Nanoemulsions (NEs) are lipophilic systems with nanoscale globules that can be absorbed e.g. through the nasal mucosa, and are therefore being explored for nose-to-brain delivery of drugs. These can be either oil-in-water (o/w) or water-in-oil (w/o) emulsions. Especially, o/w NEs are a promising option for the encapsulation of lipophilic drugs, protecting them from enzymatic degradation, increasing their solubility in liquid media, modulating their drug release, and improving their bioavailability [30].
- nanoemulsions
- nose-to-brain delivery
- nasal administration
- blood-brain barrier
- neurogenerative disorders
- CNS diseases
- intranasal
- brain targeting
- poorly soluble drugs
- bioavailability enhancement
1. Nanoemulsions
Nanoemulsions may be modified to mucoadhesive systems to increase the residence time of the formulation and to overcome the nasal clearance to achieve enhanced mucosal absorption. NEs have also been proven to mitigate the side effects and toxicity of drugs. Several NE formulations, primarily of the o/w type, have been developed for nose-to-brain delivery [1][31]. Some significant parameters of NEs for intranasal administration are presented in Figure 13. NEs may be developed through different methods by using oil, surfactants, cosurfactants, and water, all of which play a significant role in the permeation of drugs through the nasal mucosa.
Figure 13. Significant features of nanoemulsions for nasal administration.
1.1. Overview of Nanoemulsion Components
1.1.1. Oils
The major problem of new molecular entities in the drug discovery and development pipeline is their poor water solubility, which affects several key properties of therapeutic agents, such as the pharmacokinetic and pharmacodynamic parameters. Hence, oils are used for the development of NE to achieve the maximum solubility of drugs. The lipophilicity of oils is directly proportional to the solubility of drugs [2][32]. The solubilizing capacity of oils decreases in the order of vegetable oils > medium-chain triglycerides > medium-chain mono- and diglycerides [3][33]. Further, the solubility of drugs also depends on the concentration of oils in the NE formulations. The globule size of NE increases with an increase in the oil concentration [4][34]. At the same time, a larger globule size reduces drug permeation from the nasal mucosa. Some oils have permeation-enhancing properties as well. NEs showed selectivity in the uptake of some drugs, such as linolenic acid, polyunsaturated and omega-6 fatty acids, and pinolenic acid [5][35]. Edmond et al. proved that oleic acid with one cis-double bond did not get transported across the BBB, while linoleic acid with two cis-double bonds and 18 monocarboxylic acids efficiently entered the brain after intranasal administration [6][36].
The maximum solubility of a drug can be achieved by striking a correct balance between the concentration of the emulsifying agent (surfactant) and oils. Here, one needs to select the region having maximum emulsification in the phase diagram [4][34]. Better drug permeation can be achieved through a minimum globule size; hence, formulations having higher globule sizes have less permeation through the nasal mucosa. Several oils have significant permeation-enhancing properties through the nasal mucosa. For example, NE of quetiapine fumarate containing butter oil showed significant nose-to-brain delivery [7][37]. It was deduced that polar lipids from butter oil enhanced the permeation through the nasal mucosa.
1.1.2. Surfactants
Surfactants are essential components of NE formulation and signify an important role in the surface tension reduction. Surfactants stabilize NE by preventing the phase separation and coalescence of globules. Further, surfactants affect solubilization of the drugs and increase the permeation of drugs through the nasal mucosa due to alterations of the fluidity and damage to the tight junctions of epithelial layers [8][38]. Several studies report that the globule size of NEs decreases with increasing the concentrations of the surfactants. The lower the globule size, the better the permeation and, hence, the drug concentration in the brain. Nevertheless, the structural integrity of the nasal mucosa is critically affected by surfactants. Hence, the surfactant concentration should be selected carefully, keeping in view the safety considerations of the nasal mucosa [9][10][39,40].
