Adult neurogenesis, involving the generation of functional neurons from adult neural stem cells (NSCs), occurs constitutively in discrete brain regions such as hippocampus, sub-ventricular zone (SVZ) and hypothalamus. The intrinsic structural plasticity of the neurogenic process allows the adult brain to face the continuously changing external and internal environment and requires coordinated interplay between all cell types within the specialized microenvironment of the neurogenic niche. NSC-, neuronal- and glia-derived factors, originating locally, regulate the balance between quiescence and self-renewal of NSC, their differentiation programs and the survival and integration of newborn cells. Extracellular Vesicles (EVs) are emerging as important mediators of cell-to-cell communication, representing an efficient way to transfer the biologically active cargos (nucleic acids, proteins, lipids) by which they modulate the function of the recipient cells. At present, little is known on the physiological role of EVs in neurogenic niches.
- adult neurogenesis,extracellular vesicles,neural stem cell,neuron,astrocyte,microglia
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
1. Introduction
It is now commonly accepted that discrete regions of the adult mammalian brain host neural stem cells that divide in situ and give rise to new neurons, a phenomenon referred to as “"adult neurogenesis”".
The two most characterized neurogenic niches are the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), a brain region in which adult neurogenesis was confirmed in humans [1] and the subventricular zone (SVZ) of the lateral ventricles, whose relevance in adult human physiology is debated. Although SGZ and SVZ neural stem cells (NSCs) and neural progenitor cells (NPCs) share many features at cellular and molecular levels, the route for adult-born neuron integration in pre-existing neuronal circuits and their ultimate outcome in the two regions are distinctive. SVZ neurogenesis involves neuroblast migration along the rostral migratory system (RMS) to the olfactory bulb (OB), where they terminally differentiate into distinct types of olfactory neurons that are mainly inhibitory neurons [2]. In the last decades, pivotal preclinical studies, especially in rodents, have contributed to the idea that the integration of adult-born olfactory neurons facilitates continuous adaptation to environmental olfactory cues [3]. Conversely, mature granule neurons that originate from SGZ NSCs are excitatory neurons that are restricted to the granule cell layer (GCL), with minimal migration. At present, newborn DG neurons are considered to be crucially involved in specific types of hippocampal-dependent learning and memory, in stress and emotional responses [4,5][4][5]. Recently, adult neurogenesis has also been identified in the hypothalamus [6,7][6][7]. Here, tanycytes, which line the walls of the infundibular recess of the third ventricle, have been suggested as putative hypothalamic NSCs since they share the characteristics of SVZ and SGZ stem cells. In this region, adult-born neurons are regarded of crucial importance for the regulation of metabolism, energy balance [8] and systemic aging [9].
Regardless of the neurogenic region and the underlying complex functions, under physiological conditions, each step of adult neurogenesis needs to be tightly controlled by both niche-derived signals and by extrinsic environmental cues, which, together, ensure appropriate rates of NSC proliferation, differentiation, migration, neurite extension and integration of newborn cells into preexisting circuits [10,11][10][11]. This extensive modulation underlies the functional plasticity that is intrinsic to the neurogenic process, by which the brain outcome can be optimized for the needs of a given environment and/or experience.
It is generally accepted that a complete understanding of brain plasticity requires consideration of glial cells in the overall picture, and adult neurogenesis intriguingly connects neuronal and glial biology. Although all types of glial cells are directly or indirectly related to this process, astrocytes and microglia take on a prominent and active role. Astrocytes provide the closest link between adult neurogenesis and glial biology. In fact, several “"astroglial”" properties characterize NSCs in both neurogenic zones. The additional presence of essential non-neurogenic astrocytes within adult niches is also crucial for proper neurogenic process [12]. Astrocytes—which represent the most abundant cell type of the neurogenic niche—have been largely described as key regulators of the neurogenic process [12,13,14][12][13][14]. In the adult niche, astrocytes physically interact with NSCs [15,16,17][15][16][17] and with both developmentally and adult-born granule neurons [18]. In this context, they regulate NSC proliferation, differentiation and the functional integration of newborn neurons into the pre-existing network. Astrocyte communication with neurogenic niche cells also greatly depends on their paracrine activity. As one of the main secretory cells of the CNS [19], astrocytes release a myriad of gliotransmitters, neuromodulators and morphogens as well as metabolic, trophic and neuroprotective factors [13,14][13][14], by which they finely and positively regulate multiple steps of the neurogenic process. On the other hand, astrocytes can negatively modulate neurogenesis by both cell–cell contact and paracrine activity [17].
From being “"silent”" in healthy brain, microglia active role in adult neurogenesis has been profoundly reassessed in recent years. Evidence indicates that activated microglia plays a Janus-faced role in the context of adult neurogenesis, by favouring or counteracting NSC proliferation, differentiation and survival of adult-born neurons. These actions are mediated by both direct contact and paracrine mechanisms. For example, microglia have been shown to phagocyte newborn cells that undergo apoptotic death in SGZ and SVZ, thus ensuring the homeostasis of the neurogenic process [20,21][20][21]. In addition, microglia act as antigen-presenting cells interacting with peripherally derived immune cells. This interaction mainly occurs in the SVZ that is highly vascularized [22[22][23],23], thereby influencing NSC final commitment toward neuronal or glial phenotypes depending on the different kinds of activating T-cell stimuli (e.g., IL-4 or INF-y) [24]. On the other hand, microglia can influence adult neurogenesis through secretion of proneurogenic and/or antineurogenic molecules, whose balance determines the net outcome of adult-born neurons [25]. In particular, as the main driver of inflammatory processes in the brain, cytokines released by microglia can dramatically affect adult neurogenesis [26]. Altogether, astrocyte and microglia plasticity—which is reflected by their ability to acquire an anti- or pro-neurogenic phenotype—M1- and M2-states for microglia [27] and A1- and A2-states for astrocytes [28]—make these cells crucial actors in influencing NSC as well as responding to the complex and continuously changing neurogenic niche microenvironment.
An underestimated actor of the adult neurogenic niche is the neuronal component, which can participate in the regulation of neurogenesis dynamics. Recent evidence indeed suggests a bidirectional communication between developmentally and adult-born neurons [29,30][29][30]. Additionally, mature neurons were proposed to modulate adult neurogenesis by sending chemical signals to NSC [31].
2. Biogenesis and Function of Extracellular Vesicles
Extracellular Vesicles (EVs) are a heterogeneous population of membrane-bound entities that are released by both eukaryotic and prokaryotic cells [32] and that, by transporting different types of biomolecules, are key players in intercellular communication. Although much progress has been made in recent years in dissecting the molecular mechanisms underlying cargo packaging in recipient cells [33], further investigations are required to fully characterize the machineries and cellular pathways that determine the ultimate function (signaling or disposal) of cargo sorting in EVs.
The generation of EVs requires the fine-tuning of several intracellular molecular machineries and trafficking processes (as schematized in Figure 1). The best-characterized EVs are exosomes and microvesicles (MVs). Although the biogenesis of exosomes and EVs occurs at distinct sites within the cell, some common intracellular pathways and sorting machineries are involved in the generation of both types of EVs, thus hindering the possibility of discriminating between the different vesicle subpopulations [34].
