Zoonotic diseases, defined as diseases or infections that are naturally transmissible from vertebrate animals to humans, represent a significant threat to global health. Among the species recognized as pathogenic to humans, more than half originated in animals, and some have been characterized as emerging or re-emerging pathogens. Most zoonotic pathogens originated in wild and domesticated mammalian hosts such as bats, rodents, and primates. The analysis of global trends indicates that new zoonotic threats will continue to emerge at an accelerating rate, and are mainly associated with an growthing population, changes in land use, climate changes, increased intercontinental travel, and expanded trade networks. Poxviruses are among mankind’s longest and best-known viruses mainly because of their most feared and lethal representative, Variola virus (VARV), the causative agent of smallpox. Orthopoxvirus is the most important and well-characterized poxvirus genus, mainly due to its impact on human and animal health. Orthopoxviruses are remarkable for their wide host spectrum, ranging from humans to domestic and wild animals.
- Orthopoxvirus
- Poxviridae
- zoonosis
- Monkeypox virus
- Cowpox virus
- Vaccinia virus
- host range
- wild and domestic animals
- emergent viruses
- outbreak
1. Poxvirus
- Poxvirus
Poxviruses are of great veterinary and human importance and infect numerous vertebrate and invertebrate animals, including humans. The Poxviridae family is divided into two subfamilies, namely: Chordopoxvirinae, which infect vertebrates, and Entomopoxvirinae (A–C), which infect invertebrates. The Chordopoxvirinae subfamily is further divided into 18 genera (Avipoxvirus, Capripoxvirus, Centapoxvirus, Cervidpoxvirus, Crocodylidpoxvirus, Leporipoxvirus, Macropopoxvirus, Molluscipoxvirus, Mustelpoxvirus, Orthopoxvirus, Oryzopoxvirus, Parapoxvirus, Pteropopoxvirus, Salmonpoxvirus, Sciuripoxvirus, Suipoxvirus, Vespertilionpoxvirus, and Yatapoxvirus), distinguishable by their serological reactions
Poxviruses are of great veterinary and human importance and infect numerous vertebrate and invertebrate animals, including humans. The Poxviridae family is divided into two subfamilies, namely: Chordopoxvirinae, which infect vertebrates, and Entomopoxvirinae (A–C), which infect invertebrates. The Chordopoxvirinae subfamily is further divided into 18 genera (Avipoxvirus, Capripoxvirus, Centapoxvirus, Cervidpoxvirus, Crocodylidpoxvirus, Leporipoxvirus, Macropopoxvirus, Molluscipoxvirus, Mustelpoxvirus, Orthopoxvirus, Oryzopoxvirus, Parapoxvirus, Pteropopoxvirus, Salmonpoxvirus, Sciuripoxvirus, Suipoxvirus, Vespertilionpoxvirus, and Yatapoxvirus), distinguishable by their serological reactions
[1]
[2]
. The family Poxviridae comprises large, brick-shaped or ovoid enveloped viruses containing a linear, double-stranded DNA genome approximately 200 kilobase pairs in length
. Poxviruses are among mankind’s longest and best-known viruses mainly because of their most feared and lethal representative, Variola virus (VARV), the causative agent of smallpox. Before its remarkable eradication in 1980, VARV represented a centuries-old threat to humans worldwide and killed approximately 300–500 million people during the 20th century
[5]
. The global eradication of smallpox marked the culmination of an intensive vaccination program and quarantine measures promoted by the World Health Organization (WHO)
. Although VARV was eradicated 40 years ago, many challenges regarding poxvirus infections persist, including the worrisome possibility of VARV reintroduction by accidental release, its use as a biological weapon, or biological weapon, or the emergence and re-emergence of zoonotic orthopoxviruses worldwide
. Among Poxviruses, the Orthopoxvirus genus is the most important and well-characterized, mainly due to its impact on human and animal health, and for its remarkable wide host spectrum, ranging from humans to domestic and wild animals
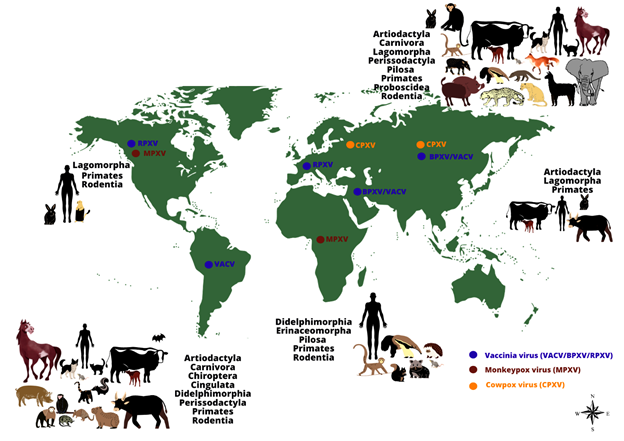
(Figure 1).
Figure 1:
The worldwide distribution and host range of monkeypox, cowpox and vaccinia viruses. The image shows the range of animal hosts (represented by orders) that have been demonstrated to be naturally infected by some Orthopoxvirus species, according to different regions of the world (except by Monkeypox virus in the United States of America, represented by imported cases). Orthopoxvirus infections have been demonstrated in animals belonging to different orders, using different methods (virus isolation, molecular detection of viral genomes or serological screening for antibodies against orthopoxviruses). The occurrence of some zoonotic orthopoxviruses has already been confirmed (by virus isolation or molecular detection of the viral genome) in some geographical regions (indicated by colored dots: blue: vaccinia virus (including buffalopox and rabbitpox viruses) in South America, Europe, Asia, and the Middle East; brown: monkeypox virus in Africa and North America; orange: cowpox virus in Europe and Asia).
2. Orthopoxvirus
- Orthopoxvirus
The Orthopoxvirus genus comprises VARV, vaccinia (VACV) and vaccinia-like, cowpox (CPXV), monkeypox (MPXV), camelpox, and Akhmeta viruses, and other species with zoonotic potential. All orthopoxviruses share significant DNA sequence similarity and are immunologically cross-reactive and cross-protective. Infection with any orthopoxvirus is considered to generate protection against exposure or re-exposure to any other member of the genus
The Orthopoxvirus genus comprises VARV, vaccinia (VACV) and vaccinia-like, cowpox (CPXV), monkeypox (MPXV), camelpox, and Akhmeta viruses, and other species with zoonotic potential. All orthopoxviruses share significant DNA sequence similarity and are immunologically cross-reactive and cross-protective. Infection with any orthopoxvirus is considered to generate protection against exposure or re-exposure to any other member of the genus
. Orthopoxvirus species are named primarily according to the hosts from which they were first isolated and identified; however, the name does not necessarily represent its natural reservoir or complete host range
. Despite a large number of studies, little is known about the primary hosts and reservoirs of zoonotic orthopoxviruses in nature, or their transmission and maintenance cycles
[17]
. Regarding the host range, orthopoxviruses can be both highly specialized and host restricted or generalists with a broad host range. For instance, VARV is a highly specialized virus that infects only humans, whereas MPXV, CPXV, and VACV are examples of generalist zoonotic orthopoxviruses that can infect several mammalian host species and also spillover into humans
[17]
. The evolution of generalist pathogens requires the successful crossing of host transmission barriers
[18]
. These include geographical, ecological, and behavioral constraints that separate a virus from its possible recipient hosts; virus-host cell incompatibility, such as tissue tropism, differences in receptor binding, genome replication, production, and shedding of infectious particles; and host immunity evasion, which includes cellular barriers or responses that restrict the infection and/or evasion of a virus from the innate immune system of its host
[19]
. To overcome these barriers, orthopoxviruses have different biological features that can synergistically contribute to the transmission to, and exploitation of, a broad range of new hosts species as observed for CXPV, MPXV, and VACV.