1.1.3. Cosurfactants
Surfactants alone are not able to reduce the surface tension to the desired level, because most of the surfactants used in the development of NEs are single-chain surfactants. Therefore, cosurfactants are incorporated to achieve the desired hydrophilic-lipophilic balance (HLB) [11][12][41,42]. Cosurfactants increase the fluidity of the formulations by reducing the interfacial tension, which can facilitate emulsification and stabilize the NE. For the development of stable NE, a judicious combination of surfactant and cosurfactant is crucial. The construction of ternary-phase diagrams is a commonly used methodology to optimize the working range and the optimum concentration(s) of oil, surfactant, and cosurfactant. An increase in the concentration of the cosurfactant deceases the globule size of the NE, and, ultimately, the drug concentration will be enhanced. Some most commonly used cosurfactants in the development of NEs for intranasal administration are transcutol-P, butan-1-ol, chiral alcohols, sorbitol, and polyethylene glycol [3][33].
1.2. Significant Factors of Nanoemulsions for Nose-to-Brain Delivery
Several research reports have shown evidence for better drug permeation to the brain from the nasal mucosa by NEs than after conventional oral drug delivery. Apart from the drug permeation-enhancing properties of the surfactants and cosurfactants, NEs have several significant features tailored for brain targeting [13][10]. Some major features of the NEs are outlined below.
1.2.1. Globule Size
The globule size of the NE plays a very significant role in drug permeation through the nasal cavity. As stated earlier, olfactory and trigeminal transport routes are the major channels for drug delivery to the brain by nasal drug delivery. The average diameter of an olfactory axon is approximately 200 nm in different preclinical species, but in humans, it ranges between 100 to 700 nm [14][43]. Hence, the globule size of novel formulations should be below 200 nm for successful drug permeation. Ahmad et al. reported that NEs with an average globule size of 100 nm exhibited a higher rate and extent of drug absorption than the average globule size of 700 nm through the olfactory pathway [14][43]. In addition, the globule size of the NE also affects the retention time of the formulations on the nasal mucosa. Smaller globule size formulations have longer retention times than the larger globule NEs that can be easily removed by nasal clearance, thus having reduced drug absorption [15][44]. For example, while NEs with average globule sizes larger than 200 nm may exhibit retention times up to 4 h after intranasal administration, NEs having globule sizes of 80 and 200 nm have shown retention times of 16 and 12 h, respectively. Hence, globule sizes of nanoemulsions play a significant role for drug delivery to the brain through intranasal administration [16][45].
1.2.2. Zeta Potential
The colloidal stability of NE is connected to the zeta potential of the developed formulations. For any colloidal system, zeta potential values exceeding ±30 mV provide electrostatically stabilized systems [17][46]. Moreover, several reports have shown that the zeta potential has a significant role in the drug retention time of the NE formulations. Mucin found in the nasal mucosa bears a negative charge; hence, formulations carrying positive charges depict good attachment to the nasal mucosa [18][25]. Several studies have shown that, usually, most of the nasal NEs developed for brain delivery bear negative charges. The values of zeta potential higher than −10 mV of the emulsion indicate the instability of NEs. Therefore, zeta potential is also an important consideration in the development of NEs for nose-to-brain delivery [10][40].
2. Intranasal NEs for Brain Disorders
Even though the nose-to-brain pathway is a proven direct drug delivery approach to the brain, the absorption efficacy of NEs through this pathway is still questionable and awaiting further validation with proof. In one study, the nose-to-brain transport of NEs was traced by the fluorescence bioimaging technique. The localization of NEs in the biological tissues was based on the on-off switching of signals of environment-sensitive embedded dyes (P2 and P4) and two probes (coumarin-6 and DiR) to represent the cargoes. NEs translocation in rats was established by either through an in vitro histological analysis or live imaging. The results evidenced that ≈100 nm globule-sized NEs, decorated or non-decorated with chitosan, had long retention times in rat nostrils and slower mucociliary clearance than larger ones. The P2 signals were traced in the mucosa and trigeminal nerves for all globule-sized groups, while weak P2 signals were identified for chitosan-decorated NEs of 100-nm size in the olfactory bulb. Confocal laser scanning microscopy confirmed the active transport of integral globules in the nasal mucosa and along the trigeminal nerve as attenuated signals. The low intensity of the P4 probe, also representing integral NEs, was identified in the olfactory bulb, and very few signals were detected in the brain. The study demonstrated that NEs as large as 900 nm could not be delivered to the olfactory bulb. However, the coumarin-6 or DiR signals were found in significant quantity along the nose-to-brain pathway that finally reached the brain. Thus, it was evidenced that integral NEs can be delivered to the olfactory bulb, but few were transported to the brain [16][45]. Therefore, the cargoes should permeate into the brain in greater amounts, proving intranasal administration as a promising drug delivery strategy. Several intranasal NEs have been studied for the treatment of CNS ailments such as Alzheimer’s disease, epilepsy, Parkinson’s disease, migraines, depression, brain tumors, and other related disorders. Some examples of intranasal NEs with their potential outcomes for brain disorders have been compiled in Table 1.