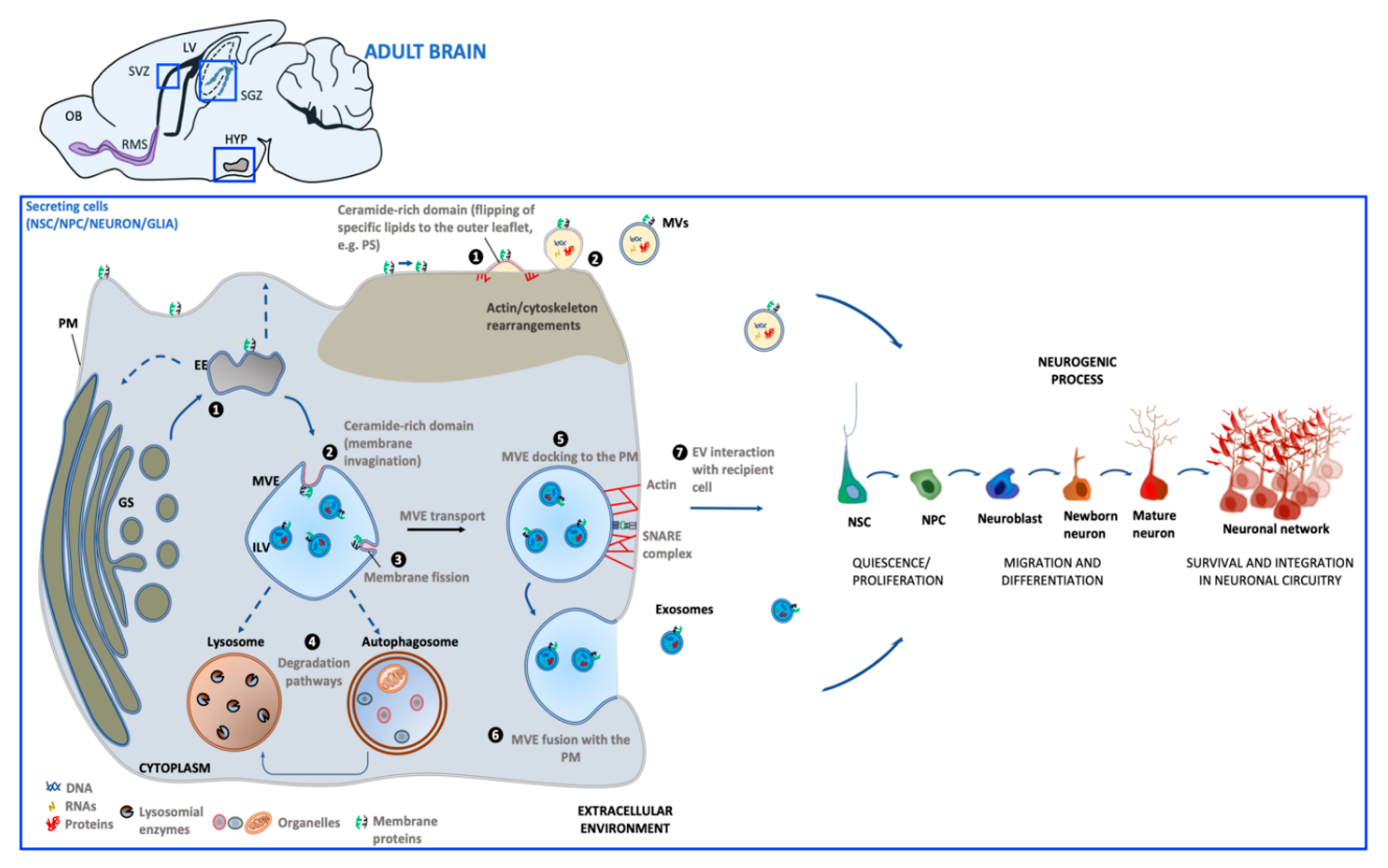
Schematic representation of extracellular vesicles' (EV) biogenesis and release in the adult neurogenic niche. Biogenesis of microvesicles (MVs) (
) involves molecular machineries and membrane microdomains that promote the outward budding of the PM (1), followed by MV release in the extracellular environment (2). Exosome biogenesis (
) occurs upon maturation of early endosomes (EE) derived from the Golgi system (GS) into multivesicular endosomes (MVEs). Alternatively, EE can undergo retrograde transport to GS (
) or recycling back to the PM (
) (1). Exosomes are generated through membrane invagination of MVE (2), followed by ILV formation (3). Once matured, MVEs can be targeted to lysosomes/autophagosomes for cargo degradation (4), or be directed towards the PM (5). MVE fusion with PM (6) allows for exosome extracellular release (7). Both MV and exosome interaction with recipient cells can influence steps of the neurogenic process. [PM: plasma membrane; PS: phosphatidylserine; ILV: intraluminal vesicle; OB: olfactory bulb; SVZ: subventricular zone; SGZ: subgranular zone; HYP: hypothalamus; LV: lateral ventricle; RMS: rostral migratory system].
Exosomes (30–100 nm) derive from the endosomal compartment. Their formation starts with the generation of multivesicular endosomes (MVEs), spherical endosomes consisting of a limiting membrane and intraluminal vesicles (ILVs). The formation of MVEs is orchestrated by a complex of proteins called the endosomal sorting complex required for transport (ESCRT) which participates in the channeling of molecules into ILVs as well as the budding and fission of ILVs within MVEs [35]. However, there is evidence that exosome formation can also occur in a ESCRT-independent process [36]. MVE docking at the plasma membrane (PM) is regulated by RABs, actin and SNARE proteins, which finally promotes MVE fusion with PM and the release of the contained ILVs in the extracellular milieu as exosomes.
Microvesicles (MVs) (50–1000 nm) originate directly from PM by an outward budding which requires redistribution in lipid and protein composition and modifications in Ca2+ levels [37]. In MV biogenesis, Ca2+-dependent enzymes such as aminophospholipid translocases (flippases and floppases), scramblases and calpain drive the externalization of phosphatidylserine, which then drives changes in local membrane curvature and restructuring of the underlying actin cytoskeleton. These events are followed by the ATP-dependent fission process that leads to vesicle budding off from the PM and its subsequent release in the extracellular space [38,39][38][39].
Once released into the extracellular environment EV docking on target cell is regulated by specific interaction between membrane receptors on the recipient cell and EV enriched proteins. The uptake mode of EVs may be dependent on cell type, its physiological state as well as on the molecular composition at the PM of the target cell [40]. EVs can bound to the cell surface and initiate intracellular signaling pathways, be internalized or directly fuse with PM [41]. If internalized, EVs can fuse with the PM and release their contents into the cytoplasm of the recipient cell. Alternatively, EVs can target the endosomal pathway of the receiving cell and be directed toward the lysosome for the degradation of EV content to provide recipient cells with essential biological metabolites.
After interaction with target cells, EVs can elicit a variety of functional responses by delivering a wide array of biologically active molecules. These include lipids, proteins and nucleic acids, mRNA and other RNA species [(transfer RNA (tRNA), long non-coding RNA (lnRNA), micro RNA (miRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA)], which can be translated into proteins or regulate transcription in recipient cells, resulting in transient or persistent cellular phenotypic changes [42,43][42][43]. In particular, increasing evidence suggests that the effect of EVs on target cells is mainly dependent on the profile of intravesicular miRNA content [44]. By transferring miRNAs to target cells, EVs are now recognized as active players in intercellular gene regulation [45] because of their key natural roles in several cellular processes, including proliferation, differentiation, survival and apoptosis [46[46]
EVs can support normal physiology by affecting stem cell maintenance [42], tissue repair [47], immune response [48], and blood coagulation [49], lipid metabolism [50], synaptic plasticity [51]. Under pathological situations, EVs can transport disease-associated proteins [52], thus contributing to propagate detrimental signals. Finally, since several molecular constituents in EVs have been found to be associated with specific diseases and treatment responses, EVs may represent reliable biomarkers which could serve as a diagnostic tool [53].
3. Extracellular Vesicles Generated in Adult Neurogenic Niches
The first publication of EVs released by neural cells was in 2004, when Février and colleagues demonstrated that glial cell lines overexpressing a prion protein released EVs that were capable of transferring infectivity in vitro and in vivo [54]. This work paved the way for the study of EVs as new tools exploited by neural cells for communicating with each other to guarantee normal brain function.
More recently, it has become increasingly evident that EVs may represent an additional key component of intercellular neurogenic niche communication. NSCs, neurons and glia have all been reported to release EVs that, in turn, can mediate a generalized cross-talk by niche components.
3.1. NSC-Derived Extracellular Vesicles
Endogenous adult NSC can generate EVs (NSC-EV).
A large array of studies have suggested that the exogenous administration of NSC-EVs in relevant animal models of acute and chronic neurodegeneration can foster neuroprotection and neuroplasticity [55,56,57,58,59][55][56][57][58][59]. Interestingly, these in vivo beneficial effects might largely depend on EV’'s intrinsic properties that contribute to re-creating an immune-permissive environment that promotes brain repair and neurogenesis. Surprisingly, as of today, a much more limited number of studies have directly focused on the molecular/functional characterization and on the endogenous role of NSC-EVs on neuroplasticity and neurogenesis.