Orthopoxviruses can cause both local lesions on the skin and systemic infections, resulting in direct and indirect transmission routes. When accompanied by viral particle stability in the environment, this can increase the likelihood of potential hosts being exposed to the virus independently of direct contact with infected hosts. In addition, orthopoxviruses can infect a variety of mammalian cells in a manner that is mostly independent of species-specific receptors and have large genomes that carry the information essential for viral replication, thereby increasing the possibility of successful infection in a new cell/host. Although the double-stranded DNA genomes of orthopoxviruses have low mutation rates when compared with other viruses, such as RNA viruses, orthopoxviruses possess a genetic arsenal comprising several immune-regulatory, virulence, and host range genes
Orthopoxviruses can cause both local lesions on the skin and systemic infections, resulting in direct and indirect transmission routes. When accompanied by viral particle stability in the environment, this can increase the likelihood of potential hosts being exposed to the virus independently of direct contact with infected hosts. In addition, orthopoxviruses can infect a variety of mammalian cells in a manner that is mostly independent of species-specific receptors and have large genomes that carry the information essential for viral replication, thereby increasing the possibility of successful infection in a new cell/host. Although the double-stranded DNA genomes of orthopoxviruses have low mutation rates when compared with other viruses, such as RNA viruses, orthopoxviruses possess a genetic arsenal comprising several immune-regulatory, virulence, and host range genes
[17]
. The variety of host-genes among poxviruses enables them to express different viral proteins with important roles in cell tropism, as well as in the modulation of host signaling pathways and immunomodulatory responses, thereby establishing optimal cellular conditions for viral replication
[20]
. Finally, many of the strategies employed by orthopoxviruses to evade host immune defenses target conserved elements of the immune system in different potential hosts
[17]
. Combined, these features altogether are crucial for virus-cell and virus-host interactions and can contribute to the success of viral replication and transmission. Despite the eradication of smallpox, the possibility of its re-emergence or the emergence of other orthopoxviruses in human and animal populations is a relevant global health issue. As smallpox vaccination is no longer mandatory, most of the world’s population that is under 40 years of age lacks immunity against orthopoxviruses
. This scenario is highlighted by numerous reports in recent years of human diseases caused by zoonotic orthopoxviruses such as MPXV
[23][24][25][26][27][28][29][30]
, CPXV
[31][32][33][34][35][36][37][38]
, VACV-like
[39][40][41][42][43][44][45][46]
, and Akhmeta virus
[16]
. To date, the circulation of orthopoxviruses among wild and domestic animals has been recorded in different regions of the world, including South America, Africa, Europe, the Middle East, and Asia
[24][37][39][47][48][49][50][51][52][53][54][55]
. These facts raise concerns regarding the host ranges and distribution of orthopoxviruses, as well as their potential to cause outbreaks in animals and human populations, thereby further impacting animal and public health.
2.1. Monkeypox Virus
Monkeypox virus isolates are subdivided into two clades, namely, the West African and the Congo Basin clades, based on genetic and phenotypic (virulence) differences
[56]
. Notably, several studies have indicated that the clinical signs are similar between infections caused by viruses from either clade
[57]
. The first observation of MPXV infection was reported in 1958 during an outbreak of pustular rash illness in cynomolgus macaques (
Macaca fascicularis
) arriving in Copenhagen, Denmark, from Singapore
[58]
. Despite being named after the first described host, non-human primates are accidental hosts for MPXV
[59]
. In 2003, an MPXV outbreak occurred in the United States of America (USA). Human infection was associated with direct contact with ill pet prairie dogs that were kept near to infected exotic animals imported from Ghana, West Africa
[60]
. This episode, as well as the infection of rodents, heightened concerns regarding the introduction of MPXV into the Americas. Meanwhile, the susceptibility of several African rodents to MPXV raised worries about the transmission of the virus to humans, as these animals are sometimes kept as pets
. Although humans are also accidental hosts
[59]
, MPXV became the most significant pathogenic zoonotic orthopoxvirus for humans since the eradication of smallpox, given its associated morbidity (systemic infection) and lethality. The natural source of MPXV and its maintenance cycle in nature remains unknown as the virus has only been isolated twice in nature (wild animals): once from the rope squirrel (
Funisciurus anerythrus
), Zaire, in 1985
[63]
, and once from the sooty mangabey (
Cercocebus atys
), Côte d’Ivoire, in 2012
[64]
. To date, naturally occurring MPXV infections remain confined to the forest regions of West and Central Africa
[65]
. Consequently, a higher proportion of human MPXV cases are reported in regions (mainly African villages) where humans and non-human primates live in close proximity. The consumption, hunting, and handling of meat derived from non-human primates, rodents, and other small mammals have also been associated with human cases of MPXV infection
. Close contact with rodents has also been implicated as a source of human infection
. As monkeypox is an emerging zoonotic disease with epidemic potential and much of its host range and maintenance cycle in nature remains obscure, advances are urgently needed to better understand the natural cycle of MPXV.
2.2. Cowpox Virus
Edward Jenner was the first to document CPXV infection after observing local lesions on the teats of cows, which he called “cow-pox”. Then, in 1798, Jenner demonstrated the efficacy of “true cow-pox” scarification in inducing immunity against smallpox
Edward Jenner was the first to document CPXV infection after observing local lesions on the teats of cows, which he called “cow-pox”. Then, in 1798, Jenner demonstrated the efficacy of “true cow-pox” scarification in inducing immunity against smallpox
. There were frequent reports of bovine cowpox cases until the early 1970s in Europe, with sporadic transmission to humans, mainly milkers, occurring via contact with infected cows
[75]
. CPXV is currently mostly found in Europe and northern and central Asia where cases of infections in rodents, cats, and humans continue to be reported. In Great Britain, CPXV is endemic in rodents such as bank voles (
Myodes glareolus
) and wood mice (
Apodemus sylvaticus
), while in Turkmenistan and Russia CPXV was isolated in the laboratory as well as in wild rodents
[76]
. Furthermore, serological surveys have also detected orthopoxvirus infections in France, Austria, and Norway in voles and wood mice
[76]
. Antibodies against orthopoxviruses were also detected in red foxes
(Vulpes vulpes
) in Western Europe being possibly related to CPXV infection, although red foxes are also known to be susceptible to ectromelia virus
. These reports of CPXV infection have occurred alongside an increasing number of reported infections in different animal species, leading to the designation of CPXV as an emerging health threat
[78]
. Cats are the most affected domestic animals, mainly due to their predatory behavior against rodents, which are the CPXV reservoir in domestic and peridomestic environments
. However, the exact prevalence of feline cowpox is uncertain. CPXV infections in cats are mostly observed after increases in the rat population density
. The infection of pet rats and domestic cats by CPXV brings a higher risk of exposure to humans in the domestic environment, but rural or wild areas may be important as the source of infection
[33]
. Even though human cowpox cases are usually self-limiting and not lethal, most people are susceptible to the disease, particularly children who are more often in close contact with pet animals
. The zoonotic potential of CPXV and its capacity to cause infection in wild and domestic environments are well established; however, many aspects of its natural maintenance cycle remain unknown.
