The majority of cancer patients will be treated with radiotherapy, either alone or together with chemotherapy and/or surgery. Optimising the balance between tumour control and the probability of normal tissue side effects is the primary goal of radiation treatment. Therefore, it is imperative to understand how irradiation affects both normal and cancer tissue. Here, we discuss how organoids, three-dimensional tissue-resembling structures derived from tissue-resident, embryonic or induced pluripotent stem cells, have a growing importance in the field of radiation biology research.
- Radiation, radiobiology, organoids
- radiation
- radiobiology
- organoids
1. Introduction
With an ever-aging population the number of people diagnosed with cancer is constantly growing [1]. Therefore, there is an even greater onus on the need to develop both current and new methods to enhance the efficacy of cancer treatments. Traditional cancer treatments, such as radiotherapy, chemotherapy and surgery, are still the most common modalities, but newer treatments such as immunotherapy are becoming more and more prevalent. Radiotherapy (either alone or in combination with surgery and/or chemotherapy) is used to treat over half of all cancer patients, with a curative intent in the majority of these cases [2,3][2][3]. Furthermore, the number of patients undergoing radiotherapy is predicted to increase even further due to an aging and growing population, as well as rapid technological advances in radiotherapy delivery practices [4]. The primary goal of radiotherapy, as with all other forms of cancer treatment, is to maximise the therapeutic window. The therapeutic window describes the balance between the probability of increasing tumour cell kill while minimising the probability of normal tissue complications. This can be achieved by using drugs which target the intrinsic vulnerabilities of a tumour to make it more susceptible than healthy tissue, or alternatively by physically targeting the tumour with greater accuracy and minimising the co-irradiated normal healthy tissue (Figure 1).
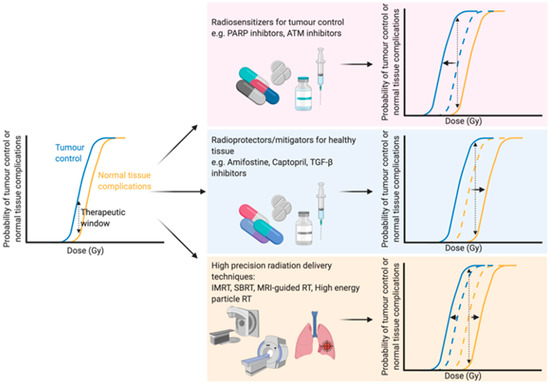
Optimising the therapeutic window of radiotherapy. The therapeutic window describes the balance between the probability of tumour control (blue line) and normal tissue complications (yellow line). There are three main rationales behind broadening the therapeutic window in radiation treatment: (1) Increasing tumour sensitivity using radiosensitisers, reducing the dose required for tumour kill (blue line shifts to the left), (2) protecting normal tissue using radioprotectors or mitigators, thus increasing the tolerable dose of normal tissue (shifting the yellow line to the right) or (3) high precision dose delivery which can reduce the volume of co-irradiated normal tissue (effectively shifting the yellow line to the right) while in the case of charged particles an increased relative biological effectiveness reduces the dose required for tumour control. Abbreviations: PARP; poly-ADP ribose polymerase, ATM; ataxia telangiectasia mutated, TGF-β; transforming growth factor beta, IMRT; intensity-modulated radiation therapy, SBRT; stereotactic body radiation therapy, MRI; magnetic resonance imaging. Created with BioRender.com.
The development of high precision means of dose delivery, such as intensity modulated radiation therapy [5], stereotactic radiation therapy [6] [6] and charged particle radiotherapy [7], have allowed for substantial reductions in co-irradiated normal tissue during therapy. These strategies enable better sparing of crucial organs [8] or sub-regions [9] within organs during treatment or dose escalation to the tumour. Furthermore, real-time advanced imaging, such as magnetic resonance imaging (MRI), during radiation therapy has been suggested as a means to further optimise the delivery of radiation to the target tumour with an increased sparing of the surrounding healthy tissue. Initial in vitro studies showed no changes in survival in response to X-rays when a magnetic field of 1.5 T was applied [10]. Indeed, combining X-ray therapy with MRI-guidance has been successfully applied in clinical practice to increase the accuracy of dose delivery and thus spare a greater proportion of healthy tissue [11]. Particle therapies, such as proton therapies, can modulate the dose to encompass the whole tumour in a so-called “spread-out Bragg peak” with a minimised entrance dose and negligible exit dose, sparing healthy tissue [12,13][12][13]. Furthermore, MRI-guided proton therapy has also been proposed [14][15] [14,15] and early in vitro findings suggest that a magnetic field perpendicular to the radiation beam has no effect on the radiobiological effectiveness of the dose [16], while a magnetic field longitudinal to the beam slightly changes the effectiveness [17], emphasising the potential of such advances in a clinical setting. Further advances in radiation delivery include FLASH radiotherapy, which delivers ultra-high dose rates of ionising radiation which are believed to reduce normal tissue complications compared to conventional dose rates [18], although the therapeutic window of FLASH therapy still needs to be addressed [19].
