was substantially lower than the air level. This indicates the presence of an oxygen diffusion barrier in the extracellular medium as suggested previously by Metzen et al. [1].
The

Figure 1. presence(A). of mRetastasis is an important prognostic factor in the majority of tumor patients. Distant metastasis begins with the intravasation of primary tumor cells into intratumoral blood and lymphatic vessels. Local tumor cell proliferations elevate hydrostatic pressure in the interstitium, which tends to push tumor cells into microvessels. Tumor angiogenesis and lymphangiogenesis further enhance passive intravasation. In addition to passive cell movement, active cell migrations guided by chemokines are also occurring, in which cancer-associated fibroblasts play a role [1][2][3].
Cpresentative data indicating changes in oxygen concentration in the extracellular medium alonsidering the analogy of chemotaxis, where component movement depends on the concentration gradient of specific chemoattractants, it has long been postulated that the gradients of nutrients and metabolic waste might guide tumor cell migration. This prediction is reinforced given the metabolic characteristics peculiar to solid tumors. In solid tumors, the structures of blood vessels are immature and their topological configuration in tissues is generally irregular [4]. As a roxygen diffusion path as measured by the oxygen sensor foil. The ratio of red and green fluorescence is represult, the convectional and diffusional transports of substances between individual cells and blood are considerably hindered and steep concentration gradients of nutrients and metabolic wastes are established within the tissue [4][5]nted in pseudo-color. Although <0.1% of primary tumor cells succeed in intravasation in the local tissue [6], dire 10 min after plactional cell migration guided by these metabolic gradients, if any, could elevate the probability of intravasation and, ultimately, hematogenous metastasis. However, evidence indicating the status of extracellular nutrient/metabolite ng the gap cover glass (GCG), no gradients as guiding cues for cancer cell migration is scarce at the present time.
Given th was demonstrated unde above, our initial hypothesis is that the metabolic gradients in the tissue might be a cue for guiding the tumor cells to nearby blood vessels. Because acidosis and hypoxia are the hallmark of an invasive hypoxic tuneath. At six hours after placing the GCG, a −6.2% O2/mor [7][8], we focused, among numerous cues in tissues [9], on H+ and oxygen particularly their concentration gradients. Hereafter, we refer to the gradient of pH and onear oxygen concentration as metabolic gradients.
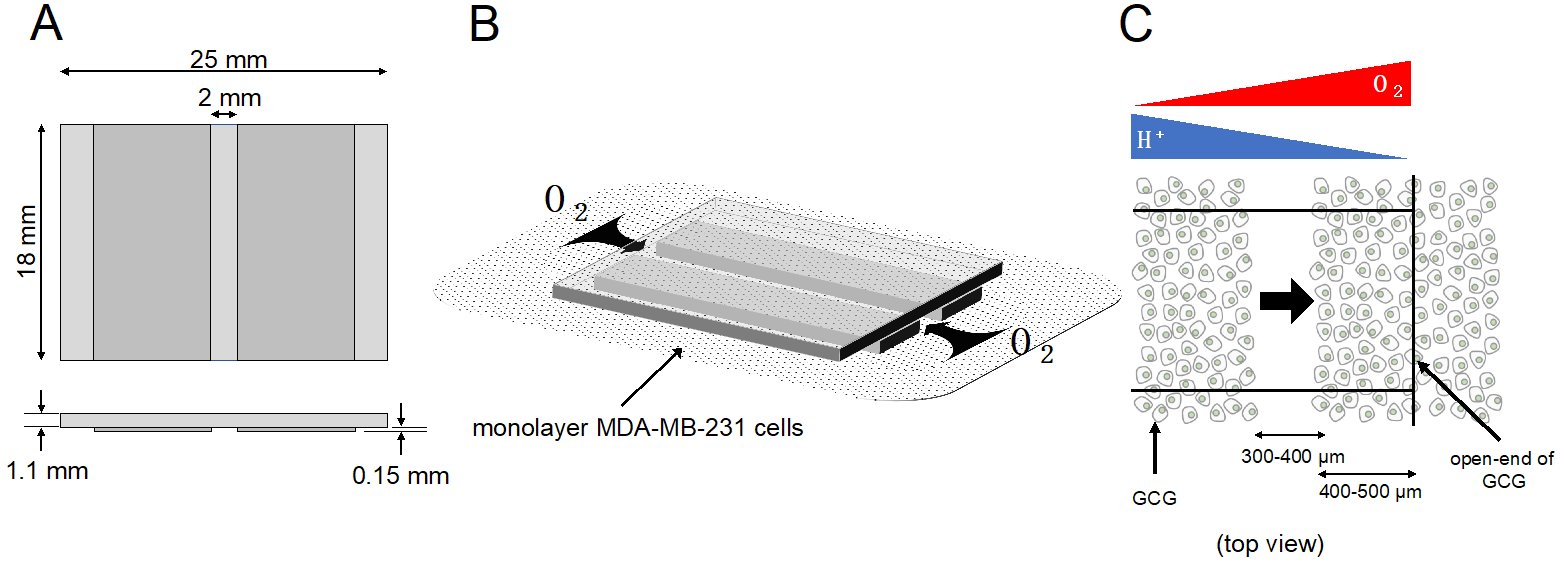
To test this hypothesis in vitro, we used a glasswareas product that we previously devised and call gap cover glass (GCG; Figure 1) [10] to apply in producing metabolic gradients in cultured monolayer cellsed under the GCG. In fact, we demonstrated tThat gradients of extracellular pH with a magnitude of 0.2–0.3 units/mm are established at three hours after placing the GCG onto confluent monolayer e number of MDA-MB-231 cells [11], whilen gradients of oxygen concentration under the GCG has not yet been demonstrated. Furthermore, we demonstrated a tendency that MDA-MB-231the culture dish was 5.2 × 106 cells /migrate toward the opening of GCG; in other words, toward areas of higher pH and/or oL. Note that the oxygen concentration. One of the serious drawbacks of our approach, however, was that migrating cells under at the opening of the GCG frequently underwent collisions with each other and subsequently changed their direction of migration. Thus, we thought that our tentative conclusions regarding directional migration of MDA-MB-231 cells were far from promising. To overcome the collision artifacts, we refined our technique by combining our GCG with the conventional wound-healing assay for cell migration [12].