Table 1. Summarized examples of nanoemulsion-based approaches for brain targeting through intranasal drug delivery.
| Drug | Therapy for | Characterization Parameters |
Study Model (s) | Relevant Therapeutic Outcomes | Ref. |
|---|
| Donepezil | Alzheimer’s disease | GS = 127.13 ± 4.14 nm PDI = 0.182 ± 0.011 |
In vitro drug diffusion study. Ex vivo drug permeation study. Tolerability study through in vitro and in vivo models. |
The permeation of donepezil was found to be significant through intranasal NE. The polymers can be used as an effective strategy to improve the bioadhesion and drug penetration through nasal mucosa, which enhances the bioavailability of donepezil. | [19] | [47] | ||||
| Rivastigmine | Alzheimer’s disease | GS = 35.75 ± 0.21 nm PDI = 0.247 ± 0.04 ZP = −24.4 ± 0.67 mV |
In vitro drug release study. Ex vivo diffusion study. In vivo pharmacokinetic and biodistribution study in rat. Nasal ciliotoxicity studies in goat nasal mucosa. |
Rivastigmine-loaded NE showed significantly higher drug concentration in brain than the solution. The optimized formulation was devoid of nasal ciliotoxicity. | [20] | [48] | ||||
| Resveratrol | Parkinson’s disease | GS = 176.3 ± 3.5 nm PDI = 0.17 ± 0.03 ZP = 18.5 ± 1.77 mV |
In vitro drug release study. Ex vivo diffusion study. In vivo drug biodistribution study in Wistar rat’s brain. |
Diffusion controlled release of resveratrol was for 6 h with flux of 2.86 mg/cm | 2 | h through sheep nasal mucosa. The drug level in the brain from intranasal resveratrol mucoadhesive NE was higher than the resveratrol solution. Bioavailability was seven times higher through this approach. | [21] | [49] | ||
| Selegiline | Parkinson’s disease | GS = 61.43 ± 4.10 nm PDI = 0.203 ± 0.005 ZP = −34.00 ± 0.17 mV |
In vitro drug release study. Ex vivo diffusion study. Behavioral activities of Parkinson’s disease in Wistar rats. |
Selegiline NE showed 3.7-fold more penetration than the drug solution. Haloperidol-induced Parkinson’s disease in animals with selegiline intranasal NE showed significant improvement in behavioral activities in comparison to conventional drug delivery. | [22] | [50] | ||||
| Letrozole | Epilepsy | GS = 95.59 ± 2.34nm PDI = 0.162 ± 0.012 ZP = −7.12 ± 0.12 mV |
In vitro and ex vivo drug release study. A behavioral seizure; biochemical and histopathological studies were performed. |
Intranasal administration of NE showed the prolonged drug release profile as compared to suspension. High concentration of drug was found in brain. |
[23] | [51] | ||||
| Amiloride | Antiepileptic | GS = 89.36 ± 11.18 nm PDI = 0.231 ± 0.018 ZP = −9.83 ± 0.12 mV |
In vitro drug release study. Ex vivo diffusion study. In vivo pharmacodynamic and pharmacokinetic study in Wistar rats. |
Bioavailability and brain-targeting efficiency with efficacy of developed amiloride NE was enhanced though nasal administration. | [24] | [52] | ||||
| Zolmitriptan | Migraine | GS = 54.63 ± 3.24 nm ZP = −0.086 ± 0.014 mV PDI = 0.17 ± 0.01 |
In vitro mucoadhesion study. Ex vivo drug permeation studies. In vivo pharmacokinetic and biodistribution studies. |
