Cyberlindnera jadinii
is widely used as a source of single-cell protein and is known for its ability to synthesize a great variety of valuable compounds for the food and pharmaceutical industries. Its capacity to produce compounds such as food additives, supplements, and organic acids, among other fine chemicals, has turned it into an attractive microorganism in the biotechnology field.
- Cyberlindnera jadinii
- phylogeny
- life cycle
- genome
- physiology
- biotechnology applications
- membrane transporter systems
1. Introduction
The future of our society challenges researchers to find novel technologies to address global environmental problems, mitigate ecosystems’ damage, and biodiversity losses, as the current model of development based on natural resources exploitation is unsustainable. Exploring microorganisms for the production of platform chemicals constitutes an alternative approach to avoid the use of nonrenewable petrochemical-based derivatives. Developing applications for the industrial sphere using biological systems instead of classical chemical catalysts is the main focus of white biotechnology [1]. In microbial-based industrial processes, several features have to be addressed to obtain robust cell factories capable of achieving superior metabolic performances, such as the optimization of metabolic fluxes, membrane, and transporter engineering, and increased tolerance against harsh industrial conditions and toxic compounds [2]. In addition, specifications like the cost of feedstock, product yield and productivity, and downstream processing have to be taken in account to develop successful industrial approaches [1]. Withal the fact that
Saccharomyces cerevisiae
is by far the most relevant industrial yeast species,
Cyberlindnera jadinii
is an example of the so-called non-
Saccharomyces yeasts [3] claiming for a place as a relevant contributor to the industrial biotechnological sector. The yeast
yeasts [3] claiming for a place as a relevant contributor to the industrial biotechnological sector. The yeast
C. jadinii is able to produce valuable bioproducts being an attractive source of biomass enriched in protein and vitamins. The richness of protein content, around 50% of dry cell weight, and amino acid diversity turn its biomass ideal as a source of protein supplement for animal feed and human consumption [4]. The high degree of tolerance to environmental changes occurring during fermentation turn
is able to produce valuable bioproducts being an attractive source of biomass enriched in protein and vitamins. The richness of protein content, around 50% of dry cell weight, and amino acid diversity turn its biomass ideal as a source of protein supplement for animal feed and human consumption [4]. The high degree of tolerance to environmental changes occurring during fermentation turn
C. jadinii an alternative to other established cell factory systems [5]. As a Crabtree-negative yeast, it has one of the highest respiratory capacities among characterized yeast species, being considered ideal for continuous cell cultures [6]. The Food and Drug Administration (FDA) attributed the “General Regarded as Safe” (GRAS) status to this yeast, recognizing it as safe and suitable for supplying food additives and dietary supplements for humans [5][7][8][9]. The ability to produce relevant compounds, to grow in a wide range of temperatures, to use inexpensive media with high productivity levels turns it an industrially relevant microorganism [8][10][11][12]. Recent efforts have developed
an alternative to other established cell factory systems [5]. As a Crabtree-negative yeast, it has one of the highest respiratory capacities among characterized yeast species, being considered ideal for continuous cell cultures [6]. The Food and Drug Administration (FDA) attributed the “General Regarded as Safe” (GRAS) status to this yeast, recognizing it as safe and suitable for supplying food additives and dietary supplements for humans [5,7,8,9]. The ability to produce relevant compounds, to grow in a wide range of temperatures, to use inexpensive media with high productivity levels turns it an industrially relevant microorganism [8,10,11,12]. Recent efforts have developed
C. jadinii molecular tools for metabolic engineering processes and the overexpression of proteins. In the past, the uncertainty of this yeast polyploidy, together with the lack of suitable selection markers and expression cassettes, [10] delayed its widely use as cell factory. With this review, we intend to compile the existing knowledge on this yeast, that will allow the development of future strategies to strengthen the role of
molecular tools for metabolic engineering processes and the overexpression of proteins. In the past, the uncertainty of this yeast polyploidy, together with the lack of suitable selection markers and expression cassettes, [10] delayed its widely use as cell factory. With this review, we intend to compile the existing knowledge on this yeast, that will allow the development of future strategies to strengthen the role of
C. jadinii
in the biotechnology sector. We start by reviewing the nomenclature of this species, altered several times over time. An update on the current nomenclature of the most relevant strains is also presented. We establish the evolutionary relationship of
C. jadinii
within other fungi with a complete genome available. The most relevant morphological and physiological traits are also here described, together with the genetic manipulation tools and expression systems currently available. Moreover, we present a summary of all the plasma membrane transporter systems so far characterized in this yeast, as they are key-players for cell factory optimization. Finally, we will focus on the biotechnological potential of this yeast and highlight the future challenges to achieve the full exploitation of this industrially valuable microorganism.