2.3. Vaccinia Virus and Related Viruses
Although VACV is the most extensively studied orthopoxvirus, its origin remains unknown
Although VACV is the most extensively studied orthopoxvirus, its origin remains unknown
[84]
. Vaccinia virus, the prototype species of the Orthopoxvirus genus, is best known as the live attenuated virus used worldwide by the WHO in the smallpox vaccine
. Despite the successful use of VACV as a vaccine, several vaccine strain-dependent complications have been reported, including progressive vaccinia, eczema vaccinatum, vaccinia gangraenosum, and neurological complications
. During smallpox eradication campaigns, various VACV strains with different degrees of virulence were used. The highly attenuated and modified VACV Ankara is a well-stablished third-generation smallpox vaccine
. For a long time, VACV vaccine strains were assumed to be incapable of establishing a natural cycle due to their attenuation in the laboratory. However, several VACVs have been isolated from different host species, and in different locations around the world
. Similarly, sub-lineages of VACV (as buffalopox virus (BPXV) and rabbitpox virus (RPXV)) have been consistently isolated in different countries and from a wide range of hosts
. In India, BPXV was first described in 1934 and was responsible for infections that mainly affected domestic buffaloes (Bubalus bubalis), but also cows and humans
[97]
. Since its first description, outbreaks of BPXV have been reported in India, Pakistan, Nepal, Egypt, Bangladesh, Indonesia, Russia, and Italy
. Humans become infected with BPXV through close contact with infected animals and no human-to-human transmission has been reported to date. Additionally, a variety of animal species, such guinea pigs, BALB and white Swiss mice, cows, buffalo calves, rabbits, and chickens have been experimentally demonstrated to be susceptible to BPXV. Nevertheless, the role of these species in BPXV transmission and maintenance in nature remains unknown
and requires clarification. RPXV is another VACV described as affecting different animal species worldwide. RPXV was first described between 1930–1933 after outbreaks in laboratory rabbits in the United States of America. Additional outbreaks were later reported in 1941 in the Netherlands, while several other cases were also reported in Europe and the USA
. To date, no human transmission has been described for RPXV
. Different VACV isolates also circulate in South American countries, including Uruguay, Argentina, Colombia, and Brazil
. In the last few decades, several outbreaks of VACV infection have occurred in Brazil where the disease caused by VACV is popularly known as “bovine vaccinia”, due to its association with dairy cattle
. Bovine vaccinia is characterized by vesiculopustular exanthematous disease in cattle and dairy workers who have direct contact with infected animals
. Initially, VACV outbreaks were described as affecting dairy cattle and humans in rural environments. Consequently, the epidemiology of the bovine vaccine in Brazil is associated with economic losses resulting from compromised milking herds
. Nevertheless, VACV circulation in Brazil has already been documented for all the regions, affecting farm animals other than cattle, as well as wild animals
[39]
. Although VACV is known to have a broad range of hosts, many aspects of its natural history remain unknown. Although VACV infection is usually self-limiting and not lethal, the disease profile in immunocompromised individuals may be differentially affected, presenting with severe and generalized manifestation
[112]
, similar to that observed for cowpox. Although farm animals are important sources of infection, the commercialization and consumption of dairy products could be alternative routes of zoonotic VACV transmission. In addition, VACV circulation in domestic animals such as cats and dogs bring the risk of viral transmission to humans in the domestic environment. The urban emergence of VACV could be an important health burden due to the unpreparedness of healthcare professionals to correctly identify and handle emerging cases
[113]
.
References
- Frank Fenner; Adventures with poxviruses of vertebrates. FEMS Microbiology Reviews 2000, 24, 123-133, 10.1016/S0168-6445(00)00027-9.
- Moss, B.. Poxviridae: the viruses and their replication. In Fields virology; B.N., Knipe, D.M., Howley, P.M., Griffin, D.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, 2013; pp. 2126–2159 .
- Moss, B.. Poxviridae: the viruses and their replication. In Fields virology;; Fields, B.N., Knipe, D.M., Howley, P.M., Griffin, D.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, 2013; pp. 2126–2159.
- Fenner, F.. The Poxviruses. In Portraits of Viruses. A History of Virology; Gibbs, A, Eds.; Basil, Karger: ., 1988; pp. 1-23.
- Catherine Thèves; Philippe Biagini; E. Crubézy; The rediscovery of smallpox. Clinical Microbiology and Infection 2014, 20, 210-218, 10.1111/1469-0691.12536.
- Fenner, F.. The Poxviruses. In Portraits of Viruses. A History of Virology; Gibbs, A., Eds.; Basil, Karger: ., 1988; pp. 1-23.
- Ladnyi, I.D.; Breman, J.G.; Smallpox eradication: progress and problems. Dev. Biol. Stand. 1978, 41, 281–290.
- Damon, I.K.. Poxviruses. In Manual of Clinical Microbiology, 10th Edition; Versalovic, J., Carroll, K., Funke, G., Jorgensen, J., Landry, M., Warnock, D., Eds.; American Society of Microbiology: Washington, 2011; pp. 1647–1658.
- E.J. Lefkowitz; C. Wang; C. Upton; Poxviruses: past, present and future. Virus Research 2006, 117, 105-118, 10.1016/j.virusres.2006.01.016.
- B.W.J Mahy; An overview on the use of a viral pathogen as a bioterrorism agent: why smallpox?. Antiviral Research 2002, 57, 1-5, 10.1016/s0166-3542(02)00194-8.
- Moss, B.. Poxviridae: the viruses and their replication. In Fields virology; Fields, B.N., Knipe, D.M., Howley, P.M., Griffin, D.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, 2013; pp. 2126–2159.
- Caroline Gubser; Stéphane Hué; Paul Kellam; Geoffrey L. Smith; Poxvirus genomes: a phylogenetic analysis. Journal of General Virology 2003, 85, 105-117, 10.1099/vir.0.19565-0.
- Sandra Essbauer; Martin Pfeffer; Hermann Meyer; Zoonotic poxviruses. Veterinary Microbiology 2009, 140, 229-236, 10.1016/j.vetmic.2009.08.026.
- Sergei N. Shchelkunov; An Increasing Danger of Zoonotic Orthopoxvirus Infections. PLOS Pathogens 2013, 9, e1003756, 10.1371/journal.ppat.1003756.
- Abdelmalik I. Khalafalla; Fatima Abdelazim; Human and Dromedary Camel Infection with Camelpox Virus in Eastern Sudan. Vector-Borne and Zoonotic Diseases 2017, 17, 281-284, 10.1089/vbz.2016.2070.