Zolmitriptan mucoadhesive NE showed higher permeability coefficients than the solution through the nasal mucosa. In vivo study of zolmitriptan mucoadhesive NE showed higher AUC | 0–8 | and shorter T | max | in the brain in comparison to intravenous and nasal solutions. | [25] | [53] |
| Rizatriptan | Migraine | GS = 20–120 nm | In vitro drug diffusion study. Nasal irritation study on sheep nasal mucosa. In vivo brain-targeting potential. |
Ex vivo drug diffusion-defined controlled release with 86% in 4 h. Brain targeting through intranasal NE (AUC = 302.52 μg min/g) was more than intranasal gel (AUC = 115 μg min/g) and intravenous route (AUC = 109.63 μg min/g). | [26] | [54] | ||||
| Cyclosporine-A | Neuroprotective | GS = 158.47 ± 3.02 nm ZP = −30 mV |
In vitro drug diffusion study. In vivo brain uptake study. |
The brain/blood ratios of cyclosporine-A by intranasal and intravenous was found to be 4.49 and 0.01, respectively. Cyclosporine-A NE can be used for direct nose-to-brain delivery, bypassing the BBB. |
[27] | [55] | ||||
| Kaempferol | Neuroprotective and anti-tumor | GS = 170.4 ± 4.1 nm PDI = 0.155 ± 0.015 ZP = −18.71 ± 1.72 |
Ex vivo diffusion study. In vivo drug biodistribution study in Wistar rats. |
The drug concentration through intranasal NE was found to be 4 to 5-fold higher than the solution. Ex vivo permeation and in vivo biodistribution studies showed higher drug concentrations in the brain with chitosan NE through intranasal administration in compared to NE and the kaempferol solution. | [28] | [56] | ||||
| Ziprasidone hydrochloride | Antipsychotic | GS = 145.24 ± 4.75 nm PDI = 0.186 ± 0.40 ZP = −30.2 ± 3.21 mV DC = 0.3418 ± 0.03 CM | 2 | /min | Ex vivo diffusion study. In vivo pharmacodynamic study in Wistar rats. Nasal ciliotoxicity studies in goat nasal mucosa. |
Higher drug diffusion of ziprasidone NE than the solution was found. Pharmacodynamic study revealed the superiority of mucoadhesive NE than NE in the locomotor activity and paw test. Formulation was devoid of acute nasal ciliotoxicity. | [29] | [57] | ||
| Quetiapine | Antipsychotic | GS = 144 ± 0.5 nm | In vitro dissolution study. In vivo drug distribution study in Wistar rats. |
Higher drug transport efficiency (DTE%) via intranasal NE. | [30] | [58] |
Abbreviations: GS = globule size, PDI = polydispersity index, ZP = zeta potential, DC = diffusion coefficient, NE = nanoemulsion, BBB = blood–brain barrier, and AUC = area under the curve.
2.1. NEs for Alzheimer’s Disease
Many characteristics of the BBB are affected in Alzheimer disease (AD), and these changes, in turn, have implications for the onset, progression, control, and treatment of the disease. In such circumstances, the BBB itself becomes a therapeutic target, and at the same time, it also acts as a formidable barrier against the delivery of drugs to the brain in the treatment of AD. Drugs like acetylcholinesterase inhibitors (galantamine, rivastigmine, and donepezil) and memantine used for AD treatment show poor brain delivery due to unfavorable pharmacokinetics and pharmacodynamics of drugs [31][32][59,60].
