Prunus species, as climacteric fruits, have a short ripening period and shelf-life after harvest, with a fast period of softening.
- Prunus
- fruit quality
- Prunus,fruit quality
1. Introduction
In the plant kingdom, fruit ripening is a coordinated process that requires the change in expression of hundreds to thousands of genes to modify many biochemical and physiological signals including carbohydrate and organic acids metabolism, cell wall restructuring, ethylene production, stress response, and organoleptic compound formation. Several studies have tried to comprehend the main processes that take place during the transformation between a green fruit to ripe fruit, understanding horticultural maturity as the moment when the “plant or plant part possesses the prerequisites for utilization by consumers for a particular purpose” [1]. Ripening was also described by this author as “the composite of processes that occur from the later stages of growth and development through the early stages of senescence and that results in characteristic aesthetic and/or food quality, as evidenced by changes in composition, color, texture, or other sensory attributes” [1]. Ripening is a well-known step of fruit development and has been extensively studied because of the importance of accurately describing the exact point where the fruit expresses greater nutritional and economic value. Senescence is the process that follows fruit ripening and leads to the death of tissue. Though it is not lethal, senescence increases the susceptibility to disease, injury, dehydration, or microbial invasion. It is hard to discriminate between these stages since they sometimes overlap, but ripening includes processes like pigment accumulation and cell wall changes that are not usually involved in senescence processes [2].
Fruit ripening leads to the breakdown of complex carbohydrates into sugars, fruit firmness reductions (softening by cell wall degradation and cuticle properties alteration), color changes (loss of green color by chlorophylls degradation and increase in non-photosynthetic pigments like anthocyanins and carotenoids), acidity decreases, and aroma increases (production and release of organic volatile compounds) [3][4][3,4]. This process contributes to increased attractiveness and acceptance by consumers (Figure 1). Mainly, the composition of sugars, acids, color, and aroma depends on genetics; however, culture practices and environmental factors strongly influence fruit quality characters [5]. Understanding ripening mechanisms will make it feasible to implement agronomical strategies optimized to climatic conditions, enhance production, increase fruit quality, and facilitate the selection of new apricot varieties. Therefore, in this review, ripening was extensively studied because of the importance of defining exactly when a fruit displays the highest nutritional and economic value, thus rendering fruits more attractive and palatable due to the acquisition of fruit quality traits.

Figure 1.
Traditional and new Spanish apricot cultivars at consumer ripening state.
Additionally, fruit development is a regulated process unique to plants that involves three distinct steps: fruit set, fruit growth, and ripening. Fruit development finishes when the fruit is ripe and is defined as the optimum moment for harvest the fruit from the point of view of the consumer. Each step is controlled by genetic and environmental factors, influenced by culture practices. Each of these factors interacts in a multilevel process and triggers the coordinated action of master regulators, including hormone signaling, microRNAs, and epigenetic modifying genes [6]. On the other hand, the epigenetic regulation of gene expression has been recognized as remodeling gene expression during the ripening process [7][8][7,8], mainly by the DNA methylation of targeted loci involved in gene expression regulation. The presence of tissue-specific methylation patterns and the progressive demethylation of ripening-related gene promoters give tissue specificity and developmental stage-dependent gene expression that may be used as a strategy to monitoring ripening process [8][9][8,9].
In Prunus species including peaches, nectarines, (Prunus persica (L.) Batsch), prunes (Prunus domestica L.), Japanese plums (Prunus salicina Lindl), apricots (Prunus armeniaca L.) and sweet (Prunus avium L.) (2n = 2x = 16) and sour (Prunus cerasus L.) cherry fruits, four stages have been described during fruit development and ripening: S1: fruit growth; S2: green fruit; S3: changing color; and S4: physiological ripening (Figure 2). The beginning of S1 is characterized by fruit set and growth. A decrease in fruit growth at the S1/S2 transition is followed by endocarp lignification (stone hardening), from the middle of S2 to its end. The S3 phase begins with additional growth activation, mainly due to the increase in the number of cells, thus generating the second exponential growth phase. The maturation of the fruit is completed at the end of S3, and then the last ripening phase S4 occurs when the fruit fully develops its quality traits. The fruit developmental phases are determined using a mathematical model based on the first derivative of the growth cumulative curve, represented by a double sigmoid pattern. The identification and characterization of each phase of fruit growth are necessary for development studies and the precision harvesting of high quality fruits [10].