Figure 1. GCG. (distance = 0) was substantially lower than that in the microincubator (A21%), Three thin glass plates were assembled into the GCG. (B) The GCG was gentlprobably placdued on to the monolayer of MDA-MB-231 cells. The diffusional supply of energy substrates such as oxygen to cells in the narrow channel of the GCG is restricted. Thus, cellular oexistence of an oxygen consumption produces gradients of oxygen concentration in the extracellular medium in the narrow channel. Washout of metabolites such as CO2/H+ to thdiffusion barrier in the bulk medium ias similarly restricted, and pH gradients are produced in the narrowuggested by Metzen et al. channel[1]. (CB) GCGEffect combined with wound-healing assay for cell migration. Gradients of oxygen and H+ of mitochondrial respiratory concmplentration are also illustrated. In the present study, we demonstrated a vectorial movement of MDA-MB-231 cells towardx III inhibitor (antimycin A, 2 µM) on the open-end of GCG (i.e., higher oxygen concentration or lower H+ concentrgration; indicated by the thick arrow)ent.
We demonstrated wElith a new improved technique that MDA-MB-231 cells under GCG migrate toward higher pH/O2 regiminations while directional migration was abolished after eliminating gradients of pH. Thus, relatively small gradients of pH in the extracellular medium compared to those found in Nof mitochondrial respiration a+/H+ exchanger-driven cell migratibon were sufficient to guide MDA-MB-231 cells in vitro.
2. Results
2.1. Extracellular Gradients of Oxygen Concentration under GCG
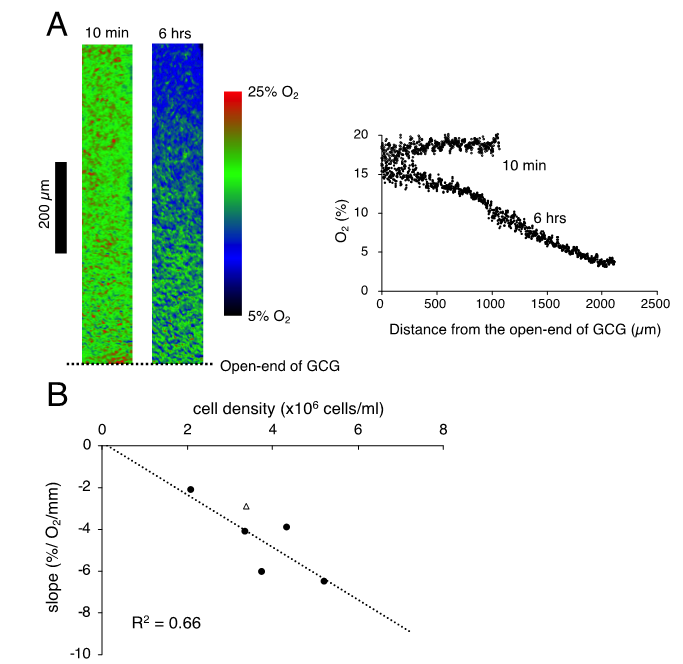
We isucchessfully visualized the oxygen concentration gradient under GCG. As shown in Figure 2A, immediately after placing the GCG, the oxygen concentration along the oxygen diffusion path was constant up to 1200 µm inside the GCG. Subsequently, the oxygen concentration gradients developed slowly and, at lthough the cell shape changed slightly six hours after placement of the GCG, a −6.5% O2/mm linear gradient was established under GCG. Magnitude of the oxygen concentration gradient increased in proportion to the number of spiratory inhibition, the cells per unit volume (Figure 2B).
Figure 2. (A). Representwere viativble data indicating changes in oxygen concentration in the extracellular medium along the oxygen diffusion path as measured as judged by the oxygen sensor foil. The ratio of red and green fluorescence is represented in pseudo-color. At 10 min after placing the gap cover glass (GCG), no gradient was demonstrated underneath. At six hours after placing the GCG, a −6.2% O2/mm liLIVE/DEAD cell imaging kit (R37601, Thermo Fisher Scienear oxygen concentration gradient was produced under the GCGific). The number of MDA-MB-231 cells in the culture dishcell density was 5.25 × 106 cells/mL. (BC) Relationship between the magnitude of the oxygen concentration gradient and cell density. The linear relationship indicates that the oxygen concentration gradient depends upon oxygen consumption of the cell per unit volume. The open triangle represents the slope in which L-15 medium was buffered with 15 mM hepes.
Effects of Hepes-buffered L-15 on pH and Oxygen Gradients under GCG
2.2. Effects of Hepes-buffered L-15 on pH and Oxygen Gradients under GCG
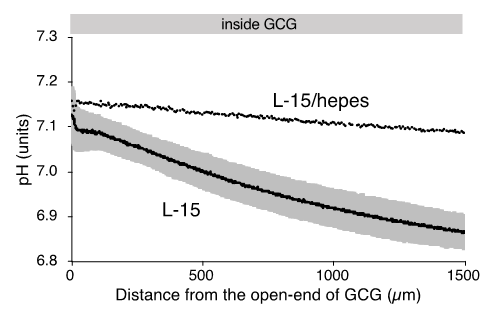
As reported elsewhere [112], extracellular pH gradients along the diffusion path slowly developed in GCG and reached the steady state in three hours. Gradients of pH with a magnitude of 0.2–0.3 units/mm were consistently demonstrated (Figure 2A). Supplementing hepes into the L-15 medium abolished the pH gradient (Figure 3 2A). On the other hand, the addition of hepes in L-15 medium did not affect oxygen concentration gradients under GCG (Figure 2B and the open triangle in Figure 2C).

Figure 32. Gradients of extracellular pH and oxygen concentration under GCG in L-15 and in hepes (15 mM)-buffered L-15 medium. (A) Representative pH data in L-15/hepes medium. Average pH gradients in L-15 medium are alshowno shown, where the range of ±standard deviation (SD) is represented in gray (n = 4). Data were collected three hours after placing the GCG. (B) Representative oxygen gradients in L-15/hepes medium. Cell density-corrected oxygen gradients represented as the open triangle in Figure 1C were comparable to those in L-15 medium. Data were collected six hours after placing the GCG.