2. Ecology, Taxonomy, and Evolution
The natural environment of
Cyberlindnera jadinii is still an open question. It is thought that it may be associated with the decomposition of plant material in nature, as it is able to assimilate pentoses and tolerate lignocellulosic by-products [3], displays great fermentative ability, and is copiotrophic [13]. The current laboratory strains were isolated from distinct environments, namely, from the pus of a woman abscess (CBS 1600/NRRL Y-1542), a cow with mastitis (CBS 4885/NRRL Y-6756), a yeast deposit from a distillery (CBS 567), yeast cell factories (CBS 621), and flowers (CBS 2160) [5][6][7][8][9][12]. The extensive nomenclature revisions of this species are well described in “The tortuous history of
is still an open question. It is thought that it may be associated with the decomposition of plant material in nature, as it is able to assimilate pentoses and tolerate lignocellulosic by-products [3], displays great fermentative ability, and is copiotrophic [13]. The current laboratory strains were isolated from distinct environments, namely, from the pus of a woman abscess (CBS 1600/NRRL Y-1542), a cow with mastitis (CBS 4885/NRRL Y-6756), a yeast deposit from a distillery (CBS 567), yeast cell factories (CBS 621), and flowers (CBS 2160) [5,6,7,8,9,12]. The extensive nomenclature revisions of this species are well described in “The tortuous history of
Candida utilis
” by Barnet [14]. In 1926, this yeast was isolated from several German yeast factories, which had been cultivated without a systematic name during the time of World War I for food and fodder [14]. It was first named
Torula utilis
being later referred to as
Torulopsis utilis
(1934). The “food yeast” was also designated as
Saccharomyces jadinii
(1932),
Hansenula jadinii
(1951),
Candida utilis
(1952),
Pichia jadinii
(1984), and
Lindnera jadinii (2008) [12][14][15][16]. From the aforementioned,
(2008) [12,14,15,16]. From the aforementioned,
C. utilis
was the nomenclature most commonly used, having almost 1000 published papers in PubMed (results available at:
https://pubmed.ncbi.nlm.nih.gov/?term=%22candida+utilis%22
; Accessed 16 October 2020). The
Candida genus comprised species that form pseudohyphae or true hyphae with blastoconidia, among other standard characteristics [17][18][19]. In the classification system implemented in 1952 by Lodder and Kreger-van Rij, the
genus comprised species that form pseudohyphae or true hyphae with blastoconidia, among other standard characteristics [17,18,19]. In the classification system implemented in 1952 by Lodder and Kreger-van Rij, the
Candida genus included yeasts that produce only simple pseudohyphae [14]. At that time, the majority of the isolates was renamed as
genus included yeasts that produce only simple pseudohyphae [14]. At that time, the majority of the isolates was renamed as
C. utilis [18]. Later, the
[18]. Later, the
C. utilis
was established as an asexual state of a known ascosporogenous yeast,
Hansenula jadinii
, as it was found to share some similarities between phenotypic traits [20]. In 1984, even though concurring with a publication of an extensive chapter of the genus
Hansenula
, Kurtzman moved most of the
Hansenula
species to the
Pichia
genus, due to their “deoxyribonucleic acid relatedness.” Thus,
C. utilis
was renamed
Pichia jadinii
. A quarter of a century later, this yeast species was again renamed as
Lindnera jadinii based on analyses of nucleotide sequence divergence in the genes coding for large and small-subunit rRNAs [12]. Species integrated into the