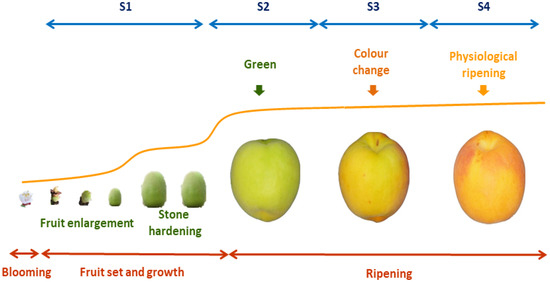
Figure 2. Stages of fruit development and ripening in apricot fruits. After flower bloom, pollination and fruit set, fruit growth begin with a fruit enlargement, stopping the size increase during stone hardening (S1). The reactivation of grown at green stage (S2) is followed by a color change in half-ripe fruit (S3). Maturation and ripening end at physiological ripening, when the fruit reaches the maximum sucrose accumulation and definitive color (S4).
On the other hand, fruits have been classified into two physiological groups with two distinct ripening fruit mechanisms—climacteric and non-climacteric—based on the presence or absence of a ripening-associated rise of ethylene and an increase of the respiration ratio. In climacteric fruits, such as Prunus species, the ripening process is marked by an increased respiration ratio induced by ethylene, while in non-climacteric fruits, it is controlled by an ethylene-independent process with little change in the respiration rate [11]. The attribute acquisition during the ripening process in climacteric fruit differs among species, varieties, nutritional feeds, and environments. The most significant changes during ripening are represented in Table 1 [2][3][2,3]. In the final phase of fruit ripening, oxidative processes with the accumulation of hydrogen peroxide and membrane lipid peroxidation occur. The identification of enzymes from the cell antioxidant systems including catalase (CAT), peroxidase (POX), and superoxide dismutase (SOD)involved in the scavenging of reactive oxygen species (ROS), is a symptom of the onset of the senescence [12][13][12,13].
Table 1. Main altered processes during the ripening of apricot fruit.
| Changes | Character | Events |
|---|
| Biochemical | Color | Chlorophyll degradation |
| Dismantling of photosynthetic apparatus | ||
| Biosynthesis of anthocyanins Accumulation of carotenoids |
||
| Texture | Solubilization of pectin and cellulose | |
| Starch hydrolysis | ||
| Changes in protein content | ||
| Hydration of cell walls | ||
| Cell wall enzyme activity | ||
| Flavor and aroma | Biosynthesis, accumulation and degradation of organic acids | |
| Biosynthesis, accumulation and degradation of sugars | ||
| Acidity loss | ||
| Production of volatile organic compounds (VOCs) | ||
| Alcohol ester synthesis | ||
| Metabolic | Control of pathways | Increase in respiration ratio |
| Ethylene biosynthesis | ||
| Changes in the metabolism of starch and organic acids | ||
| Altered regulation of existing metabolic pathways | ||
| Molecular | Gene expression | Ripening-specific messanger RNA (mRNA) synthesis |
| Small and interference RNA appearance | ||
| Disappearance of mRNAs DNA demethylation |
||
| Protein expression | Synthesis of de novo ripening-specific proteins | |
| Disappearance of proteins | ||
| Biosynthesis of allergenic compounds | ||
| Increase pathogen susceptibility |
Prunus species, as climacteric fruits, have a short ripening period and shelf-life after harvest, with a fast period of softening. The fruit firmness, soluble sugar content, and color changes are the parameters used to define the maturity stage, where a loss of firmness during ripening is the main limitation for apricot harvesting and commercialization. Hence, apricot fruit is often harvested unripe and still firm, stored at low temperature for some time and then moved to market to extend the supply period [5][14][5,14].