All of these technological advances in the field of radiation beam delivery have significantly reduced the amount of co-irradiated healthy tissue during radiation treatment; however, none of these developments can completely eliminate dose to the surrounding tissue. Therefore, it is still necessary to develop in vivo and in vitro models to improve understanding of the mechanisms involved to better protect and/or regenerate normal tissue or to target intrinsic vulnerabilities of a tumour to enhance radiotherapy efficacy. These models should also take the therapeutic window into account as there is often an overlap between these mechanisms in both normal tissue and tumours, a feature which is regretfully often overlooked.
2. Organoids and Regeneration of Radiation-Induced Damaged Tissue
Since the identification of Lgr5 as a marker for intestinal stem cells [42][20], one of the most studied and established organoid models are the gastrointestinal “mini-gut” organoids. Originally established from mouse small intestinal stem cells [43][21], organoid “mini-gut” models have subsequently been established from human stem cells [44][22], as well as from various different locations along the gastrointestinal tract, including stomach [45][23], colon [44][22] and oesophagus [46][24]. Furthermore, pluripotent stem cells have been utilised to successfully generate intestinal [25] [47] and oesophageal [26] [48] organoid cultures. These models have opened novel avenues of study for intestinal development, cancer progression [49] [27] and other diseases, such cystic fibrosis [50][28].
While there have been only a limited number of studies using organoids to investigate radiation-induced gastrointestinal injury, some recent studies have used organoids to complement and reinforce important insights from in vivo mouse studies [51,52,53][29][30][31]. Wang et al. [51][29] demonstrated using intestinal crypt organoids that selective inhibition of radiation-induced p53-mediated apoptosis using CHIR99021, an inhibitor of glycogen synthase kinase-3 (GSK-3), can protect intestinal stem cells against radiation due to an increased survival of Lgr5+ cells. This was recapitulated in vivo, indicating a pivotal role for p53 post-translational modifications in intestinal stem cell responses to irradiation [51][29]. More recently, using intestinal organoids from mouse jejunum and human colon, Bhanja et al. [52] [30] revealed the potential of BCN057, an anti-neoplastic small molecular agent, to mitigate radiation-induced gastrointestinal syndrome in normal tissue. Interestingly, BCN057 did not have a radiomitigative effect in tumour-derived organoids with these findings again mimicking in vivo findings. The same group also investigated the potential of repurposing auranofin, an anti-rheumatoid drug containing gold, as a radioprotective agent against intestinal injury [53][31]. In both in vivo mice and ex vivo human colon organoids treatment with auranofin significantly reduced the toxicity of radiation [53][31]. Furthermore, Martin et al. [32] [54] recently demonstrated that the profile of the Lgr5+ stem cell population of the large and small intestines following irradiation of organoids could act as a marker for predicting the sensitivity of these organs to radiation. The authors validated their approach using organoids with a well-established in vivo microcolony assay which quantifies the number of regenerating crypts per small intestinal circumference [54,55][32][33]. This assay is regarded as a benchmark assay for establishing the radiosensitivity of intestinal stem cell survival and highlights the potential of intestinal organoids to predict radiation responses [54][32]. These studies demonstrate the strength of “mini-gut” organoids as a model for radiation studies of the gastrointestinal tract and also the opportunities for radiobiological studies in other organoid systems, particularly in tissues which lack accurate in vitro models for radiobiological studies.
Radiotherapy is used to treat the majority of head and neck cancer patients, either alone or in combination with surgery and/or chemotherapy [56][34]. Frequently, irradiation of head and neck tumours leads to the unavoidable co-irradiation of salivary glands, with almost half of head and neck cancer patients subsequently suffering from radiation-induced xerostomia due to hyposalivation. This drastically impacts on the quality of life of patients due to impaired chewing, swallowing, speaking and an increased risk of oral infections [57][35]. In vivo studies using rats have shown that sparing a region of the salivary gland which contains a high density of tissue specific stem/progenitor cells has been shown to reduce the effects of salivary gland irradiation [9]. Therapeutic options are available to stimulate salivary gland flow post-irradiation but are limited in their effectiveness [57][35]. Therefore, a need for a more long-term strategy for salivary gland regeneration following radiotherapy remains [58][36]. While in vivo animal models have provided a wealth of knowledge as to the mechanisms behind salivary gland regeneration following injury, including radiation-induced damage [59,60,61,62,63[37][38][39][40][41][42],64], there is a limited number of in vitro systems to accurately study salivary glands following irradiation. Thus there is a growing niche for new models such as organotypic slice cultures [43] [65] and organoids in the area of salivary gland radiation research.