based on analyses of nucleotide sequence divergence in the genes coding for large and small-subunit rRNAs [12]. Species integrated into the
Lindnera
differ considerably in ascospore morphology ranging from spherical to hat-shaped or Saturn-shaped spores. In addition, this clade includes both hetero- and homothallic species and physiological features as fermenting glucose and assimilating a variety of sugars, polyols, and other carbon sources are defining characteristics of the
Lindnera genus [12]. Finally, 1 year later, the genus
genus [12]. Finally, 1 year later, the genus
Lindnera
was replaced by
Cyberlindnera
, as the later homonym defined a validly published plant genus [15]. This substitution occurred in 21 new species, including
Cyberlindnera jadinii [15]. In summary, any of the aforementioned nomenclatures reported in the literature may refer to the same organism since
[15]. In summary, any of the aforementioned nomenclatures reported in the literature may refer to the same organism since
C. utilis
is the anamorph state and
C. jadinii the teleomorph state [14]. The anamorph represents the asexual stage of a fungus contrasting with the teleomorph form that defines the sexual stage of the same fungus [21]. The primary name of a species relies on the sexual state or teleomorph, but a second valid name may rely on the asexual state or anamorph [22]. However, this should only happen when teleomorphs have not been found for a specific species or it is not clear if a particular teleomorph is the same species as a particular anamorph. Accordingly, since 2013, the International Botanical Congress states that the system for allowing separate names for the anamorph state should end [23]. The new International Code of Nomenclature for algae, fungi, and plants, the Melbourne Code, supports the directive that fungal species or higher taxon should be assigned with a single valid name. Accordingly, anamorph yeast genera like
the teleomorph state [14]. The anamorph represents the asexual stage of a fungus contrasting with the teleomorph form that defines the sexual stage of the same fungus [21]. The primary name of a species relies on the sexual state or teleomorph, but a second valid name may rely on the asexual state or anamorph [22]. However, this should only happen when teleomorphs have not been found for a specific species or it is not clear if a particular teleomorph is the same species as a particular anamorph. Accordingly, since 2013, the International Botanical Congress states that the system for allowing separate names for the anamorph state should end [23]. The new International Code of Nomenclature for algae, fungi, and plants, the Melbourne Code, supports the directive that fungal species or higher taxon should be assigned with a single valid name. Accordingly, anamorph yeast genera like
Candida should be revised to turn the genus consistent with phylogenetic affinities [19]. Notwithstanding, the reclassification of several
should be revised to turn the genus consistent with phylogenetic affinities [19]. Notwithstanding, the reclassification of several
C. utilis
as
C. jadinii
in several culture type collections is still confusing, reaching a point where the same strain is designated as
C. utilis
and
C jadinii
in different culture type collections. This aspect, together with the previous nomenclatures used in research papers, leads to unnecessary misunderstandings. To clarify this,
presents the alternative designations of the main laboratory strains.
Table 1.
Main
C. jadinii
(former
C. utilis
) strains described in literature.