Fleshy fruits are enriched in flavor, mineral compounds, dietary fiber, vitamins, and antioxidants that make them an essential component of the human diet [15]. In this sense, fruit quality is a crucial factor for fruit consumption and acceptance by consumers, mainly due to the current situation of high competition in the markets with the presence of numerous new cultivars. In the case of Prunus, fruit quality is a complex human concept that includes sensory characteristics (including texture, taste, and aroma), aesthetic attributes (appearance, defect presence, sphericity, density, and firmness), and functional and nutritional values [16]. Consumers cherish the beauty and aromatic flavor of high-quality Prunus fruits, while other parameters, such as large fruit size, ease of handling, minimum processing, and long post-harvest shelf-life, are especially considered by the fruit industry. Therefore, the requirements by consumers and the priorities of Prunus breeding programs have led to the evolution of fruit quality parameters in the last few decades, increasing fruit taste with a good balance of sugars and acidity, improving attractiveness with orange flesh and reddish blush colors, and improving post-harvest behavior and shelf-lives [9][16][9,16]. From the point of view of the consumers, these traits increase the attractiveness and acceptance of new Prunus cultivars enriched in phenylpropanoids, carotenoids, and other nutraceutical compounds highly beneficial for human health [17][18][19][20][21][17,18,19,20,21].
At this moment, the level of information on the molecular switch that occurs during the transition from unripe to ripe fruits is limited. Increasing this knowledge will have important consequences from a breeding point of view. During fruit development, fruit becomes ripe due to changes in physiological structure and biochemical composition, resulting in unique aesthetic and fruit quality traits. The regulation of this process is affected by various internal factors (epigenetic developmental signals, hormone signaling, microRNA regulation) and external factors (light, temperature, nutrition, humidity, and biotic and abiotic stress) involving exchanges between the fruit and its environment. Therefore, fruit should be examined as an integrated system of biological process network [22]. Most Prunus fruit quality traits, such as sugar content, acidity, fruit color, firmness, and enrichment in nutraceutical and antioxidant components are controlled by a polygenic expression, quantitatively inherited, and influenced by environment stimuli and internal signaling grow regulators. From the genomic point of view, the variation of these traits has been traditionally studied following quantitative genetics approaches such as the correlation of genetic marker–trait association analysis and transcriptomic analysis for differential gene expression in order to elucidate the implied molecular mechanism. Hence, integrated phenotypic, metabolomic, genomic, and transcriptomic analysis of this complex process must be addressed because of the many applications in breeding that are oriented towards the increase of fruit quality traits related to the handling, processing, marketing, and consumption phases [23].
2. Regulation and Signal Transduction during the Ripening Process
The level of information of molecular events at the transcriptional, biochemical, hormonal, and metabolite levels underlying ripening in climacteric and non-climacteric fruits has considerably increased in recent decades. However, we still poorly understand the developmental switch that occurs in hormone responsiveness during the transition from unripe to ripe fruits [9]. One of the most critical factors in the ripening process is the developmental switch triggered by growth regulators, such as hormone response, that determine the transition from unripe to ripe fruits. Plant hormones are involved in the control of various aspects of fruit development throughout the different stages, organs, and tissues, thus constituting a complex network. Considering that every species, even cultivars, have different responses to hormones, it has been proven in Prunus species that the combined action of auxins, gibberellins, and cytokinins plays a major role in the regulation of fruit attributes; although ethylene and abscisic acid cause the main effects of fruit ripening in all the species [24][25][26][27][24,25,26,27].
2.1. Ethylene
Ethylene was the first known gas with biological significance as a signaling molecule for plant growth and development. Ethylene directly affects seed germination, cell elongation, cell differentiation, sex determination, fruit ripening, fruit senescence, and abscission [28]. Ethylene is also a fundamental regulator of responses to biotic stresses such as drought, flooding, wounding, chilling, pathogen infection, and chemical damage [29]. During fruit ripening, two ethylene-responsive elements are registered in immature fruit. In non-climacteric fruits, system I is responsible for basal rate ethylene production, which is slow, inhibited by exogenous ethylene (auto inhibition), and detected in vegetative tissues during growth processes. System II operates during ripening in climacteric fruits and is autocatalytic. The presence of two ethylene-responsive systems that differ between climacteric and non-climacteric fruits suggests that climacteric fruits evolved to synthesize ethylene in an autocatalytic manner as a positive feedback loop to control ethylene synthesis during ripening [30][31][30,31].