Cell Migration
2.3. Cell Migration
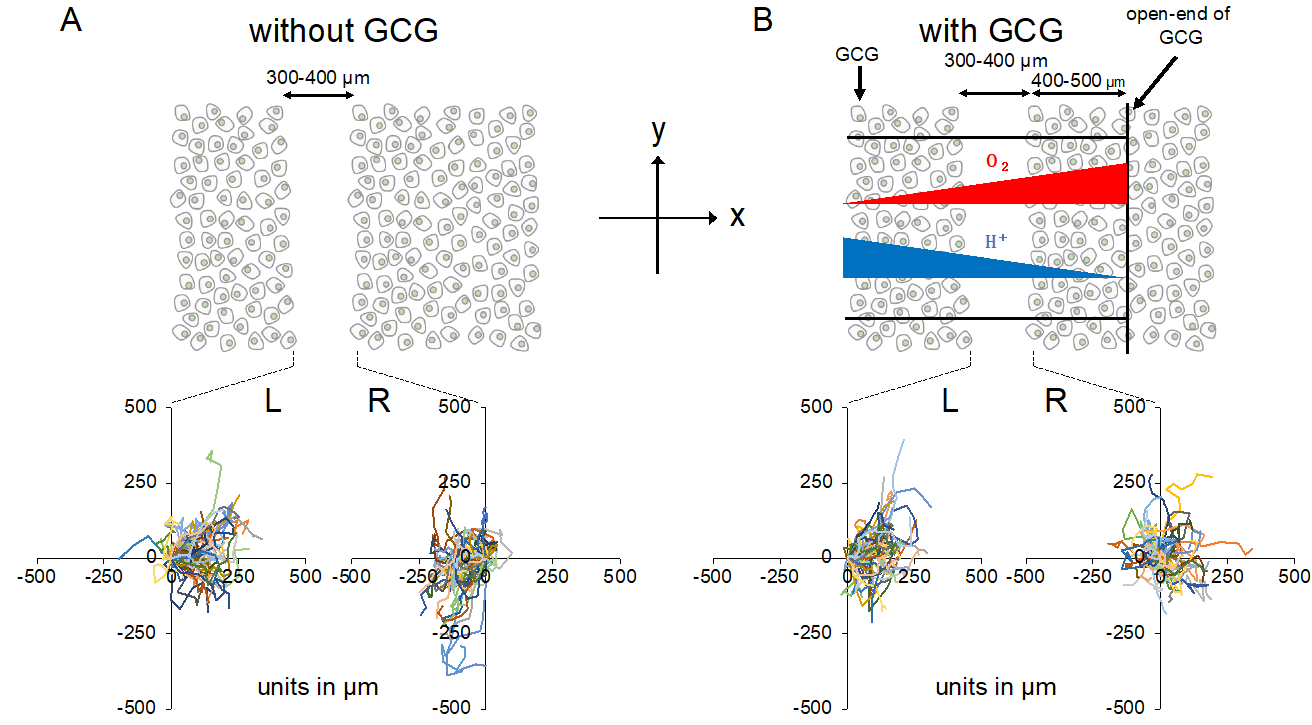
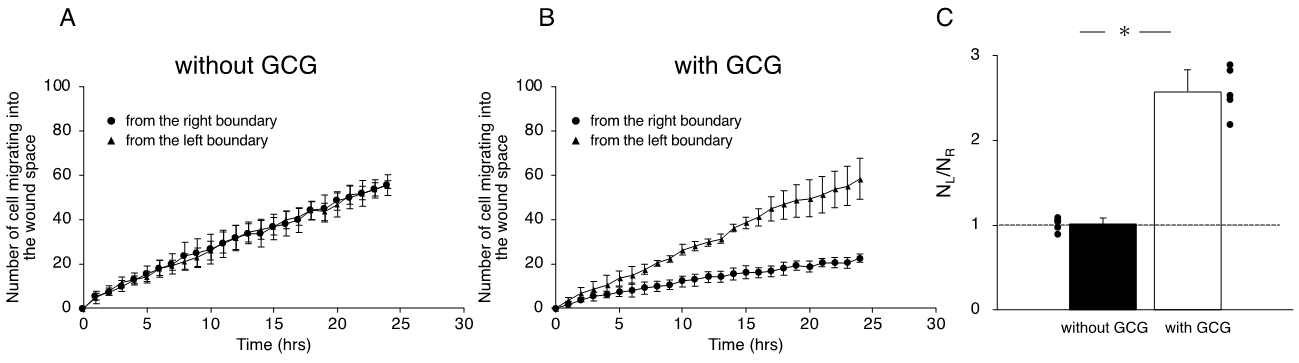
We completed five “without GCG” and five “with GCG” experiments, respectively, and representative data associated with these “without GCG” and “with GCG” experiments are shown in Supplementary Video S1 and Video S2, respectively. Figure 43 illustrates the trajectory of individual MDA-MB-231 cells for 24 h.

Figure 43. Trajectories of MDA-MB-231 cells measured for 24 h. The oxygen concentration in the microincubator was 21% (room air). Values presented in the graphs are in micrometers. (A) Without the GCG placed, cells migrated into the wound space equally from the right (R) and left (L) boundaries of the wound space. (B) With the GCG placed, the migration of the cells initially located at the right boundary (R) into the wound space was hindered and they even appeared to migrate in a direction opposite to that toward the wound space as if they were crawling into the crowd of cells. Trajectories of the 50 cells are superimposed.
W To assess the rate of cell proliferathout involvement from thion under GCG, 300 × 500 µm rectangular regions of interest (ROIs) were defined close to but outside the wound space (dashed squares) and the number of cells in the respective ROI was counted and compared at time = 0 and time = 24 h.
Without the GCG, accumulathe L-cells (ed distances for 24 h in cells migrating into the wound space from the left boundary, see Figure 4) (L-cells) and from the right boundary (R-cells) were similar (368 ± 94 µm and 358 ± 112 µm, respectively). With the GCG, the accumulated distance was significantly decreased, while values for the L-cells and R-cells (cells migratingwere not different from each other (322 ± 118 µm and 295 ± 138 µm, respectively). Without involvement from the GCG, the L-cells and R-cells similarly migrated into the wound space from the r(Figure 3A). Forward migration index (FMI) values were not different between the L-cells and R-cells (Figure 4A).