NEs have garnered considerable interest in research for AD therapeutics due to their attractive features. Sood et al. reported the intranasal delivery of curcumin–donepezil NE for brain targeting in AD. The NE was formulated using Capmul MCM as the oily phase, Cremophor EL and Tween 80 as surfactants, and PEG 400 as a cosurfactant to result in particle sizes less than 50 nm, which are regarded appropriate for intranasal administration. In vivo pharmacokinetic studies in streptozotocin-induced AD model rats revealed higher drug localization in the brain via the intranasal route compared to the intravenous route. The drug clearance from the nasal cavity was also slower after intranasal administration. The pharmacodynamic study of behavioral tasks in rats showed improved memory and learning in the test group treated with NEs compared to the drug alone. A biochemical assessment revealed that acetylcholine levels in the brain were significantly improved in the NE-treated group. The oxidative stress was much lower in animals treated with a combination therapy. Thus, the strategy of the intranasal delivery of an acetylcholinesterase inhibitor with a neuroprotective and anti-amyloid drug appeared to be a promising strategy for the management of AD. The same research group reported the optimization of mucoadhesive NE of curcumin using the Box-Behnken design, which resulted in a high permeation of curcumin across the nasal mucosal layer and was devoid of cytotoxicity in the SK-N-SH cell line [3][33][33,61].
Huperzine A (HupA), a reversible acetylcholinesterase inhibitor, is neuroprotective and enhances memory in behavioral animal models [34][62]. The commercially available oral and injectable formulations of HupA lack brain selectivity [35][63]. Hence, a new drug delivery system is required to improve the transport and distribution of the drug to the brain. Jiang et al. aimed to develop HupA NE (HupA-NE) for intranasal administration and imprsove its targeting efficiency by modifying HupA-NE with lactoferrin. The optimized HupA-NE with a globule size of 15.24 ± 0.67 nm, polydispersity index of 0.128 ± 0.025, and a zeta potential of −4.48 ± 0.97 mV was modified with lactoferrin. The lactoferrin-HupA-NE was readily taken up into hCMEC/D3 cells (in vitro model for BBB containing P-glycoprotein, multidrug resistance associated protein 1 transporters, and the breast cancer resistance protein) to a greater extent than drug NE without lactoferrin [36][37][64,65]. The mechanisms proposed for higher transport to the brain were identified as transcytosis and uptaken by specific transporters. The intranasal administration of lactoferrin-HupA-NE in rats significantly (p < 0.05) enhanced drug delivery to the brain compared to HupA-NE. The direct targeting index of lactoferrin-HupA-NE (3.21 ± 0.75) demonstrated brain targeting, and the area under the curve (AUC)0–α for lactoferrin-HupA-NE was significantly higher (p < 0.05) compared to HupA-NE [38][66].
2.2. NE for Parkinson’s Disease
In spite of several developments in drug delivery systems, the treatment of neuronal disorders such as Parkinson’s disease (PD) are still lacking, with limited treatment strategies [39][67]. Evidence that dopamine loss is the key pathological feature of PD and the subsequent introduction of levodopa revolutionized the treatment of PD (Figure 24). Levodopa is a metabolic precursor of dopamine and is capable of traversing the BBB, where it gets converted into dopamine [40][68].
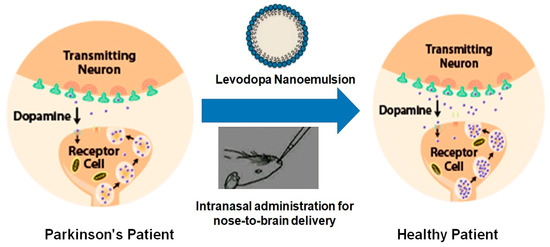
Figure 24. Diagrammatic representation of the effects of drug-loaded nanoemulsion on the dopamine levels in the brain in Parkinson’s disease.
Zainol et al. successfully developed a levodopa NE based on palm oil that has high thermodynamic and oxidative stability. Response surface methodology (RSM) was used for the investigation of the influence of the emulsion composition: a mixture of palm and medium-chain triglyceride oil (6–12% w/w), lecithin (1–3% w/w), and Cremophor EL (0.5–1.5% w/w). The researchers compared the effects of the interactions and significant factors associated with the composition of lecithin and Cremophor EL based on the RSM. The authors concluded that the RSM as a beneficial tool for carrying out the optimization study of levodopa NE formulations, and the stability of the levodopa-loaded NE, were correlated to the stabilizing effects of lecithin and Cremophor EL [41][69].