Recently, our group has established protocols for the isolation and expansion of both murine [44] [66] and human [45] [67] submandibular salivary gland stem/progenitor cells. Using these protocols, we have shown that transplantation of enriched murine or human stem/progenitor cell populations improved functional readouts of irradiated mice salivary glands [37,67,68][45][46][47]. However, this effect may not only be directly from the expansion of the stem/progenitor cells in the transplanted tissue, but also due to paracrine effects of the transplanted cells acting on the recipient tissue [45] [67]. Another recent study by Tanaka et al. has demonstrated the ability to derive salivary gland stem cells from embryonic stem cells [69][48]. Upon transplantation into parotid gland-defective mice, the induced salivary gland cells (transplanted either alone or together with mesenchymal cells) were capable of generating mature salivary gland tissue. The newly generated tissue was also shown to be functional as demonstrated by an increased saliva secretion in transplanted mice [69][48]. Combined, these studies hold significant preclinical promise for studying the mechanisms behind salivary gland regeneration and amelioration of salivary gland damage, both irradiation and non-irradiation induced damage [67,69][46][48]. However, the translation of any embryonic stem cell derived treatment [69][48] to a clinical application is always likely to be hindered by ethical concerns[49] [70] and safety concerns regarding tumorigenicity [71][50].
Our models have been successfully utilised to study the survival responses of salivary gland stem/progenitor cells [26][51]. The salivary gland stem/progenitor organoids demonstrated a disproportionate sensitivity to low dose of radiation which was recapitulated in a functional low dose sensitivity in vivo [72][52]. While low dose hypersensitivity is not a new phenomenon [73[53][54],74], this was the first study to show the relevance of this phenomenon in stem/progenitor cells, with a potential clinical relevance. Furthermore, we have recently developed a protocol for the culturing of parotid salivary gland organoids and demonstrated that parotid gland stem cells display a similar radiosensitivity as those of submandibular salivary glands [75][55]. Importantly, as organoids are derived from stem/progenitor cell populations, they allow for the study of a more stem/progenitor specific response. As stem/progenitor cells play a prominent role in tissue regeneration following irradiation, models which allow for the understanding of these cells are crucial to protecting these tissues.
3. A platform for treatment response studies; moving towards personalised treatment?
The concept of precision treatments has been of growing interest in many fields of research in recent years, particularly oncology, as there a wide variability of patient responses to standard ‘one size fits all’ treatment regimens. In some cases, genetic factors which can be specifically targeted in a ‘personalised’ manner are already known, for example non-small cell lung cancer patients with an activating mutation in tyrosine kinase are particularly sensitive to treatment with tyrosine kinases inhibitors such as gefitinib [1]. However, for other cancers, such as oesophageal cancers and locally advanced rectal cancers, there are currently no accurate predictors of patient responses to treatment. The standard of care for oesophageal cancer consists of neo-adjuvant chemoradiotherapy followed by surgery, with a complete pathological response observed in approximately a quarter at the time of surgery but no response in approximately one fifth of patients [2][3]. Similarly, for neoadjuvant chemoradiotherapy treatment of colorectal cancer while approximately one fifth of patients show a complete pathological response, almost 40% of patients show no benefit to the treatment [4]. In both cancers, patients would clearly benefit from more robust pre-treatment predictive models.
Therefore, there has been a concentrated effort in the field of organoids to establish reliable predictors of colorectal cancer treatment response to both chemotherapy alone [5][6] and neoadjuvant chemoradiotherapy [7][8]. Van de Wetering et al. [5] established colorectal cancer organoids, alongside paired healthy tissue, and demonstrated that the organoids recapitulated the genetic profiles and mutational spectra of the tumours of origin. Furthermore, by performing screening of 83 compounds, including both clinically used drugs and experimental compounds, the authors showed that the organoids facilitated the high-content drug screening [5], which could facilitate precision treatments in the future. Interestingly, a later study by Ooft et al. [6] investigating treatment response of metastatic colorectal cancer using organoids, was able to predict accuracy of irinotecan monotherapy and 5-flurouracil/irinotecan dual therapy, with 80% and 83.3% respectively. While greater accuracy is required to implement predictive models in a clinical setting, these studies show the developing potential of organoids in precision medicine. Furthermore, in recent years, there has been an increasing number of studies aimed at identifying and repurposing already available drugs as radiosensitisers [9][10][11][12]. Drugs which can be repurposed offer cheaper and quicker alternatives to developing new drugs from scratch, while many of the adverse side effects are already known [13]. The possibilities to quickly and accurately screen drugs, as shown in the studies of van de Wetering et al. [5] and Ooft et al. [6], in cancer organoids will greatly increase the possibilities in precision medicine and further benefit the search for potentiators of radiation therapy.