Figure 4. Forward migration index (FMI) parallel to tht be diffusion path (FMIx) without (A) and with the GCG in place (B). Without the GCG involved, the FMIx values fory, the L-cells and see Figure 4R-cells were not different (NS, p = 0.60). similaIn contrast, with the GCG in place, the FMIx value for the R-celly migrated into the wound space (Fis was significantly smaller compared to that of the L-cells. Data were accumulated from five independent experiments in which 10 cells were sampled in each experiment. Error bars represent the SD. *, p < 0.05, as judged by Sturedent’s 4A)t-test.
In contrast, in the “with GCG” experiments, we demonstrated distinct differences in the direction of cell migration, particularly in the R-cells. With the inclusion of the GCG, the migration of R-cells into the wound space appeared to be considerably hindered (Figure 43B). These cells even migrated in the direction opposite of that toward the wound space, as if they were instead crawling into the crowd of cells. In fact, the FMI value for R-cells was not different from the null (Figure 4B). These data are consistent with that cells under the GCG demonstrate directional migration toward the open-end of the GCG (right side). FMI values perpendicular to the diffusion path (i.e., y-axis) were not different from the null, regardless of GCG involvement. Directional cell movement can be visually confirmed by observing Video S2.
We further checked the directional migration of the cells under GCG by counting the numbers of L-cells and R-cells in the wound space. As shown in Figure 5A, without the GCG in place, the numbers of L-cells and R-cells were the same throughout the experiment. In contrast, with the GCG in place, the numbers of R-cells were smaller than that of the L-cells (Figure 5B), reaching statistical significance after five hours. Thus, the numbers of L-cells and R-cells at 24 h were highly different from one another (Figure 5C).

Figure 5. The number of cells that migrated into the wound space in 24 h. (A) Without the GCG, the numbers of cells migrating into the wound space from the left boundary and the right boundary were the same. (B) With the GCG, the numbers of cells migrating into the wound space from the left boundary and the right boundary were different, with a statistically significant difference demonstrated after five hours. (C) The ratio of the numbers of cells migrating into the wound space from the left boundary and the right boundary determined at 24 h (NL and NR, respectively). The directional migration of MDA-MB-231 cells was clearly demonstrated with involvement of the GCG. Error bars represent the SD. Data were accumulated from five independent experiments. *, p < 0.05, as judged by Student’s tt-test.
Effects of cell proliferations on heterogeneous cell migration into the wound space were also evaluated. Without GCG in place, number of the cell in the dashed square on the L-side and that in the dashed square on the R-side (see Figure 3A) similarly increased in 24 h to 111 ± 11% and 109 ± 8% compared to those at time = 0, respectively, representing proliferation of the cell. With the GCG in place, number of the cell in the dashed square on the L-side counted at 24 h increased to 112 ± 14%, while that in the dashed square on the R-side was 98±16%. The difference was not statistically significant (p = 0.055). These results indicate that the difference in the cell proliferation rate in the metabolic gradients had no significant impact on the present wound-healing assays. From these data, we concluded that MDA-MB-231 cells under testhe GCG demonstrate directional migrations toward the open-end of the GCG.
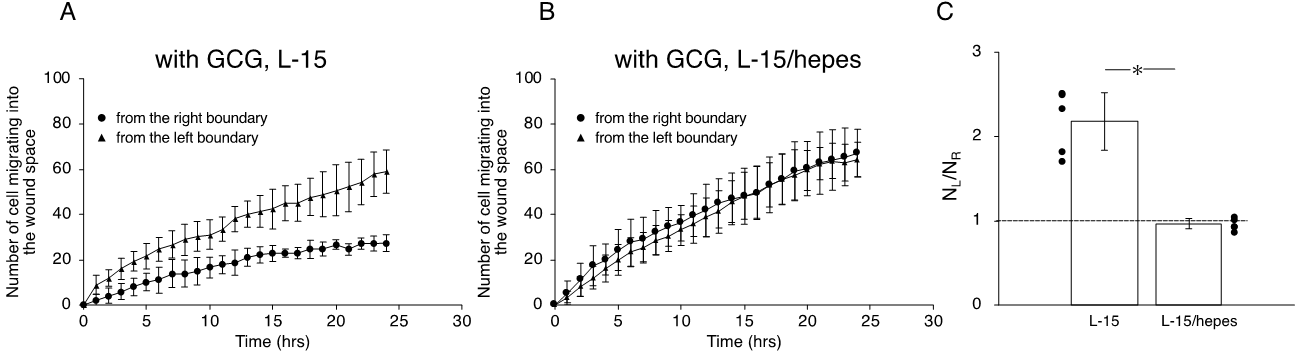
Next, we undertook another series of experiments in which the role of extracellular pH gradients in directional movements of MDA-MB-231 cells was examined. We completed five “L-15” and five “L-15/hepes” experiments, respectively. Figure 6 illustrates the analysis of the directionality of cell migration. With the GCG in place, the magnitude of the FMIn the L- for the R-cells was significantly smaller than that for the L-cells in L-15 medium, the num (Figure 6A), while FMI values for the L-cells and R-cells were not different from one another in the L-15/hepes medium (Figure 6B).

Figure 6. Effects of extracellular pH gradients on FMIx in cells underneath GCG. (A) In L-15 medium, the FMIx for the L-cells was significantly higher than that for the R-cells. This result is consistent with that in Figure 4B. (B) In L-15/hepes medium in which extracellular pH gradients disappeared, the FMIx values for the L-cells and R-cells were not different (p = 0.20), indicating that the directionality in cell migration also disappeared. Data were accumulated from five independent experiments in which 10 cells were sampled in each experiment. Error bars rer present the SD. *, p < 0.05, as judged by Student’s t-test.
In the L-15 medium, the number of cells migrating from the left boundary into the wound space was significantly higher than that from the right boundary (Figure 67A,C). In comparison, in the L-15/hepes medium, such heterogeneity disappeared (Figure 67B,C). These results indicate that the extracellular pH gradient is the dominant cue for the directional migration of MDA-MB-231 cells under our GCG.

Figure 67. The number of cells that migrated into the wound space in 24 h. (A) In L-15 medium, NL and NR were different, consistent with what is observable in Figure 45B. (B) Conversely, in L-15/hepes medium, NL and NR were the same. (C) The heterogeneity in cell migration ultimately disappeared in L-15/hepes medium. Error bars represent the SD. Data were accumulated from five independent experiments. *, p < 0.05, as judged by Student’s t-test.