- Neil M. Vora; Morgan Juliette; Marika Geleishvili; Ginny L. Emerson; Ekaterine Khmaladze; Giorgi Maghlakelidze; Archil Navdarashvili; Khatuna Zakhashvili; Maka Kokhreidze; Marina Endeladze; et al.Gela MokverashviliP.S. SatheshkumarNadia Gallardo-RomeroCynthia S. GoldsmithMaureen G. MetcalfeInger DamonEdmond F. MaesMary G. ReynoldsJuliette MorganDarin S. Carroll Human Infection with a Zoonotic Orthopoxvirus in the Country of Georgia. New England Journal of Medicine 2015, 372, 1223-1230, 10.1056/nejmoa1407647.
- Mary G Reynolds; Sarah Anne Guagliardo; Yoshinori J Nakazawa; Jeffrey B Doty; Matthew R Mauldin; Understanding orthopoxvirus host range and evolution: from the enigmatic to the usual suspects. Current Opinion in Virology 2018, 28, 108-115, 10.1016/j.coviro.2017.11.012.
- Mark Woolhouse; Louise H. Taylor; Daniel T. Haydon; Population Biology of Multihost Pathogens. Science 2001, 292, 1109-1112, 10.1126/science.1059026.
- Colin R. Parrish; Edward C. Holmes; David M. Morens; Eun-Chung Park; Donald S. Burke; Charles H. Calisher; Catherine A. Laughlin; Linda J. Saif; Peter Daszak; Cross-Species Virus Transmission and the Emergence of New Epidemic Diseases. Microbiology and Molecular Biology Reviews 2008, 72, 457-470, 10.1128/mmbr.00004-08.
- Steven J. Werden; Masmudur M. Rahman; Grant McFadden; Chapter 3 Poxvirus Host Range Genes. Advances in Virus Research 2007, 71, 135-171, 10.1016/s0065-3527(08)00003-1.
- Stephanie Gallwitz; Ted Schutzbank; Richard L. Heberling; S. S. Kalter; Jeffrey E. Galpin; Smallpox: Residual Antibody after Vaccination. Journal of Clinical Microbiology 2003, 41, 4068-4070, 10.1128/jcm.41.9.4068-4070.2003.
- Sergei N Shchelkunov; Emergence and reemergence of smallpox: The need for development of a new generation smallpox vaccine. Vaccine 2011, 29, D49-D53, 10.1016/j.vaccine.2011.05.037.
- Melissa E. Dubois; Mark K. Slifka; Retrospective Analysis of Monkeypox Infection. Emerging Infectious Diseases 2008, 14, 592-599, 10.3201/eid1404.071044.
- Reena H. Doshi; Sarah Anne J. Guagliardo; Jeffrey B. Doty; Angelie Dzabatou Babeaux; Audrey Matheny; Jillybeth Burgado; Michael B. Townsend; Clint Morgan; Panayampalli Subbian Satheshkumar; Nestor Ndakala; et al.Therese KanjingankoloLambert KitemboJean MalekaniLem’S KalembaElisabeth PukutaTobi N’KayaFabien KangoulaCynthia MosesAndrea M. MccollumMary G. ReynoldsJean-Vivien MombouliYoshinori NakazawaBrett W. Petersen Epidemiologic and Ecologic Investigations of Monkeypox, Likouala Department, Republic of the Congo, 2017. Emerging Infectious Diseases 2019, 25, 281-289, 10.3201/eid2502.181222.
- Gregory D. Huhn; Audrey M. Bauer; Krista Yorita; Mary Beth Graham; James Sejvar; Anna Likos; Inger K. Damon; Mary G. Reynolds; Matthew J. Kuehnert; Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clinical Infectious Diseases 2005, 41, 1742-1751, 10.1086/498115.
- Emmanuel Nakouné; Emmanuel Lampaert; Séverin Gervais Ndjapou; Carole Janssens; Isabel Zuniga; Michel Van Herp; Jean Paul Fongbia; Thomas Daquin Koyazegbe; Benjamin Selekon; Giscard Francis Komoyo; et al.Sandra Miriella Garba-OuangoleCasimir ManenguJean-Claude ManuguerraMirdad KazanjiAntoine GessainNicolas Berthet A Nosocomial Outbreak of Human Monkeypox in the Central African Republic. Open Forum Infectious Diseases 2017, 4, ofx168, 10.1093/ofid/ofx168.
- Weekly Bulletin on Outbreaks and Other Emergencies, week 48, 2017 . WHO. Retrieved 2021-1-21
- Weekly Bulletin on Outbreaks and Other Emergencies, week 52, 2017 . WHO. Retrieved 2021-1-21
- Weekly Bulletin on Outbreaks and Other Emergencies, week 01, 2020 . WHO. Retrieved 2021-1-21
- Weekly Bulletin on Outbreaks and Other Emergencies, week 37, 2020 . WHO. Retrieved 2021-1-21
- Brigitte Coras; Sandra Essbauer; Martin Pfeffer; Hermann Meyer; Josef Schröder; Wilhelm Stolz; Michael Landthaler; Thomas Vogt; Cowpox and a cat. The Lancet 2004, 365, 446-446, 10.1016/s0140-6736(05)17836-2.
- Laetitia Ninove; Yves Domart; Christine Vervel; Chrystel Voinot; Nicolas Salez; Didier Raoult; Hermann Meyer; Isabelle Capek; Christine Zandotti; Rémi Charrel; et al. Cowpox Virus Transmission from Pet Rats to Humans, France. Emerging Infectious Diseases 2009, 15, 781-784, 10.3201/eid1505.090235.
- A. Y. Popova; R. A. Maksyutov; O. S. Taranov; T. V. Tregubchak; A. V. Zaikovskaya; A. A. Sergeev; I. V. Vlashchenko; S. A. Bodnev; V. A. Ternovoi; N. S. Alexandrova; et al.A. L. TarasovN. V. KonovalovaA. A. KorolevaL. E. BulychevO.V. PyankovY. V. DeminaA. P. AgafonovS. N. ShchelkunovV. N. Miheev Cowpox in a human, Russia, 2015. Epidemiology and Infection 2016, 145, 755-759, 10.1017/s0950268816002922.
- C Nardin; A S Dupond; F Pelletier; E Puzenat; F Aubin; Skin Lesions in a Child After Contact With a Domestic Rat.. Clinical Infectious Diseases 2019, 68, 1063-1064, 10.1093/cid/ciy388.
- C. Haddadeen; M. Van Ouwerkerk; T. Vicek; A. Fityan; A case of cowpox virus infection in the UK occurring in a domestic cat and transmitted to the adult male owner. British Journal of Dermatology 2020, 183, 19319, 10.1111/bjd.19319.
- Tom F.W. Wolfs; Jaap A. Wagenaar; Hubert G.M. Niesters; Albert D.M.E. Osterhaus; Rat-to-Human Transmission of Cowpox Infection. Emerging Infectious Diseases 2002, 8, 1495-1496, 10.3201/eid0812.020089.
- Corinne Ducournau; Audrey Ferrier-Rembert; Olivier Ferraris; Aurélie Joffre; Anne-Laure Favier; Olivier Flusin; Dieter Van Cauteren; Kaci Kecir; Brigitte Auburtin; Serge Védy; et al.Maël BessaudChristophe Nicolas Peyrefitte Concomitant Human Infections with 2 Cowpox Virus Strains in Related Cases, France, 2011. Emerging Infectious Diseases 2013, 19, 1996-1999, 10.3201/eid1912.130256.