Indeed, recent studies by Ganesh et al. [7] and Yao et al. [8] have focussed on rectal cancer organoids for predicting patient responses to neoadjuvant chemoradiotherapy (Table 1 summarises the different cancer organoids that have been used in studies of radiation responses). Both studies further consolidated other evidence that rectal organoids faithfully recapitulate the tumours of origin, performing histopathological and mutational comparisons between the two [7][8]. Moreover, Ganesh et al. showed that upon xenotransplantation of the organoids into mice they were found to metastasise to the same locations as the original tumours. Importantly, upon treating the organoids with chemotherapeutic drugs (such as 5-Flurouracil and oxaliplatin) heterogeneous treatment responses correlated with the clinical progression-free survival of patients. Interestingly, organoids which displayed resistance to radiation were derived from patients who either were resistant to therapy or showed disease recurrence following treatment [7]. Yao et al. [8] also correlated the therapeutic clinical outcomes to the standard neoadjuvant chemoradiotherapy with the organoid outcomes following treatment 5-Flurouracil, irinotecan or radiation. In sixty-eight out of the 80 patient-derived organoid lines generated, at least one of the three treatment courses was found to be predictive of the patients tumour regression score after surgery [8]. Furthermore, in a recent study, Pasch et al. established patient-derived cancer organoids and were prospectively able to predict the treatment response of a patient with metastatic colon cancer [14]. These studies combined with the works of van de Wetering et al. and Ooft et al. provide a significant step towards a model for patient-specific response prediction.
4. Concluding remarks
While it could be questioned if a response prediction accuracy of approximately 80-85% is good enough, this will surely only improve as the models themselves are further optimised. Combining clinical patient imaging techniques currently used to predict patient responses, such as PET/CT, with the in vitro predictions from organoids may in the future bring around more accurate means to forecast treatment outcomes. Understanding the mechanisms behind tissue regeneration are key to mitigating radiation-induced side effects, whether it is by stem cell therapy or through druggable targets to protect against damage, and organoids have already proven themselves as excellent models for such studies.
References
- Thomas J. Lynch; Daphne W. Bell; Raffaella Sordella; Sarada Gurubhagavatula; Ross A. Okimoto; Brian W. Brannigan; Patricia L. Harris; Sara M. Haserlat; Jeffrey G. Supko; Frank G. Haluska; et al.David N. LouisDavid C. ChristianiJeff SettlemanDaniel A. Haber Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non–Small-Cell Lung Cancer to Gefitinib. New England Journal of Medicine 2004, 350, 2129-2139, 10.1056/nejmoa040938.Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study Global Burden. JAMA Oncol. 2017, 3, 524–548.
- P. (Pieter) Van Hagen; M.C.C.M. (Maarten) Hulshof; J.J.B. (Jan) Van Lanschot; E.W. (Ewout) Steyerberg; M.I. (Mark) Van Berge Henegouwen; B.P.L. (Bas) Wijnhoven; D.J. (Dirk) Richel; Grard A. Nieuwenhuijzen; G.A.P. (G. A P) Hospers; J. (Han) Bonenkamp; et al.M.A. (Miguel) CuestaR.J.B. BlaisseO.R.C. (Olivier) BuschF.J.W. Ten KateG.J. CreemersC. J. A. PuntJohn Th. M. PlukkerH.M.W. (Henk) VerheulErnst J. Spillenaar BilgenH. (Herman) Van DekkenM.J.C. (Maurice) Van Der SangenT. (Tom) RozemaK. (Katharina) BiermannJ.C. (Jannet) BeukemaA.H.M. (A. H M) PietC.M. Van RijJ.G.P. (Jurrien) ReindersH.W. (Hugo) TilanusAte Van Der Gaast Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. New England Journal of Medicine 2012, 366, 2074-2084, 10.1056/nejmoa1112088.Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 2013, 12, 526–542.
- Katrin M Sjoquist; Bryan H Burmeister; B Mark Smithers; John R Zalcberg; R John Simes; Andrew Barbour; Val Gebski; Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. The Lancet Oncology 2011, 12, 681-692, 10.1016/s1470-2045(11)70142-5.Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425.