Discussion
3. Discussion
Directional migration of primary cancer cells toward intratumor blood/lymphatic vessels should elevate the probability for intravasation and ultimate hematogenous metastasis. On the analogy of chemotaxis, many presume that the gradients of nutrients and metabolic waste in the local tissue might guide tumor cells to nearby microvessels. However, at the present time, presence of such metabolic cues still remains an open question.
In the present study, we specifically focused on the gradients of H+ and oxygen as candidates for the metabolic cue. To monitor migratory behaviors of the cell in gradients of pH and/or oxygen in vitro, we previously proposed a simple microfluidic glassware, GCG, which is capable of producing gradients of energy substrates and metabolites, including H+ and oxygen in monolayer cultured cells [103]. Simultaneous changes in H+ and oxygen concentrations under the GCG are similar to those found in solid tumors and, therefore, experimentation using the GCG reflects a clinically relevant setting. Unlike the recent microfluidic devices designed for investigating cell migration under oxygen concentration gradients [4][5][6][7], the magnitude of the metabolic gradients under our GCG depends on the metabolic activity of cells per unit volume and cannot be easily manipulated; control of the gradient would require a redesign of the GCG or an accurate adjustment of the cell density (Figure 1C). It is also impossible for our GCG to produce concentration gradients of a specific molecule in the extracellular space. Thus, additional experiments are required to specifically pinpoint the molecule-of-importance among various metabolic substrates/metabolites.
Previous in vitro studies demonstrated the possibility that oxygen concentration gradients may act as a guiding cue for cell migration. Mosadegh et al. [138] used a unique paper-based 3D cell culture system in which oxygen and nutrient gradients were produced along a stack of eight 40-µm-height cell culture layers. They compared metastatic potential in various subtypes of human alveolar adenocarcinoma A549 cells and demonstrated in one A549 subpopulation that oxygen was the primary chemoattractant in their invasion stack; the cells migrated toward the higher oxygen layers. Neither oxygen concentration in each individual layer nor the magnitude of oxygen concentration gradients along the stack was reported. Lewis et al. [149] observed migration of individual sarcoma cells embedded in oxygen-controllable hydrogels in which gradients of oxygen were established. With a 2.5 mm-thick hydrogel (hypoxic gel), the measured oxygen concentrations at the bottom of the hydrogel layer decreased to 0.4%–4% O2 from air level corresponding to oxygen gradients ranging from 3.4%–5.4% O2/mm. They found that individual sarcoma cells in the hypoxic gel migrate more quickly, across longer distance, in the direction of increasing oxygen concentration compared to those in the normoxic hydrogel with much smaller oxygen gradient. Chang et al. [155] demonstrated using a sophisticated microfluidics platform that under the combination of oxygen and chemokine (SDF-1α) gradients A549 cells migrated toward lower oxygen regions in a 5% O2/mm oxygen gradient. Sleeboom et al. [167] demonstrated that both MDA-MB-231 cells and their stem cell enriched population similarly migrate toward low oxygen levels in a 2% O2/mm oxygen gradient as determined by cell migration trajectory and the forward migration index. The average local oxygen concentration along the oxygen gradient varied from 1% to 7%, which did not significantly affect the forward migration index of the individual cell. Shih et al. [176] proposed a microfluidic device in which oxygen concentration gradients with a magnitude of 18% O2/mm were established along the 900 µm-wide cell channel using oxygen scavenging chemical reactions. HUVECs located at the boundaries of 300 µm-wide cell regions migrated differently in the oxygen gradient; cells initially located at the boundary with higher oxygen concentration in the gradient migrated slowly compared to those with lower oxygen concentration, resulting in a collective cell migration toward lower oxygen.
Results of these in vitro studies appear conflicting in terms of the direction of cell migration in oxygen gradients. IndHoweedver, effects of oxygen gradient on the cell migration should differ according to cell type, culture conditions (2D or 3D, culture media) and spatial profiles of oxygen concentration, including the magnitude and the local oxygen concentration at which cell is exposed. Furthermore, in most microfluidic devices, meta profile of oxygen concentration gradients may, more or less, change after loading cells in the device due to metabolic activitiey of the cell. More importantly, metabolic activities of the cell may also change spatial distribution of nutrients and metabolites that may affect the cellular migratory behavior in addition to the effect of the oxygen concentration gradient.
Initially, we hypothesized that the extracellular gradients of oxygen might be a cue for MDA-MB-231 cells to migrate directionally because steep gradients of oxygen concentration (~10 mmHg/100 µm) have been demonstrated in vivo [510]. Hypoxia-inducible factor 1α (HIF-1α) is an intracellular oxygen sensor that has been reported to affect the intracellular machinery for cell migration [1811]. Therefore, it is likely that the HIF-1α pathway plays a role in directing cell migration in the steep gradient of oxygen concentration. However, in the present setting, the direct measurement of the oxygen concentration under the GCG achieved in the present study (Figure 21A) revealed oxygen gradients with relatively small magnitudes such that the oxygen concentration recorded at ~400 µm inside the GCG was much higher (~14%) than the oxygen level at which HIF-1α is responsive (5% or lower [1912]). Thus, it is difficult to attribute the directional cell migration demonstrated in the present study specifically to HIF-1α dependent mechanisms. Although we It should be noted here, however, that the present results do not exclude the possibility that oxygen gradients might direct cell migration in hypoxic microenvironment in because the oxygen concentration can drop to pathophysiological levels in hypoxic tumor tissues in vivo [10][13]. Nevertheless, directional cell migration at unphysiologically high oxygen concentrations demonstrated in the present study prompted us to seek another possible cue for directional cell migration.
A few studies to date have addressed directional cell migration under gradients of extracellular pH in vitro. Paradise et al. [2014] demonstrated using the Dunn chamber that both αVβ3 CHO-B2 cells and primary microvascular endothelial cells preferentially migrate toward acid in an extracellular pH gradient. In their research, the Dunn chamber produced a pH gradient of 6.0 to 7.5 over 1 mm. Elsewhere, Jagielska et al. [2115] determined that oligodendrocyte precursor cells migrate toward areas of acidic extracellular pH produced by the Zigmond chamber. Here the pH gradient was set to 6.0 to 7.0 over a distance of 1 mm.