- Priv.-Doz. Dr. Andreas Wollenberg; S Vogel; Miklós Sárdy; K Glos; Hc Korting; Thomas Ruzicka; The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats. Acta Dermato Venereologica 2012, 92, 126-131, 10.2340/00015555-1227.
- Jaqueline Silva De Oliveira; Poliana De Oliveira Figueiredo; Galileu Barbosa Costa; Felipe L Assis; Betânia Paiva Drumond; Flavio Guimarães Da Fonseca; Maurício Lacerda Nogueira; Erna Geessien Kroon; Giliane De Souza Trindade; Vaccinia Virus Natural Infections in Brazil: The Good, the Bad, and the Ugly. Viruses 2017, 9, 340, 10.3390/v9110340.
- Venkatesan, G.; Balamurugan, V.; Prabhu, M.; Yogisharadhya, R.; Bora, D.P.; Gandhale, P.N.; Sankar, M.S.; Kulkarni, A.M.; Singh, R.K.; Bhanuprakash, V.; et al. Emerging and re-emerging zoonotic buffalopox infection: a severe outbreak in Kolhapur (Maharashtra), India. Vet Ital 2010, 46, 439–448.
- Raj Kumar Singh; M. Hosamani; V. Balamurugan; V. Bhanuprakash; T. J. Rasool; M. P. Yadav; Buffalopox: an emerging and re-emerging zoonosis. Animal Health Research Reviews 2007, 8, 105-114, 10.1017/s1466252307001259.
- Yogesh K. Gurav; Chandrashekhar G. Raut; Pragya D. Yadav; Babasaheb V. Tandale; Aruna Sivaram; Milind D. Pore; Atanu Basu; Devendra T. Mourya; Akhilesh C. Mishra; Buffalopox outbreak in humans and animals in Western Maharashtra, India. Preventive Veterinary Medicine 2011, 100, 242-247, 10.1016/j.prevetmed.2011.03.008.
- Andre Tavares Silva-Fernandes; Carlos Eurico Pires Ferreira Travassos; Jaqueline Maria Siqueira Ferreira; Jônatas Santos Abrahão; Eliseu Soares De Oliveira Rocha; Flávia Viana-Ferreira; João Rodrigues Dos Santos; Claudio Antônio Bonjardim; Paulo César Peregrino Ferreira; Erna Geessien Kroon; et al. Natural human infections with Vaccinia virus during bovine vaccinia outbreaks. Journal of Clinical Virology 2009, 44, 308-313, 10.1016/j.jcv.2009.01.007.
- Teresa Keico Nagasse-Sugahara; Jonas José Kisielius; Marli Ueda-Ito; Suely Pires Curti; Cristina Adelaide Figueiredo; Áurea Silveira Cruz; Maysa Madalena J. Silva; Carmen Helena Ramos; Maria Claudia C. Silva; Tiyo Sakurai; et al.Luis Florêncio Salles-Gomes Human vaccinia-like virus outbreaks in São Paulo and Goiás States, Brazil: virus detection, isolation and identification. Revista do Instituto de Medicina Tropical de São Paulo 2004, 46, 315-322, 10.1590/s0036-46652004000600004.
- Jônatas Santos Abrahão; Rafael Kroon Campos; Giliane De Souza Trindade; Flávio Guimarães Da Fonseca; Paulo César Peregrino Ferreira; Erna Geessien Kroon; Outbreak of Severe Zoonotic Vaccinia Virus Infection, Southeastern Brazil. Emerging Infectious Diseases 2015, 21, 695-698, 10.3201/eid2104.140351.
- Danilo Bretas Oliveira; Jônatas Santos Abrahão; Cláudio Antônio Bonjardim; Erna Geessien Kroon; Felipe Lopes Assis; Paulo Cesar Peregrino Ferreira; Giliane De Souza Trindade; Group 1 Vaccinia virus Zoonotic Outbreak in Maranhão State, Brazil. The American Journal of Tropical Medicine and Hygiene 2013, 89, 1142-1145, 10.4269/ajtmh.13-0369.
- Venkatesan, G.; Balamurugan, V.; Prabhu, M.; Yogisharadhya, R.; Bora, D.P.; Gandhale, P.N.; Sankar, M.S.; Kulkarni, A.M.; Singh, R.K.; Bhanuprakash, V; et al. Emerging and re-emerging zoonotic buffalopox infection: a severe outbreak in Kolhapur (Maharashtra), India. Vet Ital 2010, 46, 439–448.
- Christian Becker; Andreas Kurth; Frank Hessler; Harald Kramp; Michael Gokel; Rudolf Hoffmann; Annette Kuczka; Andreas Nitsche; Cowpox Virus Infection in Pet Rat Owners. Deutsches Aerzteblatt Online 2009, 106, 329-334, 10.3238/arztebl.2009.0329.
- Bing Lu; Lun-Biao Cui; Min-Hua Gu; Chao Shi; Chuan-Wu Sun; Kang-Chen Zhao; Jun Bi; Zhong-Ming Tan; Xi-Ling Guo; Xiang Huo; et al.Chang-Jun Bao Outbreak of Vaccinia Virus Infection from Occupational Exposure, China, 2017. Emerging Infectious Diseases 2019, 25, 1192-1195, 10.3201/eid2506.171306.
- Nikola Sklenovská; Marc Van Ranst; Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Frontiers in Public Health 2018, 6, 241, 10.3389/fpubh.2018.00241.
- Raj Kumar Singh; V. Balamurugan; V. Bhanuprakash; G. Venkatesan; M. Hosamani; Emergence and Reemergence of Vaccinia-Like Viruses: Global Scenario and Perspectives. Indian Journal of Virology 2012, 23, 1-11, 10.1007/s13337-012-0068-1.
- Ana Paula Moreira Franco-Luiz; Alexandre Fagundes-Pereira; Galileu Barbosa Costa; Pedro Augusto Alves; Danilo Bretas Oliveira; Claudio Antônio Bonjardim; Paulo César Peregrino Ferreira; Giliane De Souza Trindade; Carlos Javier Panei; Cecilia Mónica Galosi; et al.Jônatas Santos AbrahãoErna Geessien Kroon Spread of Vaccinia Virus to Cattle Herds, Argentina, 2011. Emerging Infectious Diseases 2014, 20, 1576-1578, 10.3201/eid2009.140154.
- Ana Paula Moreira Franco-Luiz; Danilo Bretas Oliveira; Alexandre Fagundes Pereira; Mirela Cristina Soares Gasparini; Cláudio Antônio Bonjardim; Paulo César Peregrino Ferreira; Giliane De Souza Trindade; Rodrigo Puentes; Agustin Furtado; Jônatas Santos Abrahão; et al.Erna Geessien Kroon Detection of Vaccinia Virus in Dairy Cattle Serum Samples from 2009, Uruguay. Emerging Infectious Diseases 2016, 22, 2174-2177, 10.3201/eid2212.160447.