| Nomenclature in Literature |
Current Nomenclature | Isolation Source | Reference |
|---|---|---|---|
| Candida utilis NBRC 0988 |
C. jadinii ATCC 9950; CBS 5609; DSM 2361; NBRC 0988; NCYC 707; NRRL Y-900 |
Yeast factory in Germany |
[24] |
| C. utilis ATCC 9256 a | C. jadinii NRRL Y1084; CBS 841; CCRC 20334; DSM 70167; NCYC 359; VKM Y768; VTT C79091 | Unknown | [25][26][25,26] |
| C. utilis ATCC 9226 a NBRC 1086 |
C. jadinii VTT C-71015; FMJ 4026; NBRC 1086 | Unknown | [25][27][28][25,27,28] |
| C. utilis IGC 3092 | C. jadinii PYCC 3092; CBS 890; VKM Y-33 | Unknown | [29][30][31][29,30,31] |
| C. utilis CCY 39-38-18 | C. jadinii CCY 029-38-18 b | Unknown | [32] |
| C. utilis NCYC 708 | C. jadinii NCYC 708; ATCC 42181; CBS 5947; VTT C-84157 | Unknown | [33] |
| C. utilis CBS 4885 NRRL Y-6756 |
C. jadinii CBS 4885; NRRL Y-6756; NBRC 10708 | Cow with mastitis | [34] |
| C. utilis CBS 567 NRRL Y-1509 |
C. jadinii CBS 567; NRRL Y-1509 | Yeast deposit in distillery |
[34] |
| C. utilis CBS 2160 | C. jadinii CBS 2160 | Flower of Taraxacum sp. | [34] |
| C. utilis CBS 621 | C. jadinii CBS 621; NRRL Y-7586; ATCC 22023; PYCC 4182 | Yeast factories | [35] |
| C. utilis CBS 1600 | C. jadinii CBS 1600; NRRL Y-1542; ATCC 18201 | Pus of a woman abscess |
[16] |
a This strain has been discontinued in ATCC. b The strain number reported in the literature is not available in the Culture Collection of Yeasts (CCY), all C. jadinii strains are registered as 029-38-XX, including C. jadinii 029-38-18, the likely match to CCY 39-38-18.
C. jadinii belongs to the phylum Ascomycota, subphylum Saccharomycotina. The members of this subphylum constitute a monophyletic group of ascomycetes that are well defined by ultrastructural and DNA characteristics [13]. These include lower amounts of chitin in overall polysaccharide composition at cell walls, being unable to stain with diazonium blue, low content of guanine and cytosine (G + C < 50%) at nuclear DNA, and presence of continuous holoblastic bud formation with wall layers.
belongs to the phylum Ascomycota, subphylum Saccharomycotina. The members of this subphylum constitute a monophyletic group of ascomycetes that are well defined by ultrastructural and DNA characteristics [13]. These include lower amounts of chitin in overall polysaccharide composition at cell walls, being unable to stain with diazonium blue, low content of guanine and cytosine (G + C < 50%) at nuclear DNA, and presence of continuous holoblastic bud formation with wall layers.
C. jadinii
belongs to the Saccharomycetes class, Saccharomycetidae family, Saccharomycetales order, and the
Cyberlindnera genus. However, a comprehensive phylogenetic analysis and evolutionary relationship are still missing for this species [36][37][38]. Aiming at filling this gap, we performed a robust phylogeny reconstruction [36][39][40][41]. As can be depicted in
genus. However, a comprehensive phylogenetic analysis and evolutionary relationship are still missing for this species [36,37,38]. Aiming at filling this gap, we performed a robust phylogeny reconstruction [36,39,40,41]. As can be depicted in
, this yeast localizes in the Phaffomycetaceae clade together with
Cyberlindnera fabianii
and
Wickerhamomyces ciferri
. The nearest neighbors belong to the Saccharomycetaceae family, which includes a clade with
S. cerevisiae/Torulaspora delbrueckii
species and another clade with
Eremothecium gossypii
(former
Ashbya gossypii
),
Kluyveromyces lactis/marxianus
, and
Lachancea
species. Despite the previous genus nomenclature adopted for
C. jadinii
(Candida and Pichia), it is phylogenetically distant from the Debaryomycetaceae and Pichiaceae families that include the
Candida
species, except
C. glabrata
, the
Pichia kudriavzevii
, and
Ogataea
species.
Komagataella phaffi
is as expected included in the Pichiaceae clade, together with
Komagataella pastoris [36][40]. The Trichomonascaceae/ Dipodascaceae clade, formerly known as the
[36,40]. The Trichomonascaceae/ Dipodascaceae clade, formerly known as the
Yarrowia
clade, includes now the
Sugiyamaella lignohabitans
species together with
Yarrowia lipolytica
, and is the most distant yeast clade, except for the Schizosaccharomycetaceae that clusters with all the Basiodiomycota. The filamentous fungi
Neurospora crassa
and
Fusarium graminearum are in different clades as members of the Sordariaceae and Nectriaceae clades, respectively [40]. In addition, in the Sordariaceae clade, the phylogenetic position of
are in different clades as members of the Sordariaceae and Nectriaceae clades, respectively [40]. In addition, in the Sordariaceae clade, the phylogenetic position of
Thermothelomyces thermophila
species (
Myceliophthora thermophila) was uncovered [39][42].