The intranasal administration of an antioxidant vitamin E loaded with naringenin for the direct brain delivery for PD therapeutics was explored [42][70]. The characteristics of optimized NE were a droplet size of 38.70 ± 3.11 nm and narrow polydispersity index of 0.14 ± 0.0024. Behavioral studies in Wistar rats showed that 6-OHDA-induced PD symptoms were successfully reversed after the intranasal NE administration of naringenin + levodopa. A biochemical investigation revealed a significant increase in the levels of glutathione and superoxide dismutase, while the levels of malondialdehyde were significantly lowered in the test group treated with naringenin + levodopa [43][71].
Mustafa et al. (2012) developed the intranasal nanoemulsion of ropinirole, a dopamine agonist approved for use to treat symptoms of early and advanced PD. The isotropic area identified by a pseudoternary-phase diagram was used for formulation development. The optimized formulation contained 2 mg of ropinirole, along with Sefsol 218 (10% v/v), Tween 80 (18% v/v), Transcutol (18% v/v), and water (54% v/v) as the oily matrix, surfactant, cosurfactant, and aqueous phase, respectively. The optimized formulation with a globule size of 58.61 ± 5.18 nm depicted a cumulative drug release of 72.23 ± 9.56%, a viscosity of 31.42 ± 6.97 mpas, and infinite dispersion capability. The ex vivo study evidenced significantly high (p < 0.005) drug translocation to the Wistar rat brain in comparison to the drug itself. The authors concluded ropinirole-loaded NE as a promising perspective for the management of PD when administered intranasally [44][72].
Resveratrol is known for its clinical efficacy for reducing the production of amyloid peptides, in addition to the reduction of cognitive defects and its cytoprotective actions [45][73]. Resveratrol-loaded vitamin E NE was developed for brain-delivered modality in PD. The research group formulated a kinetically stable nanoemulsion (o/w) using vitamin E and propylene glycol mono-caprylic ester (Sefsol®) in a 1:1 ratio as the oil phase and Smix (Tween 80 as the surfactant and Transcutol P as the cosurfactant). Resveratrol NEs showed significantly high ex vivo mucosal flux across the porcine nasal mucosa in Franz diffusion cell assembly. Brain-targeting studies in Wistar rats demonstrated a higher drug concentration in the brain after the intranasal administration of resveratrol NEs. Furthermore, histopathological studies affirmed diminutive degenerative changes in the brain during the intranasal administration of resveratrol NE [42][70].
Despite the promising benefits of conventional NEs, lipidic NEs are preferred, as these require considerably lower amounts of surfactants and cosurfactants for their formulation. Lipid NEs are a relatively new drug delivery system prepared from soybean oil, triglyceride, and egg yolk lecithin [46][74]. An example is a hyaluronic acid-based mucoadhesive lipidic NE co-encapsulating two polyphenols for the nasal treatment of neurodegenerative diseases. Nasr (2016) co-encapsulated two polyphenols, curcumin and resveratrol (1:1 weight ratio), in mucoadhesive NEs made of hyaluronic acid for nose-to-brain targeting (Table 1). The optimized NE was subjected to an antioxidant potential assessment, in vitro and ex vivo release of both the polyphenols, in vivo quantification of the two drugs in rat brains, and safety on nasal mucosa. The optimized hyaluronic acid-based NE with a globule size of 115.2 ± 0.15 nm and a zeta potential of −23.9 ± 1.7 mV displayed higher mucoadhesive strength compared to its non-mucoadhesive counterpart, preserved the antioxidant ability of the two polyphenols, and conferred protection from degradation. In vivo studies evidenced about seven- and nine-fold increases in the AUC(0–7 h) in the brain for resveratrol and curcumin, respectively, with respect to the drug given in the solution. Thus, hyaluronic acid-based lipidic NE was a successful carrier in enhancing the solubility, stability, and brain targetability of polyphenols.