- Jose A. Usme-Ciro; Andrea Paredes; J. A. Usme-Ciro Et Al.; Erica Natalia Tolosa-Pérez; Katherine Laiton-Donato; Maria Del Carmen Pinzón; Brett W. Petersen; Nadia F. Gallardo-Romero; Yu Li; Kimberly Wilkins; et al.Whitni DavidsonJinxin GaoNishi PatelYoshinori NakazawaMary G. ReynoldsP. S. SatheshkumarGinny L. EmersonAndrés Páez-Martínez Detection and Molecular Characterization of Zoonotic Poxviruses Circulating in the Amazon Region of Colombia, 2014. Emerging Infectious Diseases 2017, 23, 649-653, 10.3201/eid2304.161041.
- Ines Eder; Patrick Vollmar; Martin Pfeffer; Philipp Naether; Arne Christian Rodloff; Hermann Meyer; Two Distinct Clinical Courses of Human Cowpox, Germany, 2015. Viruses 2017, 9, 375, 10.3390/v9120375.
- Anna M. Likos; Scott A. Sammons; Victoria A. Olson; A. Michael Frace; Yu Li; Melissa Olsen-Rasmussen; Whitni Davidson; Renee Galloway; Marina L. Khristova; Mary G. Reynolds; et al.Hui ZhaoDarin S. CarrollAaron CurnsPierre FormentyJoseph J. EspositoRussell L. RegneryInger K. Damon A tale of two clades: monkeypox viruses. Journal of General Virology 2005, 86, 2661-2672, 10.1099/vir.0.81215-0.
- Adesola Yinka-Ogunleye; Olusola Aruna; Mahmood Dalhat; Dimie Ogoina; Andrea Mccollum; Yahyah Disu; Ibrahim Mamadu; Afolabi Akinpelu; Adama Ahmad; Joel Burga; et al.Adolphe NdorerahoEdouard NkunzimanaLamin MannehAmina MohammedOlawunmi AdeoyeDaniel Tom-AbaBernard SilenouOladipupo IpadeolaMuhammad SalehAyodele AdeyemoIfeoma NwadiutorNeni AworabhiPatience UkeDoris JohnPaul WakamaMary ReynoldsMatthew R MauldinJeffrey DotyKimberly WilkinsJoy MusaAsheena KhalakdinaAdebayo AdedejiNwando MbaOlubunmi OjoGerard KrauseChikwe IhekweazuAnna MandraWhitni DavidsonVictoria OlsonYu LiKay RadfordHui ZhaoMichael TownsendJillybeth BurgadoPanayampalli S. Satheshkumar Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. The Lancet Infectious Diseases 2019, 19, 872-879, 10.1016/s1473-3099(19)30294-4.
- Preben Von Magnus; Else Krag Andersen; Knud Birkum Petersen; Aksel Birch-Andersen; A POX-LIKE DISEASE IN CYNOMOLGUS MONKEYS. Acta Pathologica Microbiologica Scandinavica 2009, 46, 156-176, 10.1111/j.1699-0463.1959.tb00328.x.
- Scott Parker; Anthony Nuara; R Mark L Buller; Denise A Schultz; Human monkeypox: an emerging zoonotic disease. Future Microbiology 2007, 2, 17-34, 10.2217/17460913.2.1.17.
- Kurt D. Reed; John W. Melski; Mary Beth Graham; Russell L. Regnery; Mark J. Sotir; Mark V. Wegner; James J. Kazmierczak; Erik J. Stratman; Yu Li; Janet A. Fairley; et al.Geoffrey R. SwainVictoria A. OlsonElizabeth K. SargentSue C. KehlMichael A. FraceRichard KlineSeth L. FoldyJeffrey P. DavisInger K. Damon The Detection of Monkeypox in Humans in the Western Hemisphere. New England Journal of Medicine 2004, 350, 342-350, 10.1056/nejmoa032299.
- Update: Multistate outbreak of monkeypox—Illinois, Indiana, Missouri, Ohio, and Wisconsin, 2003. . Centers for disease control and prevention, (CDC). Retrieved 2021-1-21
- Sklenovská, N.. Monkeypox Virus. In Animal-Origin Viral Zoonoses. Livestock Diseases and Management; Malik Y.S., Singh R.K., D.K., Eds.; Springer: Singapore, 2020; pp. 39–68.
- L. Khodakevich; Z. Jezek; K. Kinzanzka; ISOLATION OF MONKEYPOX VIRUS FROM WILD SQUIRREL INFECTED IN NATURE. The Lancet 1985, 327, 98-99, 10.1016/s0140-6736(86)90748-8.
- Aleksandar Radonić; Sonja Metzger; Piotr Wojtek Dabrowski; Emmanuel Couacy-Hymann; Livia Schuenadel; Andreas Kurth; Kerstin Mätz-Rensing; Christophe Boesch; Fabian H. Leendertz; Andreas Nitsche; et al. Fatal Monkeypox in Wild-Living Sooty Mangabey, Côte d’Ivoire, 2012. Emerging Infectious Diseases 2014, 20, 1009-1011, 10.3201/eid2006.131329.
- Christina L. Hutson; Whitni Davidson; Russell L. Regnery; Mary G. Reynolds; Yu Li; Inger K. Damon; Connie Austin; Joel M. Montgomery; James J. Kazmierczak; Jeffrey P. Davis; et al.Victoria A. OlsonDarin S. CarrollPaul SpurlockKevin L. KaremJames HowellJason AbelMichael DillonFaye E. SorhageChristine HughesZachary BradenLori MiserKemba N. Lee MONKEYPOX ZOONOTIC ASSOCIATIONS: INSIGHTS FROM LABORATORY EVALUATION OF ANIMALS ASSOCIATED WITH THE MULTI-STATE US OUTBREAK. The American Journal of Tropical Medicine and Hygiene 2007, 76, 757-768, 10.4269/ajtmh.2007.76.757.
- Marennikova, S.S.; Seluhina, E.M.; Mal’ceva, N.N.; Ladnyj, I.D.; Poxviruses isolated from clinically ill and asymptomatically infected monkeys and a chimpanzee. Bull. World Health Organ 1972, 46, 613–620.
- Nicolas Berthet; Emmanuel Nakouné; Eline Whist; Benjamin Selekon; Ana-Maria Burguière; Jean-Claude Manuguerra; Antoine Gessain; Mirdad Kazanji; Maculopapular lesions in the Central African Republic. The Lancet 2011, 378, 1354, 10.1016/s0140-6736(11)61142-2.
- Andrea M. Mccollum; Yoshinori Nakazawa; Guy Mutombo Ndongala; Elisabeth Pukuta; Stomy Karhemere; Robert Shongo Lushima; Benoit Kebela Ilunga; Joelle Kabamba; Kimberly Wilkins; Jinxin Gao; et al.Y. LiGinny EmersonInger K. DamonDarin S. CarrollMary G. ReynoldsJean MalekaniJean-Jacques Muyembe Tamfum Human Monkeypox in the Kivus, a Conflict Region of the Democratic Republic of the Congo. The American Journal of Tropical Medicine and Hygiene 2015, 93, 718-721, 10.4269/ajtmh.15-0095.