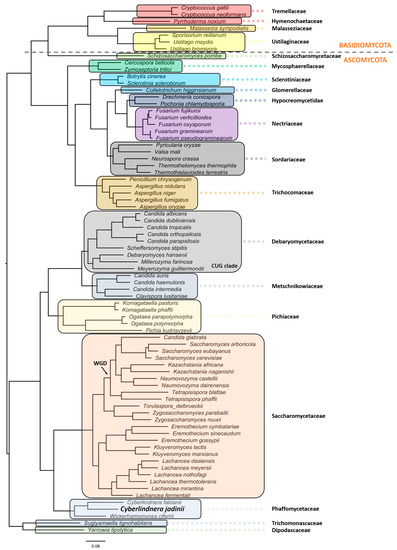
Figure 1.
Evolutionary relationship of
Cyberlindnera jadinii
, a member of the Phaffomycetaceae clade. The phylogenetic reconstruction was obtained using the following parameters: maximum likelihood in IQ-TREE (
), the model of amino acid evolution JTT (Jones-Taylor-Thornton), and four gamma-distributed rates. Homologues were detected for 1567 proteins across the proteome of 77 selected fungal species from NCBI. The 1567 set of proteins were aligned and then concatenated in order to use in the phylogenetic analysis. These proteins offer a clear high-resolution evolutionary view of the different species, as they are essential proteins beyond the specific biology of the different yeasts. Bootstrapping provided values of 100% for all the nodes. Yeast and fungi families are highlighted with different colors and shades. The phylogenetic relationships reflect evolutionary ancestries, independently of adaptations and overall gene contents within the various species. All families with more than one representative species in the analyses formed monophyletic groups.
3. Morphology and Physiology
The
C. jadinii microscopic view provided by Kurtzman et al. (2011) has shown the diversity of cell shapes and sizes [20][34] after 10–30 days at 25 °C in 5% Malt Extract Agar media. The cell patterns of
microscopic view provided by Kurtzman et al. (2011) has shown the diversity of cell shapes and sizes [20,34] after 10–30 days at 25 °C in 5% Malt Extract Agar media. The cell patterns of
C. jadinii
CBS 1600 varied from ellipsoidal to elongated occurring in single cells or in pairs. Some pseudohyphae forms were also detected with diameter balanced between (2.5–8.0) and (4.1–11.2) µm.
shows
C. jadinii DSM 2361 strain cultivated on YPD or Malt Extract Agar media for 3 (A and C) and 12 days (B and D) at 30 °C. The colonies are white, round, with a smooth texture, an entire margin, and a convex elevation trait (C and D). Cells present an ellipsoidal to elongated form, with a diameter between 5 and 7.5 µm (A and B). Yeast cell morphology can be tightly influenced by the environment. These modifications can affect the fermentation performance by inducing rheological changes that can influence mass and heat transfer alterations in the bioreactor [43]. However, in a study performed by Pinheiro et al. (2014), the CBS 621 strain cultured in a pressurized-environment triggered with 12 bar air pressure presented no significant differences in cell size and shape [35].
DSM 2361 strain cultivated on YPD or Malt Extract Agar media for 3 (A and C) and 12 days (B and D) at 30 °C. The colonies are white, round, with a smooth texture, an entire margin, and a convex elevation trait (C and D). Cells present an ellipsoidal to elongated form, with a diameter between 5 and 7.5 µm (A and B). Yeast cell morphology can be tightly influenced by the environment. These modifications can affect the fermentation performance by inducing rheological changes that can influence mass and heat transfer alterations in the bioreactor [49]. However, in a study performed by Pinheiro et al. (2014), the CBS 621 strain cultured in a pressurized-environment triggered with 12 bar air pressure presented no significant differences in cell size and shape [35].