In the current study, we found that, first, a gradient of pH was in fact established in the extracellular medium under the GCG (0.2–0.3 units/mm); second, MDA-MB-231 cells under the GCG preferentially migrated toward the open-end of the GCG (i.e., higher pH/O2 regions); and third, such findings of directional cell migration completely disappeared when the extracellular pH gradient was abolished. Albeit, while gradients of various metabolic substances should exist under the GCG, these results strongly indicate that extracellular pH gradient is the predominant cue for the migration of MDA-MB-231 cells under the GCG.
Among migrating cells, gradients of intracellular and perixtracellular pH have been demonstrated at the level of the single cell. Martin et al. [2216] observed intracellular pH (pHi) gradients within single melanoma cells incubated in hepes-buffered Ringer solution at a pH of 7.0 and reported that the mean front-to-rear pHi gradients measured ~0.15 units over a 20-µm distance in migrating human (MV3) and murine (B16V) melanoma cells, where the front (leading edge) was more alkaline. Using a combination of pH-sensitive fluorescent dye and total internal reflection microscopy, Ludwig et al. [2317] determined the pericellular pH on the surface of the plasma membrane (pHem) in polarized MV3 cells, reporting significant gradients of pHem in single cells where front (at focal adhesions)-to-rear pHem gradients were ~0.2 units (the cell front was more acidic), indicating the existence of nano-domains with distinct pH values on the surface of the plasma membrane. These subcellular heterogeneities in pHi maynd pHem arise from the heterogeneous distribution of the Na+/H+ exchanger isoform 1 (NHE1), a major plasma membrane protein that extrude protons from cytosol, where NHE1 accumulates in the leading edge of migrating cells [2216][2418][2519]. Both intracellularpHi and pericellularHem pHgradients may independently affect cell motility through effects on cytoskeletal machinery and cell-matrix interactions, respectively [2620]. In fact, a substantial role of NHE1 activities in cell motility has been demonstrated in various cells [18][21][22][23][24].
In the present experiment, we imposed 0.2–0.3 units/mm gradients of pH in the extracellular medium that correspond to a gradient of 0.02 units per single MDA-MB-231 cell. The value is far smaller, if compared at the single-cell level, than the NHE1-driven gradients of pH. This is also true in previous studies in which microfluidic devices produced ~1-unit/mm gradients of pH in the extracellular medium [2014][2115]. Therefore, it is unclear whether the relationship between cell migration and the NHE1-driven pHi and pHem heterogeneities would be directly applicable to the presentr and other experiments in which the pH of the extracellular/pe bulk medium was manipulated.
Although there is a possibility that subcellular heterogeneities in pHi and pHem might endow migrating cells with directiould be directly applicanality, we are reluctant to conclude that relatively small gradients of extracellular pH at the single-cell level could be a consistent cue for the migration behavior demonstrated in the present study. Instead, we propose a different model of directional cell migration in which stochastic cell movement is modified by macroscopic (spanning a few hundred microns) gradients in the extracellular pH. This model is based on the dependence of cell migration activity on extracellular pH [14][15][24], without assuming significant intracellular pH gradients. Stock et al. [23] demonstrated that the pH of the extracellular bule to tk solution significantly affects migratory activities in MV3 cells in vitro. At low extracellular pH values, cell-matrix interactions via integrin α2β1 appear too strong, while present and other experiments in which tthey are too weak at high extracellular pH values, both of which hinder migratory activity. Thus, cell migration was most optimally facilitated at an extracellular pH of ~7.0. Based on this bell-shaped extracellular-pH migration-velocity relationship, it is predicted that cells initially located in regions with extracellular pH values of ~7.0 would vigorously move toward either lower or higher pH regions (random directions). As cells happen to migrate into lower or higher pH regions, the migration velocity gradually decreases and, finally, cells would become trapped in the lowest or highest pH regions, respectively. Thus, a degree of heterogeneity in cell distribution across the pH of the erange would be established. It is predictable from this model that the direction of migration (toward the lower or higher pH regions) depends upon the initial extracellular pH, specifically, whether it is lower or higher than the pH at which cell migration is most optimally facilitated.
In association with hypoxia, acidosis is another metabolic hallmark of solid tumors [25][26]. A type of metabolic reprograming k nown as the Warburg effect in cancer cells and reduced washout of CO2/H+ from thedi tissue are the known major causes of an acidotic microenvironment [27]. In the process of adaptation to acidosis, cancer cells may acquire a m alignant phenotype [28][29]. A low pH in the tumor microenvironment in vivo reflects the presence of steep gras manidients of pH between cells and the blood. If the present results are applicable to in vivo conditions, acidotic tumor cells might preferentially migrate toward more alkaline intratumor vessels. With the concomitant acidotic induction of vascular endothelial growth factor and angiogenesis [30], directional cell migration would elevate the probability of intravasation and, ulatedtimately, metastasis. Thus, targeting acidosis in the tumor microenvironment may have therapeutic rationales from the standpoint of control of hematogenous metastasis.
In summary, the use of novel microfluidic devise GCG produced gradients of pH and oxygen concentration in the extracellular medium in monolayer MDA-MB-231 cells. We clearly demonstrated heterogeneous migration of the cells into the wound space in such a way that cells preferentially migrated in the direction of higher pH/oxygen concentration. Elimination of pH gradients also abolished the directional cell movement under the GCG thus indicating a possibility that extracellular pH gradients are the dominant guiding cue for migration of MDA-MB-231 in the present setting. Because, under GCG, extracellular oxygen concentration remained at unphysiologically high ranges despite the presence of significant gradients, the effect of oxygen concentration gradients on directional migration is still remain to be determined.
References
- Bockhorn, M.; Jain, R.K.; Munn, L.L. Active versus passive mechanisms in metastasis: Do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007, 8, 444–448.Eric Metzen; M Wolff; J Fandrey; Wolfgang Jelkmann; Pericellular PO2 and O2 consumption in monolayer cell cultures. Respiration Physiology 1995, 100, 101–106.