- E. Kalthan; J. Tenguere; S.G. Ndjapou; T.A. Koyazengbe; J. Mbomba; R.M. Marada; P. Rombebe; P. Yangueme; M. Babamingui; A. Sambella; et al.E.R. Nakoune Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Médecine et Maladies Infectieuses 2018, 48, 263-268, 10.1016/j.medmal.2018.02.010.
- Laudisoit, A.; Baelo, P.; Mussaw Awazi, M.; VanHoutte, N.; VanHees, M.; Amundala, N.; Leirs, H.; Biodiversity, Bushmeat and Monkeypox in the Democratic Republic of the Congo: Another viral threat upon larger cities?. Trop. Med. Int. Heal 2015, ., ..
- Kabuga, A.I.; El Zowalaty, M.E.; A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med. Virol 2019, ., ..
- Trevon Fuller; Henri A. Thomassen; Prime M. Mulembakani; Sara C. Johnston; James O. Lloyd-Smith; Neville K. Kisalu; Timothee K. Lutete; Seth Blumberg; Joseph N. Fair; Nathan D. Wolfe; et al.Robert L. ShongoPierre FormentyHermann MeyerLinda L. WrightJean-Jacques MuyembeWolfgang BuermannSassan S. SaatchiEmile OkitolondaLisa HensleyThomas B. SmithAnne W. Rimoin Using Remote Sensing to Map the Risk of Human Monkeypox Virus in the Congo Basin. EcoHealth 2010, 8, 14-25, 10.1007/s10393-010-0355-5.
- Fenner, F.. The Poxviruses. In Portraits of Viruses. A History of Virology; Gibbs, A., Eds.; Basil, Karger: ., 1988; pp. 1–23.
- Matthew R Mauldin; Markus H. Antwerpen; Ginny Emerson; Yu Li; Gudrun Zoeller; Darin S. Carroll; Hermann Meyer; Cowpox virus: What’s in a Name?. Viruses 2017, 9, 101, 10.3390/v9050101.
- Baxby, D.; Bennett, M.; Cowpox: A re-evaluation of the risks of human cowpox based on new epidemiological information. . Arch. Virol. Suppl. 1977, ., ..
- J. Chantrey; H. Meyer; D. Baxby; M. Begon; Kevin J. Bown; S. M. Hazel; T. Jones; W. I. Montgomery; Malcolm J. Bennett; Cowpox: reservoir hosts and geographic range.. Epidemiology and Infection 1999, 122, 455-460, 10.1017/s0950268899002423.
- Mahnel, H.; Holejsovsky, J.; Bartak, P.; Czerny, C.P.; Kongenitale “Ektromelie” bei Pelztieren durch Orthopoxvirus muris.. Teirarztliche Prax 1993, 21, 469–472.
- Rengina Vorou; Vassilios G Papavassiliou; Ioannis N Pierroutsakos; Cowpox virus infection: an emerging health threat. Current Opinion in Infectious Diseases 2008, 21, 153-156, 10.1097/qco.0b013e3282f44c74.
- M. Bennett; C. J. Gaskell; D. Baxbyt; R. M. Gaskell; D. F. Kelly; J. Naidoot; Feline cowpox virus infection. Journal of Small Animal Practice 1990, 31, 167-173, 10.1111/j.1748-5827.1990.tb00760.x.
- Thomsett, L.R.; Baxby, D.; Denham, E.M.; Cowpox in the domestic cat. Vet. Rec. 1978, ., ..
- U. Hinrichs; H. Van De Poel; T.S.G.A.M. Van Den Ingh; Necrotizing Pneumonia in a Cat Caused by an Orthopox Virus. Journal of Comparative Pathology 1999, 121, 191-196, 10.1053/jcpa.1999.0309.
- M. Pfeffer; O.-R. Kaaden; S. Pfleghaar; D. Von Bomhard; H. Meyer; Retrospective investigation of feline cowpox in Germany. Veterinary Record 2002, 150, 50-51, 10.1136/vr.150.2.50.
- Hartmut Campe; Pia Zimmermann; Katharina Glos; Margot Bayer; Hans Bergemann; Caroline Dreweck; Petra Graf; Bianca Kim Weber; Hermann Meyer; Mathias Büttner; et al.Ulrich BuschAndreas Sing Cowpox Virus Transmission from Pet Rats to Humans, Germany. Emerging Infectious Diseases 2009, 15, 777-780, 10.3201/eid1505.090159.
- Smith, G.L. . Poxviruses, Birkhäuser Advances in Infectious Diseases; Mercer, A.A., Schmidt, A., Weber, O., Eds.; Birkhäuser Verlag, Basel: Switzerland, 2007; pp. ..
- The global eradication of smallpox : final report of the Global Commission for the Certification of Smallpox Eradication, Geneva, December 1979 . World Health Organization (WHO). Retrieved 2021-1-21
- Henderson, D.A. . Smallpox: the death of a disease: the inside story of eradicating a worldwide killer; ., Eds.; Prometheus Books: New York, 2009; pp. ..
- Erna Geessien Kroon; Bruno E. F. Mota; Jônatas Santos Abrahão; Flávio Guimarães Da Fonseca; Giliane De Souza Trindade; Zoonotic Brazilian Vaccinia virus: From field to therapy. Antiviral Research 2011, 92, 150-163, 10.1016/j.antiviral.2011.08.018.
- Smith, G.L. . Poxviruses, Birkhäuser Advances in Infectious Diseases ; Mercer, A.A., Schmidt, A., Weber, O, Eds.; Birkhäuser Verlag, Basel: Switzerland, 2007; pp. ..
- Laboratory acquired vaccinia exposures and infections—United States, 2005– 2007 . Centers for disease control and prevention, (CDC). Retrieved 2021-1-21
- Ingo Drexler; Caroline Staib; Gerd Sutter; Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential?. Current Opinion in Biotechnology 2004, 15, 506-512, 10.1016/j.copbio.2004.09.001.
- Clement A. Meseda; Alonzo D. Garcia; Arunima Kumar; Anne E. Mayer; Jody Manischewitz; Lisa R. King; Hana Golding; Michael Merchlinsky; Jerry P. Weir; Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 2005, 339, 164-175, 10.1016/j.virol.2005.06.002.
- Giliane S. Trindade; Ginny L. Emerson; Darin S. Carroll; Erna G. Kroon; Inger K. Damon; Brazilian Vaccinia Viruses and Their Origins. Emerging Infectious Diseases 2007, 13, 965-972, 10.3201/eid1307.061404.
- Júlia B. Miranda; Iara A. Borges; Samantha P.S. Campos; Flávia N. Vieira; Tatiana M.F. De Ázara; Fernanda A. Marques; Galileu B. Costa; Ana Paula M.F. Luis; Jaqueline S. De Oliveira; Paulo César P. Ferreira; et al.Cláudio Antônio BonjardimSilvio L.M. Da SilvaÁlvaro E. EirasJônatas S. AbrahãoErna G. KroonBetânia P. DrumondAdriano P. PagliaGiliane De Souza Trindade Serologic and Molecular Evidence of Vaccinia Virus Circulation among Small Mammals from Different Biomes, Brazil. Emerging Infectious Diseases 2017, 23, 931-938, 10.3201/eid2306.161643.