Selegiline, an antioxidant and neuroprotective agent, is used for the oral therapeutics of PD, AD, depression, narcolepsy, and cocaine addiction. Of lately, it has been proposed as monotherapy for early stage PD [47][75] to hold back the treatment with levodopa to avoid its side effects for a substantial period. Other problems with the oral therapy of selegiline are poor drug bioavailability (<10%) due to its poor solubility in water and high presystemic metabolism. Owing to its poor access to the brain via oral administration, Kumar et al. investigated the intranasal NE of selegiline for direct nose-to-brain delivery [22][50]. The NE was formulated using Tween 80, Sefsol 218, and grape seed oil (1:1 by vol.). Grape seed oil has a synergistic antioxidant activity with selegiline, owing to omega-3 fatty acids that modulate the neuronal functions. Ex vivo permeation studies across the porcine nasal mucosa showed a higher flux than drug suspension. Selegiline being a substrate of P-glycoprotein could not diffuse across the nasal mucosa efficiently due to the presence of a P-glycoprotein efflux pump. The presence of P-glycoprotein in the nasal cavity may raise several challenges for drug absorption. It has been observed that P-glycoproteins are overexpressed in the nasal cavity, which comes from the respiratory mucosa in the cases of chronic rhinosinusitis. Hence, drug transport through nasal drug delivery may be affected during chronic rhinosinusitis [48][19]. However, in the presence of Tween 80 in the NE, a P-glycoprotein inhibitor, the efflux pump activity was reduced, thus allowing selegiline to diffuse across the porcine nasal mucosa. The efficacy of the NE formulation was affirmed by behavioral studies in a haloperidol-induced PD model in Wistar rats (Table 1).
NEs are also known to extend drug delivery from the nose to the brain via the olfactory region when mucoadhesive elements are added to the formulation. One such example is the report by Mustafa et al. (2015) wherein the brain-targeting potential of chitosan-coated oil in water NEs delivered intranasally in a haloperidol-induced PD rat model was investigated. The chitosan-coated NE containing a drug developed via the aqueous titration method followed by a high-pressure homogenization depicted a substantially high mucoadhesive potential in comparison to the conventional and homogenized formulations when monitored by gamma scintigraphy. Furthermore, the confocal study showed the deep localization of formulations in the brain confirming the BBB permeation potential of chitosan-coated NE. The pharmacokinetic data of the intranasal mucoadhesive NE in a Wistar rat brain and plasma indicated a significantly high (p < 0.005) AUC0–24 and amplified Cmax (peak of maximum concentration) over the intravenous treatment group. Thus, the investigation demonstrated the potential of the intranasal delivery of mucoadhesive nanocarriers in the efficient management of PD [49][76].
2.3. NE for Migraines
Migraines, a headache disorder, are characterized by moderate-to-severe pain attacks that result in several autonomic dysfunctions like nausea, photophobia, vomiting, exertion, gastric stasis, small bowel, etc. [50][77]. Oral drug delivery is not suitable for migraine therapeutics primarily due to nausea and vomiting, which result in the poor gastrointestinal absorption of drugs [51][78]. Parenteral and nasal drug deliveries are thus best-suited for the treatment of migraines. A novel drug delivery system of Imitrex nasal spray (sumatriptan nasal spray) is indicated for the acute treatment of migraine attacks with or without auras in adults. It is not intended for the prophylactic therapy of migraines or for use in the management of hemiplegic or basilar migraines [52][79]. The delivery shows a rapid onset of action of the drug by deposition in the olfactory region and then travels from the nose to the brain.
Rizatriptan is a 5HT 1B/1D receptor agonist with a half-life of two to three h and an oral bioavailability of 40%. It is commercially available as tablets and orally disintegrating tablets. Though orally disintegrating tablets are suitable for administration during a migraine attack, it would not improve the poor bioavailability of the drug. To increase the bioavailability and brain tissue deposition of rizatriptan, its intranasal delivery systems were investigated for antimigraine therapy. Bhanushali et al. developed both intranasal NE and gel formulations of rizatriptan benzoate for controlled drug release and the direct targeting of the drug to the brain. The most important findings of the study, including improved brain targeting, are tabulated in Table 1. Likewise, zolmitriptan-loaded NE for intranasal administration was developed using various gel formulations. Studies showed a higher permeability through the nasal mucosa for zolmitriptan from NE formulation than that from the solution (Table 1). Both the reports show evidence for brain targeting, but none demonstrated effective antimigraine therapeutics. There are several research reports some examples have been discussed in table.