- Elisabetta Marcuzzi; Roberta Angioni; Barbara Molon; B. Calì; Chemokines and Chemokine Receptors: Orchestrating Tumor Metastasization. InternationalEnokida, Y.; Tsuruno, Y.; Okubo, K.; Yamaoka, Y.; Takahashi, E; Directional migration of MDA-MB-231 cells under O2/pH gradients. Adv. JournalExp. of Molecular Sciences Med. Biol. 2018, 20, 96, 10.3390/ijms20010096.7, 977, 169–174, 10.3390/ijms21072565.
- Morgan O'hayre; Catherina L. Salanga; Tracy M. Handel; Samantha J. Allen; Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Yahara, D.; Yoshida, T.; Enokida, Y.; Takahashi, E; Directional migration of MDA-MB-231 cells under oxygen concentration gradients. Adv. Exp. Med. Biochemical Journal . 2008, 4016, 9, 635-649, 10.1042/bj20071493.23, 129–134.
- Peter Vaupel; Tumor microenvironmental physiology and its implications for radiation oncology. SeAcosta, M.A.; Jiang, X.; Huang, P.-K.; Cutler, K.B.; Grant, C.S.; Walker, G.M.; Gamcsik, M.P; A microfluidic device to study cancer metastasis under chronic and intermittent hypoxia. Biominacrs in Radiation Oncology ofluidics 20014, 14, 198-206, 10.1016/j.semradonc.2004.04.008., 8, 054117.
- Gabriel Helmlinger; Fan Yuan; Marc Dellian; Rakesh K. Jain; Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. NChang, C.-W.; Cheng, Y.-J.; Tu, M.; Chen, Y.-H.; Peng, C.-C.; Liao, W.-H.; Tung, Y.-C; A polydimethylsiloxane-polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Latureb Medicine Chip 201997, 3, 177-182, 10.1038/nm0297-177.4, 14, 3762–3772.
- Franziska Van Zijl; Georg Krupitza; Wolfgang Mikulits; Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutation ResearShih, H.-C.; Lee, T.-A.; Wu, H.-M.; Ko, P.-L.; Liao, W.-H.; Tung, Y.-C; Microfluidic collective cell migration assay for study of endothelial cell proliferation and migration under combinations of oxygen gradients, tensions, and drug treatments. Sch/Reviews in Mutation Research . Rep. 2011, 728, 23-34, 10.1016/j.mrrev.2011.05.002.9, 9, 8234.
- Jennifer S. Fang; Robert D. Gillies; Robert A. Gatenby; Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. SemiSleeboom, J.J.F.; Den Toonder, J.M.J.; Sahlgren, C.M; MDA-MB-231 breast cancer cells and their CSC population migrate towards low oxygen in a microfluidic gradient device. Inarst. J. in Cancer Biology Mol. Sci. 20018, 18, 330-7, 10.1016/j.semcancer.2008.03.011.9, 3047.
- Calvin R. Justus; Edward J. Sanderlin; Li V. Yang; Molecular Connections between Cancer Cell Metabolism and the Tumor Microenvironment. InMosadegh, B.; Lockett, M.R.; Minn, K.T.; Simon, K.A.; Gilbert, K.; Hillier, S.; Newsome, D.; Li, H.; Hall, A.B.; Boucher, D.M; et al. A paper-based invasion assay: Assessing chemotaxis of cancer cells in gradients of oxygen. Biomaternational Journal of Molecular Scienceals 2015, 16, 11055-11086, 10.3390/ijms160511055., 52, 262–271.
- Madeleine J. Oudin; Valerie M. Weaver; Physical and Chemical Gradients in the Tumor Microenvironment Regulate Tumor Cell Invasion, Migration, and Metastasis. CLewis, D.M.; Park, K.M.; Tang, V.; Xu, Y.; Pak, K.; Eisinger-Mathason, T.S.K.; Simon, M.C.; Gerecht, S; Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc. Natld Spring Harbor Symposia on Quantitative Biology . Acad. Sci. USA 2016, 8, 11, 189-205, 10.1101/sqb.2016.81.030817.3, 9292–9297.
- D. Yahara; T. Yoshida; Y. Enokida; Eiji Takahashi; Directional Migration of MDA-MB-231 Cells Under Oxygen Concentration Gradients. SingleHelmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K; Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Molecule and Single Cell Sequencing 20d. 16, 92997, 3, 129-134, 10.1007/978-3-319-38810-6_17., 177–182.
- Y. Enokida; Y. Tsuruno; K. Okubo; Y. Yamaoka; Eiji Takahashi; Directional Migration of MDA-MB-231 Cells Under O2/pH Gradients. AdvanGilkes, D.M.; Xiang, L.; Lee, S.J.; Chaturvedi, P.; Hubbi, M.E.; Wirtz, D.; Semenza, G.L; Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proces. in Experimental Medicine and Biology Natl. Acad. Sci. USA 2017, 977, 169-174, 10.1007/978-3-319-55231-6_23.4, 111, E384–E393.
- Yusuke Tsuruno; Kaima Okubo; Takahiro Fujiwara; Yoshihisa Yamaoka; Eiji Takahashi; An In Vitro Model for Determining Tumor Cell Migration Under Metabolic Gradients. Jiang, B.H.; Semenza, G.L.; Bauer, C.; Marti, H.H; Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Advances in Experimental Medicine and Biology 20. J. Physiol. 18, 996, 271072, 201-205, 10.1007/978-3-319-91287-5_32., C1172–C1180.
- Chia-Wen Chang; Yung-Ju Cheng; Melissa Tu; Ying-Hua Chen; Chien-Chung Peng; Wei-Hao Liao; Yi-Chung Tung; A polydimethylsiloxane–polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. LabMcKeown, S.R; Defining normoxia, physoxia and hypoxia in tumours-implications for treatment respons. Br. onJ. a Chip Radiol. 2014, 14, 3762-3772, 10.1039/c4lc00732h., 87, 20130676.
- Hsiu-Chen Shih; Tse-Ang Lee; Hsiao-Mei Wu; Ping-Liang Ko; Wei-Hao Liao; Yi-Chung Tung; Microfluidic Collective Cell Migration Assay for Study of Endothelial Cell Proliferation and Migration under Combinations of Oxygen Gradients, Tensions, and Drug Treatments.. SciParadise, R.K.; Whitfield, M.J.; Lauffenburger, D.A.; Van Vliet, K.J; Directional cell migration in an extracellular pH gradient: A model study with an engineered cell line and primary microvascular endothelial cells. Exp. Centificll Reports . 2019, 3, 319, 8234, 10.1038/s41598-019-44594-5., 487–497.