C. jadinii is a homothallic species and forming hat-shaped ascospores that can be present in a number of one to four in unconjugated deliquescent asci [34].
is a homothallic species and forming hat-shaped ascospores that can be present in a number of one to four in unconjugated deliquescent asci [34].
Cyberlindnera
species can assimilate several compounds, namely, sugars and organic acids. The robust fermentation characteristics of
C. jadinii allow growth in a diversity of substrates from biomass-derived wastes, including hardwood hydrolysates from the pulp industry, being able to assimilate glucose, arabinose, sucrose, raffinose, and D-xylose [8][9][10][44]. As previously mentioned,
allow growth in a diversity of substrates from biomass-derived wastes, including hardwood hydrolysates from the pulp industry, being able to assimilate glucose, arabinose, sucrose, raffinose, and D-xylose [8,9,10,50]. As previously mentioned,
C. jadinii is a Crabtree-negative yeast, reaching higher cell yields under aerobic conditions [45][46][47] than Crabtree-positive species. The Crabtree-negative effect favors the respiration process over fermentation, enabling the development of phenotypes relevant for protein production [48]. This species has a high tolerance to elevated temperatures, being able to grow in a broad spectrum of temperatures from 19 to 37 °C [37] and to tolerate long-term mild acid pHs (~3.5) [49]. Another relevant property is the ability to release proteins to the extracellular medium [50]. Significant lipase and protease content were achieved using a wild
is a Crabtree-negative yeast, reaching higher cell yields under aerobic conditions [51,52,53] than Crabtree-positive species. The Crabtree-negative effect favors the respiration process over fermentation, enabling the development of phenotypes relevant for protein production [54]. This species has a high tolerance to elevated temperatures, being able to grow in a broad spectrum of temperatures from 19 to 37 °C [37] and to tolerate long-term mild acid pHs (~3.5) [55]. Another relevant property is the ability to release proteins to the extracellular medium [56]. Significant lipase and protease content were achieved using a wild
C. jadinii strain isolated from spoiled soybean oil, using solid-state fermentation [51][52].
strain isolated from spoiled soybean oil, using solid-state fermentation [57,58].
C. jadinii assimilates alcohols, acetaldehyde, organic acids, namely, monocarboxylates (DL-lactate), dicarboxylates (succinate), and tricarboxylates (citrate), sugar acids (D-gluconate), and various nitrogen sources comprising nitrate, ammonium hydroxide, as well as amino acids [8][10][12][37]. A set of metabolic advantages, as the high metabolic flux in TCA cycle, the great amino acid synthesis ability, and strong protein secretion turns
assimilates alcohols, acetaldehyde, organic acids, namely, monocarboxylates (DL-lactate), dicarboxylates (succinate), and tricarboxylates (citrate), sugar acids (D-gluconate), and various nitrogen sources comprising nitrate, ammonium hydroxide, as well as amino acids [8,10,12,37]. A set of metabolic advantages, as the high metabolic flux in TCA cycle, the great amino acid synthesis ability, and strong protein secretion turns
C. jadinii
a yet underexplored host for bioprocesses. An incomplete understanding of genetics, metabolism, and cellular physiology combined with a lack of advanced molecular tools for genome edition and metabolic engineering manipulation of
C. jadinii
hampered its development for cell factory utilization.
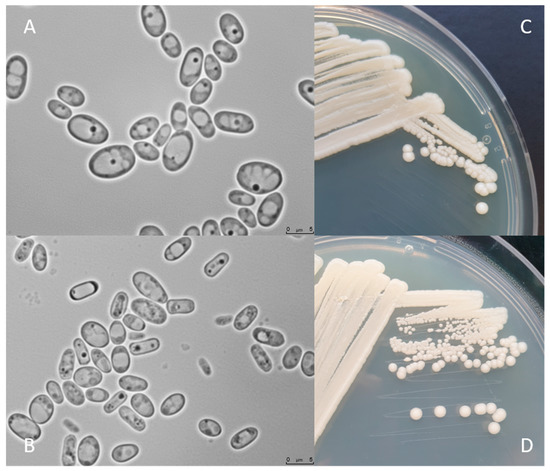
Figure 2.