Based on this lack of knowledge, herein we reviewed the current direct and indirect knowledge of how endogenous NSC-EVs may affect and modulate different cellular components of the adult niche. Although more experimental efforts are required in this field, some interesting studies have opened the way to an initial understanding of the endogenous NSC-EV cargo and function. The different classes of pro-, anti-neurogenic and glia modulatory molecules found in EVs derived from NSCs are summarized in Table 1.
Table 1. List of different classes of pro-, anti-neurogenic and glia modulatory molecules found in extracellular vesicles derived from neural stem/progenitor cells-(NSC-EVs) and neurons (NDEs).
Class of Molecule | Molecules | Cellular Process/Molecular Target | EV Type | ||||||||||
Growth factors | Growth factor receptor cysteine-rich domain, | EGF-like domain, EGF-like calcium-binding domain | ↑ NSC proliferation by activating the down-stream extracellular signal-regulated kinase (ERK) pathways [60] | NSC-EVs [60] | |||||||||
| VEGF | ↑ NSC proliferation in SGZ [61]; | ↑ survival and integration of newborn neurons in the forebrain | [ | |||||||||
NDEs | |||||||||||||
[ | |||||||||||||
] | |||||||||||||
EGF: epidermal growth factor; VEGF: vascular endothelial growth factor; GAP43: growth-associated protein 43; L1CAM: L1 cell adhesion molecule; Ndfip1: Nedd4 family-interacting protein 1; MAP1b: microtubule -associated protein 1b; Proline-rich protein 7 (PRR7); Asrgl1: asparaginase-like protein 1; STAT1/3: signal transducer and activator of transcription 1/3; INFγ: interferon-γ. ↑: increased; ↓: decreased.
3.2. Neuron-Derived EVs in Neurogenic Niches
Neuron-derived EVs (NDEs) are increasingly gaining attention as a novel mechanism of cell-to-cell communication, including inter-neuronal crosstalk. Indeed NDEs can selectively bind to other neurons [108][97]. Based on these assumptions, within adult niches, NDEs can potentially contribute to modulation of neurogenesis by acting on NSC and/or their neuronal progeny directly or indirectly, via glial cells. Table 1 summarizes a list of pro-, anti-neurogenic and glia modulatory molecules associated with EVs of neuronal origin.
3.3. Glia-Derived Extracellular Vesicles
Although the current knowledge on the role of glial-derived EVs in adult neurogenic niches is limited, growing evidence suggest that these biological entities may be major players in the communication of astrocytes and microglia with NSC and their progeny (for reviews, see [120,121][98][99]). In the following paragraphs, we will discuss the potential role of EVs derived from astrocytes [Astrocyte-Derived Extracellular Vesicles (ADEs)] and microglia [(Microglia-Derived Extracellular vesicles (MDEs)] in regulating the adult neurogenic process. In particular, we posit that glia-derived EVs may have a prominent role in regulating the dynamics in the neurogenic zones, based on their presence in the ADEs and MDEs of biomolecules that have been functionally characterized as modulators of adult neurogenesis (Table 2).
Table 2. List of different classes of pro- or anti-neurogenic molecules found in astrocyte-derived (ADEs) and/or microglia-derived (MDEs) extracellular vesicles.
Class of Molecule | Molecules | Cellular Process/Molecular Target | Glial EV Type | ||||||||||||||||||||||||||
Growth Factor | FGF-2 | ↑ NSC proliferation and differentiation in SGZ and SVZ [122] | [100] |
ADEs [123] | [101] |
||||||||||||||||||||||||
] | NSC-EVs [ | ||||||||||||||||||||||||||||
VEGF | ↑ NSC proliferation in SGZ [61]; | ↑ survival and integration of newborn neurons in the forebrain | [63] | ||||||||||||||||||||||||||
] |
ADEs [123] | [101] |
Proteins | Flotillin, GAP43, Cadherin 2 L1CAM | |||||||||||||||||||||||||
Enzymes | EAAT-1 | Regulate NSC proliferation and neuronal differentiation [64] | NDEs |
↑ NSC differentiation, maturation and integration of newly formed neurons in synaptic network in SGZ and SVZ through regulation of extracellular glutamate [124] and GABA [125,126] levels | [102] and GABA [103][104] levels | [64] |
|||||||||||||||||||||||
ADEs | [127] | [105] |
| Cystatin C | |||||||||||||||||||||||||
Asrgl1 | |||||||||||||||||||||||||||||
| NTPDases | ↑ NSC proliferation by cooperating with FGF-2 [65] | ↓ NSC proliferation in SGZ and SVZ by regulating nucleotide ATP and adenosine levels [128] | [106] | ↓ NSC proliferation in hippocampus [129] and in vitro neuronal differentiation of SVZ NSCs [130] through adenosine production | [ 107] and in vitro neuronal differentiation of SVZ NSCs [108] through adenosine production | NDEs [66] | ||||||||||||||||||||||
ADEs | [131] | [109] |
| Ndfip1 | ↑ Removal of protein during stress [s disease [142] | [120] | ↑ NSC proliferation, differentiation in DG via enhanced CREB phosphorylation and improve novel object recognition in mice [143] | [ | ↑ levels of aspartate/glutamate [72] which regulate adult neurogenesis [73,74] | [72] which regulate adult neurogenesis [73][74] |
NSC-EVs [72] | ||||||||||||||||||
Cytokines | INFγ | Regulate function of microglia and astrocytes by activating Stat1 in target cells [75,76] | [ | ||||||||||||||||||||||||||
| CD13 | 67] |
↑ NSC proliferation, differentiation and survival through regulation of cAMP levels [132–135] | NDEs [67] | |||||||||||||||||||||||||
MDEs | [136] | [114] |
| Synaptotagmin 4 | ↑ Retrograde signaling in pre-synaptic cells by releasing Syt4-bound exosomes [68] | NDEs [68] | |||||||||||||||||||||||
| PRR7 | 75][ | |||||||||||||||||||||||||||
| 76] |
NSC-EVs [77] | |||||||||||||||||||||||||||
miRNAs | miR-21a | ↑ NSC proliferation by targeting Sox2 and Stat3 [78] | NSC-EVs [78] | ||||||||||||||||||||||||||
MCT-1 | ↑ NSC survival of newly generated neurons [137] | [115] |
MDEs [136] | [114] |
|||||||||||||||||||||||||
Neuroprotectant proteins | Synapsins | ↑ Removal of excitatory synapses by acting as a Wnt inhibitor [69] | ↑ NSC proliferation and survival in adult DG [138] | [116] | ↑ synapse development [139], neurotransmitter release [140], neurite outgrowth after oxygen-glucose deprivation (OGD)/oxidative stress [141] | [ 117], neurotransmitter release [118], neurite outgrowth after oxygen-glucose deprivation (OGD)/oxidative stress [119] | NDEs [69] | ||||||||||||||||||||||
ADEs | [141] | [119] |
| MAP1b | |||||||||||||||||||||||||
| ↑ synaptic transmission and plasticity [70] | NDEs [ |
HSP7071] | ||||||||||||||||||||||||||
↑ expression of genes involved in neuronal differentiation, synaptic activity, regulation of neuronal synaptic plasticity in Alzheimer’' | ] |
ADEs [144] | [122] |
Enzymes | |||||||||||||||||||||||||
| Neuroglobin | ↑ NSC proliferation and differentiation in SVZ via Wnt signaling in murine stroke model [145] | [123] |
ADEs [146] | [124] |
||||||||||||||||||||||||
Cytokines | IL-1β | ↓ neurogenesis in DG by reducing the number of DCX+ cells [147] | [125] | ↓ neurogenesis in DG by reducing the number of Nestin + cells [148] | [ 126] | ↓ hippocampal NSC proliferation in vitro via the nuclear factor-κB signaling pathway [149] | [ ] | ↑ NSC proliferation and differentiation through the activation of SAPK/JNK pathway [150] | [ ] |