- Maria Luiza G. Medaglia; Nissin Moussatché; Andreas Nitsche; Pjotr Wojtek Dabrowski; Yu Li; Inger K. Damon; Carolina G. O. Lucas; Luciana B. Arruda; Clarissa Damaso; Genomic Analysis, Phenotype, and Virulence of the Historical Brazilian Smallpox Vaccine Strain IOC: Implications for the Origins and Evolutionary Relationships of Vaccinia Virus. Journal of Virology 2015, 89, 11909-11925, 10.1128/jvi.01833-15.
- Venkatesan, G.; Balamurugan, V.; Prabhu, M.; Yogisharadhya, R.; Bora, D.P.; Gandhale, P.N.; Sankar, M.S.; Kulkarni, A.M.; Singh, R.K.; Bhanuprakash, V.; et al. Emerging and re-emerging zoonotic buffalopox infection: a severe outbreak in Kolhapur (Maharashtra), India. . Vet Ital 2010, 46, 439–448.
- Baxby, D.; Hill, B.J.; Characteristics of a new poxvirus isolated from Indian buffaloes. Arch. Gesamte Virusforsch. 1971, 35, 70–79.
- Baxby, D.; Hill, B.J.; Characteristics of a new poxvirus isolated from Indian buffaloes. . Arch. Gesamte Virusforsch. 1971, 35, 70–79.
- Venkatesan, G.; Balamurugan, V.; Prabhu, M.; Yogisharadhya, R.; Bora, D.P.; Gandhale, P.N.; Sankar, M.S.; Kulkarni, A.M.; Singh, R.K.; Bhanuprakash, V.; et al. Emerging and re-emerging zoonotic buffalopox infection: a severe outbreak in Kolhapur (Maharashtra), India. . Vet Ital 2010, 46,, 439–448.
- Tarang Goyal; Anupam Varshney; Surrinder Kumar Bakshi; Sanjay Barua; Bidhan Chandra Bera; Raj Kumar Singh; Buffalo pox outbreak with atypical features: a word of caution and need for early intervention!. International Journal of Dermatology 2013, 52, 1224-1230, 10.1111/ijd.12120.
- Veerakyathappa Bhanuprakash; G. Venkatesan; V. Balamurugan; M. Hosamani; R. Yogisharadhya; R. S. Chauhan; A. Pande; B. Mondal; R. K. Singh; Pox outbreaks in Sheep and Goats at Makhdoom (Uttar Pradesh), India: Evidence of Sheeppox Virus Infection in Goats. Transboundary and Emerging Diseases 2010, 57, 375-382, 10.1111/j.1865-1682.2010.01158.x.
- Mahavir Singh; P. P. Bhat; B. P. Mishra; R. K. Singh; Biological Transmissibility of Buffalopox Virus. Journal of Applied Animal Research 1996, 9, 79-88, 10.1080/09712119.1996.9706107.
- Aysegul Nalca; Donald K. Nichols; Rabbitpox: a model of airborne transmission of smallpox. Journal of General Virology 2010, 92, 31-35, 10.1099/vir.0.026237-0.
- Christensen, L.R.; Bond, E.; Matanic, B.; “Pock-less” rabbit pox.. Lab. Anim. Care 1967, ., ..
- Frank Fenner; The biological characters of several strains of vaccinia, cowpox and rabbitpox viruses. Virology 1958, 5, 502-529, 10.1016/0042-6822(58)90042-4.
- Dumbell, K.; Richardson, M.; Virological investigations of specimens from buffaloes affected by buffalopox in Maharashtra State, India between 1985 and 1987. . Arch. Virol. 1993, 128, 257–267..
- Dumbell, K.; Richardson, M.; Virological investigations of specimens from buffaloes affected by buffalopox in Maharashtra State, India between 1985 and 1987. . Arch. Virol. 1993, 128, 257–267.
- Juliana A. Leite; Betânia P. Drumond; Giliane S. Trindade; Zélia I.P. Lobato; Flávio G. Da Fonseca; João R. Dos Santos; Marieta C. Madureira; Maria I.M.C. Guedes; Jaqueline M.S. Ferreira; Cláudio A. Bonjardim; et al.Paulo C.P. FerreiraErna Geessien Kroon Passatempo Virus, a Vaccinia Virus Strain, Brazil. Emerging Infectious Diseases 2005, 11, 1935-1941, 10.3201/eid1112.050773.
- Anselmo V. Rivetti; Maria Isabel M.C. Guedes; Izabelle S. Rehfeld; Tércia M.L. Oliveira; Ana Carolina Diniz Matos; Jônatas Santos Abrahão; Erna Geessien Kroon; Zélia Inês Portela Lobato; Bovine vaccinia, a systemic infection: Evidence of fecal shedding, viremia and detection in lymphoid organs. Veterinary Microbiology 2013, 162, 103-111, 10.1016/j.vetmic.2012.09.005.
- Ana Carolina Diniz Matos; Izabelle Silva Rehfeld; Maria Isabel Maldonado Coelho Guedes; Zélia Inês Portela Lobato; Bovine Vaccinia: Insights into the Disease in Cattle. Viruses 2018, 10, 120, 10.3390/v10030120.
- Jônatas S. Abrahão; Tércia M.L. Oliveira; Rafael K. Campos; Marieta C. Madureira; Erna G. Kroon; Zélia I.P. Lobato; Bovine Vaccinia Outbreaks: Detection and Isolation of Vaccinia Virus in Milk Samples. Foodborne Pathogens and Disease 2009, 6, 1141-1146, 10.1089/fpd.2009.0324.
- Izabelle Silva Rehfeld; Ana Carolina Diniz Matos; Maria Isabel Maldonado Coelho Guedes; Aristóteles Gomes Costa; Ana Luiza Soares Fraiha; Zélia Inês Portela Lobato; Subclinical bovine vaccinia: An important risk factor in the epidemiology of this zoonosis in cattle. Research in Veterinary Science 2017, 114, 233-235, 10.1016/j.rvsc.2017.03.022.
- Fassbender, P.; Zange, S.; Ibrahim, S.; Zoeller, G.; Herbstreit, F.; Meyer, H.; Generalized cowpox virus infection in a patient with HIV, Germany, 2012. Emerg. Infect. Dis. 2016, ., ..
- Jaqueline Silva De Oliveira; Galileu Barbosa Costa; Ana Paula Moreira Franco Luiz; Juliana Almeida Leite; Cláudio Antônio Bonjardim; Jônatas Santos Abrahão; Betânia Paiva Drumond; Erna Geessien Kroon; Giliane De Souza Trindade; Cross-sectional study involving healthcare professionals in a Vaccinia virus endemic area. Vaccine 2017, 35, 3281-3285, 10.1016/j.vaccine.2017.04.048.
- Jaqueline Silva De Oliveira; Galileu Barbosa Costa; Ana Paula Moreira Franco Luiz; Juliana Almeida Leite; Cláudio Antônio Bonjardim; Jônatas Santos Abrahão; Betânia Paiva Drumond; Erna Geessien Kroon; Giliane De Souza Trindade; Cross-sectional study involving healthcare professionals in a Vaccinia virus endemic area. Vaccine 2017, 35, 3281-3285, 10.1016/j.vaccine.2017.04.048.