- Harinarayanan Janakiraman; Yun Zhu; Scott A. Becker; Cindy Wang; Ashley Cross; Emily Curl; David Lewin; Brenda J. Hoffman; Graham W. Warren; Elizabeth G. Hill; et al.Cynthia TimmersVictoria J. FindlayErnest R. Camp Modeling rectal cancer to advance neoadjuvant precision therapy. International Journal of Cancer 2020, 147, 1405-1418, 10.1002/ijc.32876.Borras, J.M.; Lievens, Y.; Barton, M.; Corral, J.; Ferlay, J.; Bray, F.; Grau, C. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis. Radiother. Oncol. 2016, 119, 5–11.
- Marc Van De Wetering; Hayley E. Francies; Joshua M. Francis; Gergana Bounova; Francesco Iorio; Apollo Pronk; Winan Van Houdt; Joost Van Gorp; Amaro Taylor-Weiner; Lennart Kester; et al.Anne McLaren-DouglasJoyce BlokkerSridevi JaksaniSina BartfeldRichard VolckmanPeter Van SluisSaez-Rodriguez JulioSara SeepoChandra Sekhar PedamalluKristian CibulskisScott L. CarterAaron McKennaMichael S. LawrenceLee LichtensteinChip StewartJan KosterRogier VersteegAlexander Van OudenaardenJulio Saez-RodriguezRobert G.J. VriesGad GetzLodewyk WesselsMichael R. StrattonUltan McDermottMatthew MeyersonMathew J. GarnettHans Clevers Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell 2015, 161, 933-945, 10.1016/j.cell.2015.03.053.Wang, X.; Eisbruch, A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J. Radiat. Res. 2016, 57, i69–i75.
- Salo N. Ooft; Fleur Weeber; Krijn K. Dijkstra; Chelsea McLean; Sovann Kaing; Erik D. Van Werkhoven; Luuk Schipper; Louisa Hoes; Daniel J. Vis; Joris Van De Haar; et al.Warner PrevooPetur SnaebjornssonDaphne Van Der VeldenMichelle KleinMyriam ChalabiHenk BootMonique Van LeerdamHaiko J. BloemendalLaurens V. BeerepootLodewyk WesselsEdwin CuppenHans CleversEmile E. Voest Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Science Translational Medicine 2019, 11, eaay2574, 10.1126/scitranslmed.aay2574.Folkert, M.R.; Timmerman, R.D. Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT). Adv. Drug Deliv. Rev. 2017, 109, 3–14.
- Karuna Ganesh; Chao Wu; Kevin P. O’Rourke; Bryan Szeglin; Youyun Zheng; Charles-Etienne Gabriel Sauvé; Mohammad Adileh; Isaac Wasserman; Michael R. Marco; Amanda S. Kim; et al.Maha ShadyFrancisco Sanchez-VegaWouter R. KarthausHelen H. WonSeo-Hyun ChoiRaphael PelossofAfsar BarlasPeter NtiamoahEmmanouil PappouArthur ElghouayelJames S. StrongChin-Tung ChenJennifer W. HarrisMartin R. WeiserGarrett M. NashJose G. GuillemIris H. WeiRichard N. KolesnickHarini VeeraraghavanEduardo J. OrtizIva PetkovskaAndrea CercekKatia O. Manova-TodorovaLeonard B. SaltzJessica A. LaveryRonald P. DeMatteoJoan MassaguéPhilip B. PatyRona YaegerXi ChenSujata PatilHans CleversMichael F. BergerScott W. LoweJinru ShiaPaul B. RomesserLukas E. DowJulio Garcia-AguilarCharles L. SawyersJ. Joshua Smith A rectal cancer organoid platform to study individual responses to chemoradiation. Nature Medicine 2019, 25, 1607-1614, 10.1038/s41591-019-0584-2.Durante, M.; Loeffler, J.S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 2010, 7, 37–43.
- Ye Yao; Xiaoya Xu; Lifeng Yang; Ji Zhu; Juefeng Wan; Lijun Shen; Fan Xia; Guoxiang Fu; Yun Deng; Mengxue Pan; et al.Qiang GuoXiaoxue GaoYuanchuang LiXinxin RaoYi ZhouLiping LiangYaqi WangJing ZhangHui ZhangGuichao LiLixing ZhangJunjie PengSanjun CaiChen HuJianjun GaoHans CleversZhen ZhangGuoqiang Hua Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020, 26, 17-26.e6, 10.1016/j.stem.2019.10.010.Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.A.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136.