MDEs [151], | [129], | ADEs [152] | [ 130] |
||||||||||||||||
| IL-6 | ↓ DG NSC proliferation in vitro [153] | [131] | ↓ NSC proliferation, differentiation and survival in DG [154] | [ 132] | ↑ NSC self-renewal and maintenance in SVZ [155] | [ ] | ↑ NSC proliferation and neuronal maturation in SVZ and SGZ [156] | [ ] |
ADEs [157], | [135], | MDEs [158] | [ 136] |
| miR-9 | ↓ NSC proliferation and ↑ neural differentiation by targeting the stem cell regulator TLX [79] | |||||||||||||
| NSC-EVs [78] | ||||||||||||||||||||||||||||
TNFα | ↑ NSC proliferation and survival through TNFR2 in vitro and in vivo [159] | [137] | ↓ NSC proliferation and ↑ cell death through TNFR1 in vitro and in vivo [159,160] |
[ |
MDEs [158] | [136] |
| miR-let-7b | |||||||||||||||||||||
miRNAs | miR-302 | ↓ NSC proliferation and ↑ neural differentiation by targeting the stem cell regulator TLX and the cell cycle regulator cyclin D1 [80] | ↑ NSC proliferation, differentiation, survival through Cyclin D1/D2 and Fgf15 [161] | [139] | NSC-EVs [78] | ||||||||||||||||||||||||
ADEs | [162] | [140] |
| miR-124 | miR-137 | ||||||||||||||||||||||||
| miR-let-7d, miR-let-7a | Regulate NSC activation/proliferation, fate specification and differentiation by cooperatively targeting the pro-apoptotic protein BCL2L13 [81] | ↓ NSC proliferation and ↑ neural differentiation by targeting TLX receptor gene [163] | [141] | ↑ NSC dopaminergic differentiation in olfactory bulb by PAX6 targeting (miR-let-7a,[164]) | [ 142]) | NSC-EVs [82] | ||||||||||||||||||||||
ADEs | [163] | [141] |
| miR-let-7 | |||||||||||||||||||||||||
| miR-145 | Regulate microglia activation which negatively affect NSC proliferation in SVZ [83] | NSC-EVs [ |
↑ NSC differentiation through Sox2-Lin28/let-7 signaling pathway [165] | [143] | 83] |
|||||||||||||||||||||||
ADEs | [163] | [141] |
| ||||||||||||||||||||||||||
| miR-9, miR-let-7, miR-26a, and miR-181c | miR-146a-5p | Regulate microglia morphology and physiology [84–87] | ↓ NSC neural specification and synaptogenesis by targeting neuroligin 1 (Nlg1[86][87] | ) and synaptotagmin 1 (Syt1) [166] | [144] | NSC-EVs [83] | ||||||||||||||||||||||
MDEs | [167] | [145] |
| miR-34a | |||||||||||||||||||||||||
| miR-9 | Regulate NSC proliferation and morphology and function of newborn neurons by interacting with DCX [88] | ↓ NSC proliferation, ↑ NSC neural differentiation by targeting TLX receptor | Target genes linked to the regulation of neuronal excitability, mitochondria oxidative phosphorylation, glycolysis, and resting state functional connectivity | [ 89] |
[79] | NDEs [89] | ||||||||||||||||||||||
ADEs | [168] | [146] |
| miR-124 | |||||||||||||||||||||||||
| miR-9, miR-124 | ↑ NSC neuronal differentiation in SVZ [90] | ↑ NSC neuronal differentiation in SVZ by targeting SOX9 | [ 91] |
↑NSC neural differentiation and dendritic branching of differentiated neurons by targeting the small GTP-binding protein Rap2a [169] | [147] | NDEs [92] | ||||||||||||||||||||||
ADEs | [168], | [146], | MDEs [170] | [ 148] |
| miR-124-3p | ↑ GLT-1 expression in astrocytes [ | ||||||||||||||||||||||
| miR-184 | 93] which ↑ NSC differentiation in vitro [94] and regulate synaptic transmission |
↑ NSC proliferation, ↓ differentiation in SGZ by targeting Numblike [171] | NDEs [93] | |||||||||||||||||||||||||
ADEs | [162] | [140] |
| ||||||||||||||||||||||||||
| miR-21-5p | miR-34a | ↑ M1 polarization in microglia [96] | ↑ NSC proliferation, ↓ dendrite branching and neuronal maturation by targeting DCX [88] | ADEs [172], | [150], | MDEs [167] | [ 145] |
|||||||||||||||||||||
| miR-106b, miR-93, miR-25 | ↑ NSC proliferation and differentiation toward neuronal lineage in vitro through insulin/IGF-FoxO pathway [173] | [151] |
ADEs [162] | [140] |
FGF-2: fibroblast growth factor 2; VEGF: vascular endothelial growth factor; EAAT-1: excitatory amino acid transporter 1; NTPDases: nucleoside triphosphate diphosphohydrolases; CD13: aminopeptidase N; MCT-1: Monocarboxylate transporter 1; CREB: cAMP response element-binding protein; HSP70: heat shock protein 70; SAPK/JNK: stress-activated protein kinases (SAPK)/Jun amino-terminal kinases (JNK); TNFR1/2: tumor necrosis factor receptor 1/2; IL-1β: interleukin-1β; IL-6: interleukin-6: TNFα: tumor necrosis factor α. ↑: increased; ↓: decreased.
References
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317, doi:10.1038/3305.
- Lepousez, G.; Valley, M.T.; Lledo, P.-M. The impact of adult neurogenesis on olfactory bulb circuits and computations. Annu. Rev. Physiol. 2013, 75, 339–363, doi:10.1146/annurev-physiol-030212-183731.
- Li, W.L.; Chu, M.W.; Wu, A.; Suzuki, Y.; Imayoshi, I.; Komiyama, T. Adult-born neurons facilitate olfactory bulb pattern separation during task engagement. Elife 2018, 7, e33006, doi:10.7554/eLife.33006.
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346, doi:10.1038/nrn.2017.45.
- Bortolotto, V.; Cuccurazzu, B.; Canonico, P.L.; Grilli, M. NF-κB mediated regulation of adult hippocampal neurogenesis: Relevance to mood disorders and antidepressant activity. Biomed Res. Int. 2014, 2014, 612798, doi:10.1155/2014/612798.
- Evans, J.; Sumners, C.; Moore, J.; Huentelman, M.J.; Deng, J.; Gelband, C.H.; Shaw, G. Characterization of mitotic neurons derived from adult rat hypothalamus and brain stem. J. Neurophysiol. 2002, 87, 1076–1085, doi:10.1152/jn.00088.2001.
- Cheng, M.-F. Hypothalamic neurogenesis in the adult brain. Front. Neuroendocrinol. 2013, 34, 167–178, doi:10.1016/j.yfrne.2013.05.001.
- Sousa-Ferreira, L.; de Almeida, L.P.; Cavadas, C. Role of hypothalamic neurogenesis in feeding regulation. Trends Endocrinol. Metab. 2014, 25, 80–88, doi:10.1016/j.tem.2013.10.005.
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79, doi:10.1016/j.mad.2018.04.008.
- Lledo, P.-M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193, doi:10.1038/nrn1867.
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87, doi:10.1038/s41380-018-0036-2.
- Cassé, F.; Richetin, K.; Toni, N. Astrocytes’ Contribution to Adult Neurogenesis in Physiology and Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 432, doi:10.3389/fncel.2018.00432.
- Cvijetic, S.; Bortolotto, V.; Manfredi, M.; Ranzato, E.; Marengo, E.; Salem, R.; Canonico, P.L.; Grilli, M. Cell autonomous and noncell-autonomous role of NF-κB p50 in astrocyte-mediated fate specification of adult neural progenitor cells. Glia 2017, 65, 169–181, doi:10.1002/glia.23085.
- Spampinato, S.F.; Bortolotto, V.; Canonico, P.L.; Sortino, M.A.; Grilli, M. Astrocyte-Derived Paracrine Signals: Relevance for Neurogenic Niche Regulation and Blood-Brain Barrier Integrity. Front. Pharmacol. 2019, 10, 1346, doi:10.3389/fphar.2019.01346.
- Lim, D.A.; Alvarez-Buylla, A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 7526–7531, doi:10.1073/pnas.96.13.7526.