In both systems, ethylene is regulated through three enzymatic reactions: (a) the precursor S-adenosyl-L-methionine (AdoMet) is converted to S-adenosyl methionine (SAM) by S-adenosyl-L-methionine synthetase (MAT); (b) 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) converts SAM to ACC; and (c) ACC oxidase (ACO) oxidizes ACC to release ethylene. The conversion of ACC to ethylene catalyzed by ACO is oxygen-dependent. However, under anaerobic conditions, ethylene formation is completely suppressed. Apart from ACC, ACS also produces 5′-methylthioadenosine (MTA), which is used for the synthesis of new methionine, thus ensuring high rates of ethylene biosynthesis [32][33][34][35][36][32,33,34,35,36].
Studies on the components of ethylene signaling have revealed a complex transduction pathway where the hormone is perceived by specifics receptors that initiate a transcriptional regulation cascade of genes underlying ripening-related traits including color, firmness, aroma, taste, and post-harvest shelf-life. Ethylene is perceived by ethylene receptor (ETR) and ethylene sensor (ERS) proteins. These receptors are negative regulators of ethylene signaling, and in the absence of ethylene, ERT activates the associated constitutive triple-response serine/threonine-protein kinase (CTR), acting as a negative regulator of the ethylene transduction pathway by suppressing the ethylene response via the inactivation of ethylene-insensitive (EIN) and ethylene-insensitive-like (EIL) proteins. The ethylene signaling cascade ends with the transcriptional activation of the transcription factors ethylene-responsive factors (ERFs), activated by EIN/EIL proteins. ERFs modulate the transcription of ethylene-regulated genes by binding to GCC-box type cis-elements present in their target promoters. It has also been suggested that the tetratricopeptide repeat (TPR) binds to ethylene receptors and leads to receptor degradation. These molecular regulators have been observed in different plant species including Prunus [32][33][36][37][38][32,33,36,37,38].
Ethylene is the main trigger of climacteric fruit ripening. Most biochemical changes associated with apricot ripening are under the control of ethylene [5]. A significant number of genes expressed during the transition of nectarine fruit from the preclimacteric to climacteric stages are not ethylene-regulated, but nevertheless a high upregulation of genes encoding transcription factors (TFs) belonging to several families like MADS-box, Auxine/indole acetid acid (IAA), basic leucine zipper domain (bZIP), basic helix–loop–helix (bHLH), homeodomain (HD )and myeloblastosis (Myb) [39]. In addition, treatments with the ethylene of unripe apricot fruit storage in cold conditions complete maturation by accelerating soluble solid accumulation, organic acid degradation, volatile organic compound (VOC) production, and fruit color change, thus leading to an improvement of apricot marketing [40][41][42][40,41,42]. CO2 is a competitive inhibitor of the ethylene effect, O2 is a substrate in the biosynthesis of ethylene, and ethylene oxide acts as an antagonist inhibitor of ethylene that delays the ripening process [28]. The compound 1-methylcyclopropane (1-MCP) can bind ethylene receptors as a competitive inhibitor that reduces ethylene action in fruit by suppressing the expression of genes related to fruit ripening, thus delaying the ripening process and prolonging storage life [41]. Aminoethoxyvinylglycine (AVG) acts as the most effective competitive inhibitor in the conversion of SAM to ACC, blocking ACS activity and reducing the rate of fruit softening in apricot during post-harvest with no effect in quality traits [43]. On the other hand, the application of ethephon, a long-lasting ethylene release compound, accelerates fruit ripening and abscission [44]. Therefore, there are multiple control levels of ethylene during ripening modulation [15].