- Stephen L. Brown; Andrew Kolozsvary; Derek M. Isrow; Karine Al Feghali; Karen Lapanowski; Kenneth A. Jenrow; Jae Ho Kim; A Novel Mechanism of High Dose Radiation Sensitization by Metformin. Frontiers in Oncology 2019, 9, 247, 10.3389/fonc.2019.00247.Van Luijk, P.; Pringle, S.; Deasy, J.O.; Moiseenko, V.V.; Faber, H.; Hovan, A.; Baanstra, M.; Van Der Laan, H.P.; Kierkels, R.G.J.; Van Der Schaaf, A.; et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci. Transl. Med. 2015, 7, 305ra147.
- Linda Spiegelberg; Stefan J. Van Hoof; Rianne Biemans; Natasja G. Lieuwes; Damiënne Marcus; Raymon Niemans; Jan Theys; Ala Yaromina; Philippe Lambin; Frank Verhaegen; et al.Ludwig J. Dubois Evofosfamide sensitizes esophageal carcinomas to radiation without increasing normal tissue toxicity.. Radiotherapy and Oncology 2019, 141, 247-255, 10.1016/j.radonc.2019.06.034.Wang, L.; Hoogcarspel, S.J.; Wen, Z.; van Vulpen, M.; Molkentine, D.P.; Kok, J.; Lin, S.H.; Broekhuizen, R.; Ang, K.K.; Bovenschen, N.; et al. Biological responses of human solid tumor cells to X-ray irradiation within a 1.5-Tesla magnetic field generated by a magnetic resonance imaging–linear accelerator. Bioelectromagnetics 2016, 37, 471–480.
- Le Zhang; Milana Bochkur Dratver; Taha Yazal; Kevin Dong; Andrea Nguyen; Garrett Yu; Amy Dao; Michael Bochkur Dratver; Sara Duhachek- Muggy; Kruttika Bhat; et al.Claudia AlliFrank PajonkErina Vlashi Mebendazole Potentiates Radiation Therapy in Triple-Negative Breast Cancer. International Journal of Radiation Oncology*Biology*Physics 2019, 103, 195-207, 10.1016/j.ijrobp.2018.08.046.Raaymakers, B.W.; Jürgenliemk-Schulz, I.M.; Bol, G.H.; Glitzner, M.; Kotte, A.N.T.J.; Van Asselen, B.; De Boer, J.C.J.; Bluemink, J.J.; Hackett, S.L.; Moerland, M.A.; et al. First patients treated with a 1.5 T MRI-Linac: Clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys. Med. Biol. 2017, 62, L41–L50.
- Lindsay A. Broadfield; Katarina Marcinko; Evangelia Tsakiridis; Panayiotis G. Zacharidis; Linda Villani; James S. V. Lally; Gabe Menjolian; Danitra Maharaj; Tammy Mathurin; Marcia Smoke; et al.Thomas FarrellPaola MutiGregory R. SteinbergTheodoros Tsakiridis Salicylate enhances the response of prostate cancer to radiotherapy. The Prostate 2019, 79, 489-497, 10.1002/pros.23755.Lomax, A.J. Charged particle therapy: The physics of interaction. Cancer J. 2009, 15, 285–291.
- Sudeep Pushpakom; Francesco Iorio; Patrick A Eyers; K. Jane Escott; Shirley Hopper; Andrew Wells; Andrew Doig; Tim Guilliams; Joanna Latimer; Christine McNamee; et al.Alan NorrisPhilippe SanseauDavid CavallaMunir Pirmohamed Drug repurposing: progress, challenges and recommendations. Nature Reviews Drug Discovery 2018, 18, 41-58, 10.1038/nrd.2018.168.Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209.
- Cheri A. Pasch; Peter F. Favreau; Alexander E. Yueh; Christopher P. Babiarz; Amani A. Gillette; Joe T. Sharick; Mohammad Rezaul Karim; Kwangok P. Nickel; Alyssa K. DeZeeuw; Carley M. Sprackling; et al.Philip B. EmmerichRebecca A. DestefanisRosabella T. PiteraSusan N. PayneDemetra P. KorkosLinda ClipsonChristine M. WalshDevon MillerEvie H. CarchmanMark E. BurkardKayla K. LemmonKristina A. MatkowskyjMichael A. NewtonIrene M. OngMichael F. BassettiRandall J. KimpleMelissa C SkalaDustin A. Deming Patient-Derived Cancer Organoid Cultures to Predict Sensitivity to Chemotherapy and Radiation. Clinical Cancer Research 2019, 25, 5376-5387, 10.1158/1078-0432.ccr-18-3590.Oborn, B.M.; Dowdell, S.; Metcalfe, P.E.; Crozier, S.; Mohan, R.; Keall, P.J. Proton beam deflection in MRI fields: Implications for MRI-guided proton therapy. Med. Phys. 2015, 42, 2113–2124.