- Ashton, R.S.; Conway, A.; Pangarkar, C.; Bergen, J.; Lim, K.-I.; Shah, P.; Bissell, M.; Schaffer, D.V. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 2012, 15, 1399–1406, doi:10.1038/nn.3212.
- Wilhelmsson, U.; Faiz, M.; de Pablo, Y.; Sjöqvist, M.; Andersson, D.; Widestrand, A.; Potokar, M.; Stenovec, M.; Smith, P.L.P.; Shinjyo, N.; et al. Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells 2012, 30, 2320–2329, doi:10.1002/stem.1196.
- Krzisch, M.; Temprana, S.G.; Mongiat, L.A.; Armida, J.; Schmutz, V.; Virtanen, M.A.; Kocher-Braissant, J.; Kraftsik, R.; Vutskits, L.; Conzelmann, K.-K.; et al. Pre-existing astrocytes form functional perisynaptic processes on neurons generated in the adult hippocampus. Brain Struct. Funct. 2015, 220, 2027–2042, doi:10.1007/s00429-014-0768-y.
- Verkhratsky, A.; Matteoli, M.; Parpura, V.; Mothet, J.-P.; Zorec, R. Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO J. 2016, 35, 239–257, doi:10.15252/embj.201592705.
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J. Neurosci. 2020, 40, 1453–1482, doi:10.1523/JNEUROSCI.0993-19.2019.
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495, doi:10.1016/j.stem.2010.08.014.
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.-M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell 2008, 3, 289–300, doi:10.1016/j.stem.2008.07.026.
- Tavazoie, M.; Van der Veken, L.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3, 279–288, doi:10.1016/j.stem.2008.07.025.
- Butovsky, O.; Ziv, Y.; Schwartz, A.; Landa, G.; Talpalar, A.E.; Pluchino, S.; Martino, G.; Schwartz, M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006, 31, 149–160, doi:10.1016/j.mcn.2005.10.006.
- Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029, doi:10.1016/j.neuroscience.2008.06.052.
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science 2003, 302, 1760–1765, doi:10.1126/science.1088417.
- Nakagawa, Y.; Chiba, K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol. Ther. 2015, 154, 21–35, doi:10.1016/j.pharmthera.2015.06.010.
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487, doi:10.1038/nature21029.
- Asrican, B.; Wooten, J.; Li, Y.-D.; Quintanilla, L.; Zhang, F.; Wander, C.; Bao, H.; Yeh, C.-Y.; Luo, Y.-J.; Olsen, R.; et al. Neuropeptides Modulate Local Astrocytes to Regulate Adult Hippocampal Neural Stem Cells. Neuron 2020, 108, 349–366.e6, doi:10.1016/j.neuron.2020.07.039.
- Song, J.; Zhong, C.; Bonaguidi, M.A.; Sun, G.J.; Hsu, D.; Gu, Y.; Meletis, K.; Huang, Z.J.; Ge, S.; Enikolopov, G.; et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012, 489, 150–154, doi:10.1038/nature11306.
- Pardal, R.; López Barneo, J. Mature neurons modulate neurogenesis through chemical signals acting on neural stem cells. Dev. Growth Differ. 2016, 58, 456–462, doi:10.1111/dgd.12283.
- Deatherage, B.L.; Cookson, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957, doi:10.1128/IAI.06014-11.
- Iavello, A.; Frech, V.S.L.; Gai, C.; Deregibus, M.C.; Quesenberry, P.J.; Camussi, G. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int. J. Mol. Med. 2016, 37, 958–966, doi:10.3892/ijmm.2016.2488.
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289, doi:10.1146/annurev-cellbio-101512-122326.
- Adell, M.A.Y.; Vogel, G.F.; Pakdel, M.; Müller, M.; Lindner, H.; Hess, M.W.; Teis, D. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J. Cell Biol. 2014, 205, 33–49, doi:10.1083/jcb.201310114.
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular Endosome Biogenesis in the Absence of ESCRTs. Traffic 2009, 10, 925–937, doi:10.1111/j.1600-0854.2009.00920.x.
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51, doi:10.1016/j.semcdb.2015.02.010.
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 2010, 123, 1603–1611, doi:10.1242/jcs.064386.
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232, doi:10.1080/21541248.2016.1215283.
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108, doi:10.1186/1471-2407-11-108.
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228, doi:10.1038/nrm.2017.125.
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856, doi:10.1038/sj.leu.2404132.
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Beer Stolz, D.; Sullivan, M.L.G.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119, 756–766, doi:10.1182/blood-2011-02-338004.
- Pfeifer, P.; Werner, N.; Jansen, F. Role and Function of MicroRNAs in Extracellular Vesicles in Cardiovascular Biology. Biomed Res. Int. 2015, 2015, 161393, doi:10.1155/2015/161393.
- Xavier, L.; Anne-Clémence, V.; Tedgui, A.; Boulanger, C.M. Microvesicles as Cell–Cell Messengers in Cardiovascular Diseases. Circ. Res. 2014, 114, 345–353, doi:10.1161/CIRCRESAHA.113.300858.
- Nouraee, N.; Mowla, S.J. miRNA therapeutics in cardiovascular diseases: Promises and problems. Front. Genet. 2015, 6, 232, doi:10.3389/fgene.2015.00232.
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483, doi:10.1093/ndt/gfr015.
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Leijendekker, R.; Harding, C.V.; Melief, C.J.M.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172, doi:10.1084/jem.183.3.1161.
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; López, J.A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611, doi:10.1182/blood-2004-03-1095.
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 108–120, doi:10.1016/j.bbalip.2013.10.004.
- Chivet, M.; Hemming, F.; Pernet-Gallay, K.; Fraboulet, S.; Sadoul, R. Emerging role of neuronal exosomes in the central nervous system. Front. Physiol. 2012, 3, 145, doi:10.3389/fphys.2012.00145.
- Coleman, B.M.; Hill, A.F. Extracellular vesicles—Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin. Cell Dev. Biol. 2015, 40, 89–96, doi:10.1016/j.semcdb.2015.02.007.
- Gámez-Valero, A.; Beyer, K.; Borràs, F.E. Extracellular vesicles, new actors in the search for biomarkers of dementias. Neurobiol. Aging 2019, 74, 15–20, doi:10.1016/j.neurobiolaging.2018.10.006.
- Fevrier, B.; Vilette, D.; Archer, F.; Loew, D.; Faigle, W.; Vidal, M.; Laude, H.; Raposo, G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA 2004, 101, 9683–9688, doi:10.1073/pnas.0308413101.
- Li, B.; Liu, J.; Gu, G.; Han, X.; Zhang, Q.; Zhang, W. Impact of neural stem cell-derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. J. Neurochem. 2020, 154, e15001, doi:10.1111/jnc.15001.
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S.; et al. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl. Stroke Res. 2018, 9, 530–539, doi:10.1007/s12975-017-0599-2.
- Mahdavipour, M.; Hassanzadeh, G.; Seifali, E.; Mortezaee, K.; Aligholi, H.; Shekari, F.; Sarkoohi, P.; Zeraatpisheh, Z.; Nazari, A.; Movassaghi, S.; et al. Effects of neural stem cell-derived extracellular vesicles on neuronal protection and functional recovery in the rat model of middle cerebral artery occlusion. Cell Biochem. Funct. 2020, 38, 373–383, doi:10.1002/cbf.3484.
- Sun, X.; Jung, J.-H.; Arvola, O.; Santoso, M.R.; Giffard, R.G.; Yang, P.C.; Stary, C.M. Stem Cell-Derived Exosomes Protect Astrocyte Cultures From in vitro Ischemia and Decrease Injury as Post-stroke Intravenous Therapy. Front. Cell. Neurosci. 2019, 13, 394, doi:10.3389/fncel.2019.00394.
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 340, doi:10.1038/s41419-019-1571-8.
- Ma, Y.; Wang, K.; Pan, J.; Fan, Z.; Tian, C.; Deng, X.; Ma, K.; Xia, X.; Huang, Y.; Zheng, J.C. Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal-regulated kinase pathways. Neurobiol. Dis. 2019, 124, 322–334, doi:10.1016/j.nbd.2018.12.003.