C. jadinii
DSM 2361 morphological traits. (
A
) Microscopic photographs of
C. jadinii
cells after 3 days (
A
) and 12 days (
B
) of growth at 30 °C on yeast extract-peptone-dextrose media. Scale bars are 5.0 µm. Macro-morphological features of
C. jadinii
after 3 days of growth in YPD (
C
) and Malt Extract Agar (
D) media, at 30 °C.
) media, at 30 °C.
4. Emerging Biotechnological Applications
4.1. Therapeutic Applications
Being an edible yeast, C. jadinii has the potential to target the gastrointestinal tract in humans and animals [10,55]. C. jadinii’s robust growth characteristics including insensitivity to low pH and temperatures up to 40 °C, allow its transit in the gastrointestinal tract without losing viability [55,73]. Additionally, like other food constituents, intact/partially degraded C. jadinii cells may adhere to M cells in the small intestine. Upon this, they are translocated to antigen-presenting cells of Peyer’s plaques or to other lymphoid tissue connected with the gastrointestinal tract [74,75]. The ingestion of engineered C. jadinii cells, carrying a myelin oligodendrocyte glycoprotein antigen on its surface, promoted tolerance to self-antigens in a mouse model of the autoimmune multiple sclerosis (MS) disease [76]. C. jadinii cells expressing the immunodominant MOG35–55 epitope of a myelin protein on their surface, fused with the native fungal Gas1 cell wall protein, prevented the typical MS symptoms in this animal model [76]. The cell surface display of antigens by C. jadinii seems to modulate immune responses, either by suppression to combat autoimmune disease or through immune stimulation, enabling the creation of edible vaccines [73,76,77]. C. jadinii cells were also applied as probiotic agents against fungal infections (particularly oral candidiasis) as an antagonist to the relevant human fungal pathogens Candida albicans (strains SC5312, 10341, and GDH2346), Aspergillus sp., and Fusarium sp. [73]. The unidentified toxins secreted by C. jadinii act as antagonistic compounds conditioning the growth, systemic invasion, and disease caused by these fungal pathogens. Buerth et al. [10] reported the role of C. jadinii and also W. farinosa as antagonistic agents against C. albicans by inhibiting its growth and morphogenesis, proposing their exploitation for the formulation of new prebiotic compounds and strategies to tackle candidiasis. C. jadinii was also described to secrete heterologous proteins into the growth medium, including lipase B from Candida antarctica (CalB) [56]. The signal sequence of the enzyme invertase, one of the most predominant proteins of the C. jadinii secretome, allowed high secretion levels of recombinant CalB [5]. Lipases have a great potential application in substitution therapies, where metabolic deficiency is overcome by external administration of these enzymes in diseased conditions [78]. Furthermore, lipase activity can go from activation of tumor necrosis factor, having a relevant role over the treatment of malignant tumors, to the treatment of gastrointestinal disturbances, digestive allergies, or dyspepsias [78]. In addition, CalB is involved in enzymatic resolutions, desymmetrization, and aminolysis events with application in not only pharmaceutical but also biotech industry, having a role in polymer production [78,79]. C. jadinii is also able to synthesize (R)-phenylacetylcarbinol (L-PAC), the pharmaceutical precursor for L-ephedrine and pseudoephedrine, relevant compounds used in the treatment of nasal congestion [80,81,82,83]. The function of 30 ATP-binding cassette transporters (ABC) transporters was studied by the amplification of predicted C. jadinii gDNA ORFs. The function of putative multidrug efflux pumps was evaluated by heterologous expression in S. cerevisiae ADΔ, a strain disrupted in seven of its major multidrug efflux pumps: Pdr5p, Pdr10p, Pdr15p, Snq2p, Pdr11p, Ycf1p, and Yor1p [84]. This strategy uncovered the mechanism of action of CjCdr1, C. jadinii’s closest homolog of the multidrug efflux pump C. albicans Cdr1. The characterization of C. jadinii multidrug efflux systems can turn C. jadinii into an appealing host for the development of novel antimicrobial agents [85], as it is imperative to understand the structure, function, and expression of multidrug efflux pumps in order to develop optimal novel antimicrobial agents.