- Oborn, B.M.; Dowdell, S.; Metcalfe, P.E.; Crozier, S.; Mohan, R.; Keall, P.J. Future of medical physics: Real-time MRI-guided proton therapy: Real-time. Med. Phys. 2017, 44, e77–e90.
- Nagle, P.W.; van Goethem, M.J.; Kempers, M.; Kiewit, H.; Knopf, A.; Langendijk, J.A.; Brandenburg, S.; van Luijk, P.; Coppes, R.P. In vitro biological response of cancer and normal tissue cells to proton irradiation not affected by an added magnetic field. Radiother. Oncol. 2019, 137, 125–129.
- Inaniwa, T.; Suzuki, M.; Sato, S.; Muramatsu, M.; Noda, A.; Iwata, Y.; Kanematsu, N.; Shirai, T.; Noda, K. Effect of External Magnetic Fields on Biological Effectiveness of Proton Beams. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 597–603.
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563.
- Vozenin, M.-C.; Baumann, M.; Coppes, R.P.; Bourhis, J. FLASH radiotherapy International Workshop. Radiother. Oncol. 2019, 139, 1–3.
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007.
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265.
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772.
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve Stem Cells Drive Self-Renewal in the Stomach and Build Long-Lived Gastric Units In Vitro. Cell Stem Cell 2010, 6, 25–36.
- DeWard, A.D.; Cramer, J.; Lagasse, E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 2014, 9, 701–711.
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–110.
- Trisno, S.L.; Philo, K.E.D.; McCracken, K.W.; Catá, E.M.; Ruiz-Torres, S.; Rankin, S.A.; Han, L.; Nasr, T.; Chaturvedi, P.; Rothenberg, M.E.; et al. Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell Stem Cell 2018, 23, 501–515.
- Drost, J.; Van Boxtel, R.; Blokzijl, F.; Mizutani, T.; Sasaki, N.; Sasselli, V.; De Ligt, J.; Behjati, S.; Grolleman, J.E.; Van Wezel, T.; et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science 2017, 358, 234–238.
- Dekkers, J.F.; Wiegerinck, C.L.; De Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; De Winter-De Groot, K.M.; Brandsma, A.M.; De Jong, N.W.M.; Bijvelds, M.J.C.; Scholte, B.J.; et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013, 19, 939–945.
- Wang, X.; Wei, L.; Cramer, J.M.; Leibowitz, B.J.; Judge, C.; Epperly, M.; Greenberger, J.; Wang, F.; Li, L.; Stelzner, M.G.; et al. Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci. Rep. 2015, 5, 8566.
- Bhanja, P.; Norris, A.; Gupta-Saraf, P.; Hoover, A.; Saha, S. BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res. Ther. 2018, 9, 26.
- Nag, D.; Bhanja, P.; Riha, R.; Sanchez-Guerrero, G.; Kimler, B.F.; Tsue, T.T.; Lominska, C.; Saha, S. Auranofin protects intestine against radiation injury by modulating p53/p21 pathway and radiosensitizes human colon tumor. Clin. Cancer Res. 2019, 25, 4791–4807.
- Martin, M.L.; Adileh, M.; Hsu, K.S.; Hua, G.; Lee, S.G.; Li, C.; Fuller, J.D.; Rotolo, J.A.; Bodo, S.; Klingler, S.; et al. Organoids reveal that inherent radiosensitivity of small and large intestinal stem cells determines organ sensitivity. Cancer Res. 2020, 256, 1219–1227.
- Withers, H.R.; Elkind, M.M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int. J. Radiat. Biol. 1970, 17, 261–267.
- Nutting, C. Radiotherapy in head and neck cancer management: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S66–S67.
- Vissink, A.; Mitchell, J.B.; Baum, B.J.; Limesand, K.H.; Jensen, S.B.; Fox, P.C.; Elting, L.S.; Langendijk, J.A.; Coppes, R.P.; Reyland, M.E. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: Successes and barriers. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 983–991.
- Rocchi, C.; Emmerson, E. Mouth-Watering Results: Clinical Need, Current Approaches, and Future Directions for Salivary Gland Regeneration. Trends Mol. Med. 2020, 26, 649–669.
- Zeilstra, L.J.W.; Vissink, A.; Konings, A.W.T.; Coppes, R.P. Radiation induced cell loss in rat submandibular gland and its relation to gland function. Int. J. Radiat. Biol. 2000, 76, 419–429.