- Palmer, T.D.; Willhoite, A.R.; Gage, F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000, 425, 479–494, doi:10.1002/1096-9861(20001002)425:4<479::AID-CNE2>3.0.CO;2-3.
- Louissaint, A.J.; Rao, S.; Leventhal, C.; Goldman, S.A. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 2002, 34, 945–960, doi:10.1016/s0896-6273(02)00722-5.
- Zhong, D.; Cao, Y.; Li, C.-J.; Li, M.; Rong, Z.-J.; Jiang, L.; Guo, Z.; Lu, H.-B.; Hu, J.-Z. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp. Biol. Med. 2020, 245, 54–65, doi:10.1177/1535370219895491.
- Sharma, P.; Mesci, P.; Carromeu, C.; McClatchy, D.R.; Schiapparelli, L.; Yates, J.R.; Muotri, A.R.; Cline, H.T. Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. USA 2019, 116, 16086–16094, doi:10.1073/pnas.1902513116.
- Taupin, P.; Ray, J.; Fischer, W.H.; Suhr, S.T.; Hakansson, K.; Grubb, A.; Gage, F.H. FGF-2-Responsive Neural Stem Cell Proliferation Requires CCg, a Novel Autocrine/Paracrine Cofactor. Neuron 2000, 28, 385–397, doi:10.1016/S0896-6273(00)00119-7.
- Ghidoni, R.; Paterlini, A.; Albertini, V.; Glionna, M.; Monti, E.; Schiaffonati, L.; Benussi, L.; Levy, E.; Binetti, G. Cystatin C is released in association with exosomes: A new tool of neuronal communication which is unbalanced in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1435–1442, doi:10.1016/j.neurobiolaging.2009.08.013.
- Putz, U.; Howitt, J.; Lackovic, J.; Foot, N.; Kumar, S.; Silke, J.; Tan, S.-S. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J. Biol. Chem. 2008, 283, 32621–32627, doi:10.1074/jbc.M804120200.
- Korkut, C.; Li, Y.; Koles, K.; Brewer, C.; Ashley, J.; Yoshihara, M.; Budnik, V. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron 2013, 77, 1039–1046, doi:10.1016/j.neuron.2013.01.013.
- Lee, S.H.; Shin, S.M.; Zhong, P.; Kim, H.-T.; Kim, D.-I.; Kim, J.M.; Do Heo, W.; Kim, D.-W.; Yeo, C.-Y.; Kim, C.-H.; et al. Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat. Commun. 2018, 9, 3434, doi:10.1038/s41467-018-05858-2.
- Palenzuela, R.; Gutiérrez, Y.; Draffin, J.E.; Lario, A.; Benoist, M.; Esteban, J.A. MAP1B Light Chain Modulates Synaptic Transmission via AMPA Receptor Intracellular Trapping. J. Neurosci. 2017, 37, 9945–9963, doi:10.1523/JNEUROSCI.0505-17.2017.
- Goldie, B.J.; Dun, M.D.; Lin, M.; Smith, N.D.; Verrills, N.M.; Dayas, C.V.; Cairns, M.J. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014, 42, 9195–9208, doi:10.1093/nar/gku594.
- Iraci, N.; Gaude, E.; Leonardi, T.; Costa, A.S.H.; Cossetti, C.; Peruzzotti-Jametti, L.; Bernstock, J.D.; Saini, H.K.; Gelati, M.; Vescovi, A.L.; et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat. Chem. Biol. 2017, 13, 951–955, doi:10.1038/nchembio.2422.
- Kim, P.M.; Duan, X.; Huang, A.S.; Liu, C.Y.; Ming, G.; Song, H.; Snyder, S.H. Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3175–3179, doi:10.1073/pnas.0914706107.
- Vicini, S. The role of GABA and glutamate on adult neurogenesis. J. Physiol. 2008, 586, 3737–3738.
- Hidano, S.; Randall, L.M.; Dawson, L.; Dietrich, H.K.; Konradt, C.; Klover, P.J.; John, B.; Harris, T.H.; Fang, Q.; Turek, B.; et al. STAT1 Signaling in Astrocytes Is Essential for Control of Infection in the Central Nervous System. MBio 2016, 7, e01881-16, doi:10.1128/mBio.01881-16.
- Tsuda, M.; Masuda, T.; Kitano, J.; Shimoyama, H.; Tozaki-Saitoh, H.; Inoue, K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc. Natl. Acad. Sci. USA 2009, 106, 8032–8037, doi:10.1073/pnas.0810420106.
- Cossetti, C.; Iraci, N.; Mercer, T.R.; Leonardi, T.; Alpi, E.; Drago, D.; Alfaro-Cervello, C.; Saini, H.K.; Davis, M.P.; Schaeffer, J.; et al. Extracellular Vesicles from Neural Stem Cells Transfer IFN-γ via Ifngr1 to Activate Stat1 Signaling in Target Cells. Mol. Cell 2014, 56, 193–204, doi:10.1016/j.molcel.2014.08.020.
- Ma, Y.; Li, C.; Huang, Y.; Wang, Y.; Xia, X.; Zheng, J.C. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal. 2019, 17, 96, doi:10.1186/s12964-019-0418-3.
- Zhao, C.; Sun, G.; Li, S.; Shi, Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat. Struct. Mol. Biol. 2009, 16, 365–371, doi:10.1038/nsmb.1576.
- Zhao, C.; Sun, G.; Li, S.; Lang, M.-F.; Yang, S.; Li, W.; Shi, Y. MicroRNA regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 1876–1881. doi:10.1073/pnas.0908750107.
- Bielefeld, P.; Mooney, C.; Henshall, D.C.; Fitzsimons, C.P. miRNA-Mediated Regulation of Adult Hippocampal Neurogenesis; Implications for Epilepsy. Brain Plast. 2017, 3, 43–59, doi:10.3233/BPL-160036.
- Seguro, B.; Diogo, M.; Fitzsimons, C. Neural Stem Cell-Derived Extracellular Vesicles as Paracrine Regulators in the Hippocampus. Extended Abstract. 2018. Available online: https://fenix.tecnico.ulisboa.pt/downloadFile/1689244997258826/Extended_Abstract.pdf (accessed on 25 November 2018).
- Morton, M.C.; Neckles, V.N.; Seluzicki, C.M.; Holmberg, J.C.; Feliciano, D.M. Neonatal Subventricular Zone Neural Stem Cells Release Extracellular Vesicles that Act as a Microglial Morphogen. Cell Rep. 2018, 23, 78–89, doi:10.1016/j.celrep.2018.03.037.
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835, doi:10.1038/nn.3113.
- Yao, H.; Ma, R.; Yang, L.; Hu, G.; Chen, X.; Duan, M.; Kook, Y.; Niu, F.; Liao, K.; Fu, M.; et al. MiR-9 promotes microglial activation by targeting MCPIP1. Nat. Commun. 2014, 5, 4386, doi:10.1038/ncomms5386.
- Kumar, A.; Bhatia, H.S.; de Oliveira, A.C.P.; Fiebich, B.L. microRNA-26a modulates inflammatory response induced by toll-like receptor 4 stimulation in microglia. J. Neurochem. 2015, 135, 1189–1202, doi:10.1111/jnc.13364.
- Zhang, L.; Li, Y.-J.; Wu, X.-Y.; Hong, Z.; Wei, W.-S. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J. Neurochem. 2015, 132, 713–723, doi:10.1111/jnc.13021.
- Mollinari, C.; Racaniello, M.; Berry, A.; Pieri, M.; de Stefano, M.C.; Cardinale, A.; Zona, C.; Cirulli, F.; Garaci, E.; Merlo, D. miR-34a regulates cell proliferation, morphology and function of newborn neurons resulting in improved behavioural outcomes. Cell Death Dis. 2015, 6, e1622, doi:10.1038/cddis.2014.589.
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151, doi:10.1016/j.brainres.2016.05.026.
- Åkerblom, M.; Sachdeva, R.; Barde, I.; Verp, S.; Gentner, B.; Trono, D.; Jakobsson, J. MicroRNA-124 Is a Subventricular Zone Neuronal Fate Determinant. J. Neurosci. 2012, 32, 8879–8889, doi:10.1523/JNEUROSCI.0558-12.2012.