2.2. Abscisic Acid
Abscisic acid (ABA) is an isoprenoid plant hormone. In higher plants, ABA is traditionally used to delay blooming, regulating seed dormancy and plant growth, and promoting ripening in climacteric and non-climacteric fruits [26]. ABA biosynthesis is derived from the oxidative cleavage of epoxy carotenoids like 9-cis-violaxanthin and 9-cis-neoxanthin by 9-cis-epoxycarotenoid dehydrogenase (NCED) to produce xanthoxin, the direct C15 ABA precursor [45]. In climacteric fruits, there is an accumulation of ABA content preceding climacteric ethylene production, reaching its maximum in fully ripe fruit and activating ethylene biosynthesis genes. A decrease of ABA was found to be correlated with an increase of ethylene, which is also related to a decrease of fruit firmness. In addition, a decrease of ABA is correlated with an increase in the sugar–acid ratio during the fruit ripening process. Thus, ABA may act as an original inducer for maturation in relation to ethylene action/perception [46]. Additionally, exogenous treatment with ABA accelerates the ripening process and increases the respiration rate when applied to pre-climacteric fruit but inhibits these processes when applied to post-climacteric fruit. The reduction of ABA content by biosynthetic inhibitors effectively delays maturity and softening [47].
2.3. Auxin
Auxins are compounds with an aromatic ring and a carboxylic group. Auxin modulates the response to light and gravity, general root and shoot architecture, organ patterning, vascular development, and plant growth. The effect of auxin differs between climacteric and non-climacteric fruits. Meanwhile, in climacteric fruits, auxin seems to accelerate the ripening process, and in non-climacteric fruit, auxin can negatively control the ripening [9][39][9,39]. In climacteric fruits like peaches, a concomitant increase of ethylene and auxin has been shown to exist. Instead, auxin plays an individual role during ripening, regulating the expression of different genes; thus, the hypothesis that cross-talk between auxin and ethylene existence has been supported [3].
2.4. Gibberellins
Gibberellins (GAs) are tetracyclic diterpenoids acids with an ent-gibberellane ring system. In apricots, the presence of GA1, GA3, GA5, GA6, GA8, GA29, and GA32 [48] [48] has been described, and GA3 was found to be used in phytochemical treatments to induce the abscission of floral buds [49]. GAs play a stimulatory role in fruit development, enhancing cell division and enlargement, developing in parthenocarpic fruits when applied during fruit hardening, and delaying fruit ripening by decreasing ethylene release. This ethylene decrease is responsible for increasing fruit firmness, thus reducing susceptibility damage by mechanical compression and increasing time for fruit growth so that the fruit may reach a larger size. Gibberellin application during fruit set decreases flower bud populations for the following season, thus thinning the tree without phytotoxic effects, which may also increase fruit size due to the elimination of the competitive effect between developing fruits [50][51][52][50,51,52]. The application of GA in combination with ethephon, an ethylene long-lasting release compound used in agronomical treatments, can prevent the browning of pureed and sliced peaches [50]. On the hand, ethylene and ABA seem to be the antagonists of GA [53].
2.5. Cytokinins
Cytokinins (CKs) are N6-substituted adenine derivatives that play a crucial role during plant growth and development, including cell division, shoot initiation and growth, leaf senescence, apical dominance, sink/source relationships, nutrient uptake, phyllotaxis, vascular gametophyte and embryonic development, and responses to biotic and abiotic factors [54]. Endogenous levels of CKs induce fruit growth via the stimulation of cell division and could act by inhibiting auxin responses, at least partially, during fruit set and growth to increase fruit size [25].
2.6. Jasmonates
Jasmonates (JAs) are a class of oxylipins that induce a wide variety of higher-plant responses. In peaches, lipoxygenase (LOX), responsible for catalyzing the hydroperoxidation of polyunsaturated fatty acids to initiate the synthesis of oxylipins, was reported to increase at the early ripening stage and decrease in ripe fruit [55]. Using a peach as a model fruit regarding the effect of JAs during the ripening processes showed that exogenous JAs led to a ripening delay [56].