- Coppes, R.P.; Vissink, A.; Konings, A.W.T. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother. Oncol. 2002, 63, 321–328.
- Marmary, Y.; Adar, R.; Gaska, S.; Wygoda, A.; Maly, A.; Cohen, J.; Eliashar, R.; Mizrachi, L.; Orfaig-Geva, C.; Baum, B.J.; et al. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer Res. 2016, 76, 1170–1180.
- Emmerson, E.; May, A.J.; Nathan, S.; Cruz-Pacheco, N.; Lizama, C.O.; Maliskova, L.; Zovein, A.C.; Shen, Y.; Muench, M.O.; Knox, S.M. SOX2 regulates acinar cell development in the salivary gland. eLife 2017, 6, e26620.
- Ninche, N.; Kwak, M.; Ghazizadeh, S. Diverse epithelial cell populations contribute to the regeneration of secretory units in injured salivary glands. Development 2020, 147, dev192807.
- Lombaert, I.M.A.; Patel, V.N.; Jones, C.E.; Villier, D.C.; Canada, A.E.; Moore, M.R.; Berenstein, E.; Zheng, C.; Goldsmith, C.M.; Chorini, J.A.; et al. CERE-120 Prevents Irradiation-Induced Hypofunction and Restores Immune Homeostasis in Porcine Salivary Glands. Mol. Ther.-Methods Clin. Dev. 2020, 18, 839–855.
- Meyer, R.; Wong, W.Y.; Guzman, R.; Burd, R.; Limesand, K. Radiation treatment of organotypic cultures from submandibular and parotid salivary glands models key in vivo characteristics. J. Vis. Exp. 2019, 2019, 59484.
- Nanduri, L.S.Y.; Baanstra, M.; Faber, H.; Rocchi, C.; Zwart, E.; De Haan, G.; Van Os, R.; Coppes, R.P. Purification and Ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep. 2014, 3, 957–964.
- Pringle, S.; Maimets, M.; Van Der Zwaag, M.; Stokman, M.A.; Van Gosliga, D.; Zwart, E.; Witjes, M.J.H.; De Haan, G.; Van Os, R.; Coppes, R.P. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 2016, 34, 640–652.
- Maimets, M.; Rocchi, C.; Bron, R.; Pringle, S.; Kuipers, J.; Giepmans, B.N.G.; Vries, R.G.J.; Clevers, H.; De Haan, G.; Van Os, R.; et al. Long-Term in Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Rep. 2016, 6, 150–162.
- Nanduri, L.S.Y.; Maimets, M.; Pringle, S.A.; Van Der Zwaag, M.; Van Os, R.P.; Coppes, R.P. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother. Oncol. 2011, 99, 367–372.
- Tanaka, J.; Ogawa, M.; Hojo, H.; Kawashima, Y.; Mabuchi, Y.; Hata, K.; Nakamura, S.; Yasuhara, R.; Takamatsu, K.; Irié, T.; et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018, 9, 4216.
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213.
- Nori, S.; Okada, Y.; Nishimura, S.; Sasaki, T.; Itakura, G.; Kobayashi, Y.; Renault-Mihara, F.; Shimizu, A.; Koya, I.; Yoshida, R.; et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015, 4, 360–373.
- Nagle, P.W.; Hosper, N.A.; Ploeg, E.M.; Van Goethem, M.J.; Brandenburg, S.; Langendijk, J.A.; Chiu, R.K.; Coppes, R.P. The in Vitro Response of Tissue Stem Cells to Irradiation with Different Linear Energy Transfers. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 103–111.
- Nagle, P.W.; Hosper, N.A.; Barazzuol, L.; Jellema, A.L.; Baanstra, M.; Van Goethem, M.J.; Brandenburg, S.; Giesen, U.; Langendijk, J.A.; Van Luijk, P.; et al. Lack of DNA damage response at low radiation doses in adult stem cells contributes to organ dysfunction. Clin. Cancer Res. 2018, 24, 6583–6593.
- Marples, B.; Joiner, M.C. The response of Chinese hamster V79 cells to low radiation doses: Evidence of enhanced sensitivity of the whole cell population. Radiat. Res. 1993, 133, 41–51.
- Martin, L.M.; Marples, B.; Lynch, T.H.; Hollywood, D.; Marignol, L. Exposure to low dose ionising radiation: Molecular and clinical consequences. Cancer Lett. 2014, 349, 98–106.
- Serrano Martinez, P.; Cinat, D.; van Luijk, P.; Baanstra, M.; de Haan, G.; Pringle, S.; Coppes, R.P. Mouse parotid salivary gland organoids for the in vitro study of stem cell radiation response. Oral Dis. 2020.