- Cheng, L.-C.; Pastrana, E.; Tavazoie, M.; Doetsch, F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009, 12, 399–408, doi:10.1038/nn.2294.
- Veremeyko, T.; Kuznetsova, I.S.; Dukhinova, M.; Yung, A.W.Y.; Kopeikina, E.; Barteneva, N.S.; Ponomarev, E.D. Neuronal extracellular microRNAs miR-124 and miR-9 mediate cell–cell communication between neurons and microglia. J. Neurosci. Res. 2019, 97, 162–184, doi:10.1002/jnr.24344.
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.S.R.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 2019, 10, 4136, doi:10.1038/s41467-019-11534-w.
- Guo, Y.; Wei, Q.; Huang, Y.; Xia, W.; Zhou, Y.; Wang, S. The effects of astrocytes on differentiation of neural stem cells are influenced by knock-down of the glutamate transporter, GLT-1. Neurochem. Int. 2013, 63, 498–506, doi:10.1016/j.neuint.2013.08.003.
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105, doi:10.1016/s0301-0082(00)00067-8.
- Yin, Z.; Han, Z.; Hu, T.; Zhang, S.; Ge, X.; Huang, S.; Wang, L.; Yu, J.; Li, W.; Wang, Y.; et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain. Behav. Immun. 2020, 83, 270–282, doi:10.1016/j.bbi.2019.11.004.
- Chivet, M.; Javalet, C.; Laulagnier, K.; Blot, B.; Hemming, F.J.; Sadoul, R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 2014, 3, 24722, doi:10.3402/jev.v3.24722.
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Control. Release 2020, 323, 225–239, doi:10.1016/j.jconrel.2020.04.017.
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157, doi:10.1016/j.neuroscience.2018.04.003.
- Ohkubo, Y.; Uchida, A.O.; Shin, D.; Partanen, J.; Vaccarino, F.M. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J. Neurosci. 2004, 24, 6057–6069, doi:10.1523/JNEUROSCI.1140-04.2004.
- Proia, P.; Schiera, G.; Mineo, M.; Ingrassia Maria Rita, A.; Santoro, G.; Savettieri, G.; Di Liegro, I. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 2008, 21, 63–67, doi:10.3892/ijmm.21.1.63.
- Espósito, M.S.; Piatti, V.C.; Laplagne, D.A.; Morgenstern, N.A.; Ferrari, C.C.; Pitossi, F.J.; Schinder, A.F. Neuronal Differentiation in the Adult Hippocampus Recapitulates Embryonic Development. J. Neurosci. 2005, 25, 10074–10086, doi:10.1523/JNEUROSCI.3114-05.2005.
- Ge, S.; Goh, E.L.K.; Sailor, K.A.; Kitabatake, Y.; Ming, G.; Song, H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 2006, 439, 589–593, doi:10.1038/nature04404.
- Bordey, A. Enigmatic GABAergic networks in adult neurogenic zones. Brain Res. Rev. 2007, 53, 124–134, doi:10.1016/j.brainresrev.2006.07.004.
- Gosselin, R.-D.; Meylan, P.; Decosterd, I. Extracellular microvesicles from astrocytes contain functional glutamate transporters: Regulation by protein kinase C and cell activation. Front. Cell. Neurosci. 2013, 7, 251, doi:10.3389/fncel.2013.00251.
- Gampe, K.; Stefani, J.; Hammer, K.; Brendel, P.; Pötzsch, A.; Enikolopov, G.; Enjyoji, K.; Acker-Palmer, A.; Robson, S.C.; Zimmermann, H. NTPDase2 and purinergic signaling control progenitor cell proliferation in neurogenic niches of the adult mouse brain. Stem Cells 2015, 33, 253–264, doi:10.1002/stem.1846.
- Chauhan, G.; Ray, K.; Sahu, S.; Roy, K.; Jain, V.; Wadhwa, M.; Panjwani, U.; Kishore, K.; Singh, S.B. Adenosine A1 receptor antagonist mitigates deleterious effects of sleep deprivation on adult neurogenesis and spatial reference memory in rats. Neuroscience 2016, 337, 107–116, doi:10.1016/j.neuroscience.2016.09.007.
- Benito-Muñoz, M.; Matute, C.; Cavaliere, F. Adenosine A1 receptor inhibits postnatal neurogenesis and sustains astrogliogenesis from the subventricular zone. Glia 2016, 64, 1465–1478, doi:10.1002/glia.23010.
- Ceruti, S.; Colombo, L.; Magni, G.; Viganò, F.; Boccazzi, M.; Deli, M.A.; Sperlágh, B.; Abbracchio, M.P.; Kittel, Á. Oxygen–glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood–brain barrier. Neurochem. Int. 2011, 59, 259–271, doi:10.1016/j.neuint.2011.05.013.
- Eisch, A.J.; Barrot, M.; Schad, C.A.; Self, D.W.; Nestler, E.J. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Natl. Acad. Sci. USA 2000, 97, 7579–7584, doi:10.1073/pnas.120552597.
- Nakagawa, S.; Kim, J.-E.; Lee, R.; Malberg, J.E.; Chen, J.; Steffen, C.; Zhang, Y.-J.; Nestler, E.J.; Duman, R.S. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J. Neurosci. 2002, 22, 3673–3682, doi:10.1523/JNEUROSCI.22-09-03673.2002.
- Arguello, A.A.; Harburg, G.C.; Schonborn, J.R.; Mandyam, C.D.; Yamaguchi, M.; Eisch, A.J. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience 2008, 157, 70–79, doi:10.1016/j.neuroscience.2008.08.064.
- Xu, C.; Zheng, H.; Loh, H.H.; Law, P.-Y. Morphine Promotes Astrocyte-Preferential Differentiation of Mouse Hippocampal Progenitor Cells via PKCε-Dependent ERK Activation and TRBP Phosphorylation. Stem Cells 2015, 33, 2762–2772, doi:10.1002/stem.2055.
- Potolicchio, I.; Carven, G.J.; Xu, X.; Stipp, C.; Riese, R.J.; Stern, L.J.; Santambrogio, L. Proteomic Analysis of Microglia-Derived Exosomes: Metabolic Role of the Aminopeptidase CD13 in Neuropeptide Catabolism. J. Immunol. 2005, 175, 2237–2243, doi:10.4049/jimmunol.175.4.2237.
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403, doi:10.3389/fnins.2019.00403.
- Barbieri, R.; Contestabile, A.; Ciardo, M.G.; Forte, N.; Marte, A.; Baldelli, P.; Benfenati, F.; Onofri, F. Synapsin I and Synapsin II regulate neurogenesis in the dentate gyrus of adult mice. Oncotarget 2018, 9, 18760–18774, doi:10.18632/oncotarget.24655.
- Jovanovic, J.N.; Benfenati, F.; Siow, Y.L.; Sihra, T.S.; Sanghera, J.S.; Pelech, S.L.; Greengard, P.; Czernik, A.J. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 3679–3683, doi:10.1073/pnas.93.8.3679.
- Hilfiker, S.; Pieribone, V.A.; Czernik, A.J.; Kao, H.T.; Augustine, G.J.; Greengard, P. Synapsins as regulators of neurotransmitter release. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 269–279, doi:10.1098/rstb.1999.0378.
- Wang, S.; Cesca, F.; Loers, G.; Schweizer, M.; Buck, F.; Benfenati, F.; Schachner, M.; Kleene, R. Synapsin I Is an Oligomannose-Carrying Glycoprotein, Acts As an Oligomannose-Binding Lectin, and Promotes Neurite Outgrowth and Neuronal Survival When Released via Glia-Derived Exosomes. J. Neurosci. 2011, 31, 7275–7290, doi:10.1523/JNEUROSCI.6476-10.2011.
- Evgen’ev, M.B.; Krasnov, G.S.; Nesterova, I.V.; Garbuz, D.G.; Karpov, V.L.; Morozov, A.V.; Snezhkina, A.V.; Samokhin, A.N.; Sergeev, A.; Kulikov, A.M.; et al. Molecular Mechanisms Underlying Neuroprotective Effect of Intranasal Administration of Human Hsp70 in Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 59, 1415–1426, doi:10.3233/JAD-170398.