- Jelle J. F. Sleeboom; Jaap M.J. Den Toonder; Cecilia M. Sahlgren; MDA-MB-231 Breast Cancer Cells and Their CSC Population Migrate Towards Low Oxygen in a Microfluidic Gradient Device. InternatiJagielska, A.; Wilhite, K.D.; Van Vliet, K.J; Extracellular acidic pH inhibits oligodendrocyte precursor viability, migration, and differentiation. PLonalS Journal of Molecular Sciences ONE 2018, 19, 3047, 10.3390/ijms19103047.3, 8, e76048.
- Bobak Mosadegh; Matthew R. Lockett; Kyaw Thu Minn; Karen A. Simon; Karl Gilbert; Shawn Hillier; David Newsome; Howard Li; Amy B. Hall; Diane M. Boucher; Brenda K. Eustace; George M. Whitesides; A paper-based invasion assay: Assessing chemotaxis of cancer cells in gradients of oxygen. BMartin, C.; Pedersen, S.F.; Schwab, A.; Stock, C; Intracellular pH gradients in migrating cells. Am. J. Physiomatl. Cerials ll Physiol. 20115, 52, 262-271, 10.1016/j.biomaterials.2015.02.012., 300, ec106-ec106.
- Daniel M. Lewis; Kyung Min Park; Vitor Tang; Yu Xu; Koreana Pak; T. S. Karin Eisinger-Mathason; M. Celeste Simon; Sharon Gerecht; Intratumoral oxygen gradients mediate sarcoma cell invasion. ProcLudwig, F.T.; Schwab, A.; Stock, C; The Na+/H+-exchanger (NHE1) generates pH nanodomains at focal adhesions. J. Ceedingsll. of the National Academy of Sciences Physiol. 2016, 113, 9292-9297, 10.1073/pnas.1605317113.3, 228, 1351–1358.
- Daniele M. Gilkes; Lisha Xiang; Sun Joo Lee; Pallavi Chaturvedi; Maimon E. Hubbi; Denis Wirtz; Gregg L. Semenza; Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. PSheryl P. Denker; Diane L. Barber; Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. Jouroceedings of the National Academal of Cell Biology of Sciences 20013, 2, 111, E384-E393, 10.1073/pnas.1321510111.59, 1087-1096, 10.1083/jcb.200208050.
- B H Jiang; G L Semenza; C Bauer; H H Marti; Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. American Jensen, H.H.; Pedersen, G.A.; Morgen, J.J.; Parsons, M.; Pedersen, S.F.; Nejsum, L.N; The Na+/H+ exchanger NHE1 localizes as clusters to cryptic lamellipodia and accelerates collective epithelial cell migration. Journal. of Physiology-Legacy Content . 201996, 2, 5971, C1172-1180., 849–867.
- Ranjani K. Paradise; Matthew J. Whitfield; Uglas A. Lauffenburger; Krystyn J. Van Vliet; Directional cell migration in an extracellular pH gradient: A model study with an engineered cell line and primary microvascular endothelial cells. ExpStock, C.; Pedersen, S.F; Roles of pH and the Na+/H+ exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective. Sermimental Cell Research n. Cancer Biol. 2013, 7, 4319, 487-497, 10.1016/j.yexcr.2012.11.006., 5–16.
- Anna Jagielska; Kristen D. Wilhite; Krystyn J. Van Vliet; Extracellular Acidic pH Inhibits Oligodendrocyte Precursor Viability, Migration, and Differentiation. Klein, M.; Seeger, P.; Schuricht, B.; Alper, S.L.; Schwab, A; Polarization of Na+/H+ and Cl−/HCO3− exchangers in migrating renal epithelial cells. J. Gen. PLOS ONE hysiol. 2013, 8, e76048, 10.1371/journal.pone.0076048.00, 115, 599–608.
- Martin, C.; Pedersen, S.F.; Schwab, A.; Stock, C; Intracellular pH gradients in migrating cells. Am. J. Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C; Role of ion channels and transporters in cell migration. Physiol. CRell Physiolv. 2011, 300, C490-495.2, 92, 1865–1913.
- Florian Timo Ludwig; Albrecht Schwab; Christian Stock; The Na+/H+-exchanger (NHE1) generates pH nanodomains at focal adhesions. Stock, C.; Gassner, B.; Hauck, C.R.; Arnold, H.; Mally, S.; Eble, J.A.; Dieterich, P.; Schwab, A; Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. Journal. of Cellular Physiology . 20013, 228, 1351-1358, 10.1002/jcp.24293.5, 567, 225–238.
- Sheryl P. Denker; Diane L. Barber; Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. Stüwe, L.; Müller, M.; Fabian, A.; Waning, J.; Mally, S.; Noël, J.; Schwab, A.; Stock, C; pH dependence of melanoma cell migration: Protons extruded by NHE1 dominate protons of the bulk solution. Journal. of Cell Biology Physiol. 2002, 17, 5859, 1087-1096, 10.1083/jcb.200208050., 351–360.
- Helene Halkjær Jensen; Gitte A. Pedersen; Jeanette J. Morgen; Maddy Parsons; Stine F. Pedersen; Lene Niemann Nejsum; The Na + /H + exchanger NHE1 localizes as clusters to cryptic lamellipodia and accelerates collective epithelial cell migration. TheJustus, C.R.; Sanderlin, E.J.; Yang, L.V; Molecular connections between cancer cell metabolism and the tumor microenvironment. Int. J. Mournal of Physiology l. Sci. 2019, 597, 849-867, 10.1113/jp277383.5, 16, 11055–11086.
- Stock, C.; Pedersen, S.F. Roles of pH and the Na+/H+ exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective? Semin. Cancer Biol. 2017, 43, 5–16.Corbet, C.; Feron, O; Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593.
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J; Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019, 38, 205–222.
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535.
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L; Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J. Cell. Sci. 2017, 130, 663–669.
- Fukumura, D.; Xu, L.; Chen, Y.; Gohongi, T.; Seed, B.; Jain, R.K; Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001, 61, 6020–6024.