4.2. Bioproduction of Valuable Compounds Using Cost-Effective Carbon Sources
Recombinant C. jadinii strains were developed for the production of a variety of compounds from food supplements such as vitamins (biotin) [86], carotenoids (lycopene, β-carotene, and astaxanthin) [60,68], to proteins (α-amylase, monellin) [64], antioxidant glutathione [87], polysaccharides (glucomannan) [88,89], organic acids (L-lactic acid) [44,59], and ribonucleic acids [5,24]. Additionally, the production of secreted enzymes such as invertase and phospholipase B (NBRC 1086 strain) [27,28] was also explored. Cells of C. jadinii DSM 2361 were successfully engineered for the secretion of Penicillium simplicissimum xylanase (PsXynA) to the culture medium [65] allowing cells to grow on xylan as the sole carbon source. Cells expressing the xylose reductase from Candida shehatae and the native xylitol dehydrogenase, in combination with further multiple site-directed mutations in coenzyme binding sites, resulted in the highest titer of 17.4 g/L of ethanol from 50 g/L of xylose in 20 h [90]. Organic acids present in industrial waste streams (e.g., acetic acid, propionic, or butyric acid) have been demonstrated to be suitable substrates for biomass production, reaching biomass yields varying from 30% to 40% in batch cultures, while in continuous cultures, an average of 44–55% was achieved [91,92]. Despite its already important role as an industrial microorganism, further developments are still necessary to fully explore the biotechnological potential of this yeast.
4.3. Industrial Applications—A Patent-View
In recent years, the applications of C. jadinii were extended to cosmetic and health care products and to the chemical-process industry for the production of chiral chemicals, as well as for agriculture and wine making. In this last application, C. jadinii yeast was used in the production of loquat wine, being introduced after S. cerevisiae fermentation to reduce acid content and enhance the aroma [93,94]. It is also used in another fermentation process, for the production of an alcohol-free fruit wine rich in lovastatin [95]. In the cosmetic industry, a β-D-glucan polysaccharide produced by C. jadinii was applied in formulations of several products, i.e., body lotions [96], sunscreen cream [97], facial cleanser [98], toner [99], eye cream [100], shampoo [101], body wash [102], and hand-care cream [103]. This bioproduct is mainly added for its properties as a moisturizing agent and for conferring oxidation and radiation resistance. C. jadinii was also used for the efficient production of a recombinant uricase, active in humans and with greater stability and/or activity than naturally occurring enzymes [104]. This enzyme can be used for the treatment of hyperuricemia-related diseases or other human pathological symptoms. Considering the chemical industry, the bioproduction of methyl fluorophenyl methyl propionate was achieved with a developed reduction method using C. jadinii as a biocatalyst [105]. The obtained chemical, (2S,3S)-3-(4-fluorophenyl)-3-hydroxy-2-methyl methyl propionate, is reported to be produced in high yield and with a high level of the enantiomeric excess rate. The wide applicability of this chiral building-block chemical can go from the synthesis of chiral drugs, fine chemicals to pesticides [105]. In agriculture, a consortium of strains that include C. jadinii was incorporated in an organomineral granular fertilizer containing among other components fulvic acid, and a natural mineral component (activated natural siliceous zeolite-containing rock). Its properties allow the reduction in the amount of fertilizer introduced in the soil with a prolonging action improved [106]. As C. jadinii is capable of efficiently converting a cadmium form from contaminated soil, it is now being proposed for soil bioremediation [107]. In addition, a microbial soil conditioner for lithified soil was also developed involving a C. jadinii strain along with Bacillus megaterium, Bacillus subtilis, Rhodopseudomonas palustris, and Azotobacter chroococcum species. The full interaction among the aforementioned strains was claimed to improve the soil ecological environment and alter the soil lithiation event, thereby contributing for the purpose of turning the soil suitable for farming [108].