- Kwon, H.J.; Kim, W.; Jung, H.Y.; Kang, M.S.; Kim, J.W.; Hahn, K.R.; Yoo, D.Y.; Yoon, Y.S.; Hwang, I.K.; Kim, D.W. Heat shock protein 70 increases cell proliferation, neuroblast differentiation, and the phosphorylation of CREB in the hippocampus. Lab. Anim. Res. 2019, 35, 21, doi:10.1186/s42826-019-0020-2.
- Taylor, A.R.; Robinson, M.B.; Gifondorwa, D.J.; Tytell, M.; Milligan, C.E. Regulation of heat shock protein 70 release in astrocytes: Role of signaling kinases. Dev. Neurobiol. 2007, 67, 1815–1829, doi:10.1002/dneu.20559.
- Yu, Z.; Cheng, C.; Liu, Y.; Liu, N.; Lo, E.H.; Wang, X. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. 2018, 9, 945, doi:10.1038/s41419-018-1007-x.
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes From Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452, doi:10.3389/fphar.2019.01452.
- Wu, M.D.; Hein, A.M.; Moravan, M.J.; Shaftel, S.S.; Olschowka, J.A.; O’Banion, M.K. Adult murine hippocampal neurogenesis is inhibited by sustained IL-1β and not rescued by voluntary running. Brain Behav. Immun. 2012, 26, 292–300, doi:10.1016/j.bbi.2011.09.012.
- Wu, M.D.; Montgomery, S.L.; Rivera-Escalera, F.; Olschowka, J.A.; O’Banion, M.K. Sustained IL-1β expression impairs adult hippocampal neurogenesis independent of IL-1 signaling in nestin+ neural precursor cells. Brain Behav. Immun. 2013, 32, 9–18, doi:10.1016/j.bbi.2013.03.003.
- Koo, J.W.; Duman, R.S. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA 2008, 105, 751–756, doi:10.1073/pnas.0708092105.
- Wang, X.; Fu, S.; Wang, Y.; Yu, P.; Hu, J.; Gu, W.; Xu, X.-M.; Lu, P. Interleukin-1β mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol. Cell. Neurosci. 2007, 36, 343–354, doi:10.1016/j.mcn.2007.07.005.
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia. J. Immunol. 2005, 174, 7268–7277.
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009, 28, 1043–1054, doi:10.1038/emboj.2009.45.
- McPherson, C.A.; Aoyama, M.; Harry, G.J. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: Differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain. Behav. Immun. 2011, 25, 850–862, doi:10.1016/j.bbi.2010.09.003.
- Vallières, L.; Campbell, I.L.; Gage, F.H.; Sawchenko, P.E. Reduced Hippocampal Neurogenesis in Adult Transgenic Mice with Chronic Astrocytic Production of Interleukin-6. J. Neurosci. 2002, 22, 486–492, doi:10.1523/JNEUROSCI.22-02-00486.2002.
- Storer, M.A.; Gallagher, D.; Fatt, M.P.; Simonetta, J.V.; Kaplan, D.R.; Miller, F.D. Interleukin-6 Regulates Adult Neural Stem Cell Numbers during Normal and Abnormal Post-natal Development. Stem Cell Rep. 2018, 10, 1464–1480, doi:10.1016/j.stemcr.2018.03.008.
- Bowen, K.K.; Dempsey, R.J.; Vemuganti, R. Adult interleukin-6 knockout mice show compromised neurogenesis. Neuroreport 2011, 22, 126–130, doi:10.1097/WNR.0b013e3283430a44.
- Chen, Y.; Xia, K.; Chen, L.; Fan, D. Increased Interleukin-6 Levels in the Astrocyte-Derived Exosomes of Sporadic Amyotrophic Lateral Sclerosis Patients. Front. Neurosci. 2019, 13, 574, doi:10.3389/fnins.2019.00574.
- Yang, Y.; Boza-Serrano, A.; Dunning, C.J.R.; Clausen, B.H.; Lambertsen, K.L.; Deierborg, T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflamm. 2018, 15, 168, doi:10.1186/s12974-018-1204-7.
- Chen, Z.; Palmer, T.D. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain. Behav. Immun. 2013, 30, 45–53, doi:10.1016/j.bbi.2013.01.083.
- Iosif, R.E.; Ekdahl, C.T.; Ahlenius, H.; Pronk, C.J.H.; Bonde, S.; Kokaia, Z.; Jacobsen, S.-E.W.; Lindvall, O. Tumor Necrosis Factor Receptor 1 Is a Negative Regulator of Progenitor Proliferation in Adult Hippocampal Neurogenesis. J. Neurosci. 2006, 26, 9703–9712, doi:10.1523/JNEUROSCI.2723-06.2006.
- Yang, S.-L.; Yang, M.; Herrlinger, S.; Liang, C.; Lai, F.; Chen, J.-F. MiR-302/367 regulate neural progenitor proliferation, differentiation timing, and survival in neurulation. Dev. Biol. 2015, 408, 140–150, doi:10.1016/j.ydbio.2015.09.020.
- Jovičić, A.; Gitler, A.D. Distinct repertoires of microRNAs present in mouse astrocytes compared to astrocyte-secreted exosomes. PLoS ONE 2017, 12, e0171418, doi:10.1371/journal.pone.0171418.
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.-W.; Trout, A.; Talbot, C.C.J.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018, 9, 363, doi:10.1038/s41419-018-0369-4.
- de Chevigny, A.; Coré, N.; Follert, P.; Gaudin, M.; Barbry, P.; Béclin, C.; Cremer, H. miR-7a regulation of Pax6 controls spatial origin of forebrain dopaminergic neurons. Nat. Neurosci. 2012, 15, 1120–1126, doi:10.1038/nn.3142.
- Morgado, A.L.; Rodrigues, C.M.P.; Solá, S. MicroRNA-145 Regulates Neural Stem Cell Differentiation Through the Sox2–Lin28/let-7 Signaling Pathway. Stem Cells 2016, 34, 1386–1395, doi:10.1002/stem.2309.
- Jovičić, A.; Roshan, R.; Moisoi, N.; Pradervand, S.; Moser, R.; Pillai, B.; Luthi-Carter, R. Comprehensive Expression Analyses of Neural Cell-Type-Specific miRNAs Identify New Determinants of the Specification and Maintenance of Neuronal Phenotypes. J. Neurosci. 2013, 33, 5127–5137, doi:10.1523/JNEUROSCI.0600-12.2013.
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550, doi:10.1007/s00401-017-1803-x.
- Yang, L.; Niu, F.; Yao, H.; Liao, K.; Chen, X.; Kook, Y.; Ma, R.; Hu, G.; Buch, S. Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration. J. Neuroimmune Pharmacol. 2018, 13, 330–344, doi:10.1007/s11481-018-9779-4.
- Xue, Q.; Yu, C.; Wang, Y.; Liu, L.; Zhang, K.; Fang, C.; Liu, F.; Bian, G.; Song, B.; Yang, A.; et al. miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a. Sci. Rep. 2016, 6, 26781, doi:10.1038/srep26781.
- Li, D.; Huang, S.; Yin, Z.; Zhu, J.; Ge, X.; Han, Z.; Tan, J.; Zhang, S.; Zhao, J.; Chen, F.; et al. Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem. Res. 2019, 44, 1903–1923, doi:10.1007/s11064-019-02825-1.
- Liu, C.; Teng, Z.-Q.; Santistevan, N.J.; Szulwach, K.E.; Guo, W.; Jin, P.; Zhao, X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell 2010, 6, 433–444, doi:10.1016/j.stem.2010.02.017.
- Mao, S.; Sun, Q.; Xiao, H.; Zhang, C.; Li, L. Secreted miR-34a in astrocytic shedding vesicles enhanced the vulnerability of dopaminergic neurons to neurotoxins by targeting Bcl-2. Protein Cell 2015, 6, 529–540, doi:10.1007/s13238-015-0168-y.
- Brett, J.O.; Renault, V.M.; Rafalski, V.A.; Webb, A.E.; Brunet, A. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging 2011, 3, 108–124, doi:10.18632/aging.100285.
