Cyanobacteria (e.g., Synechocystis sp. PCC 6803, and Synechococcus elongatus PCC 7942), which are photosynthetic prokaryotes and were previously identified as blue-green algae, are currently under close attention for their abilities to capture solar energy and the greenhouse gas carbon dioxide for the production of high-value products. In the last few decades, these microorganisms have been exploited for different purposes (e.g., biofuels, antioxidants, fertilizers, and ‘superfood’ production). Microalgae (e.g., Chlamydomonas reinhardtii, and Phaeodactylum tricornutum) are also suitable for environmental and biotechnological applications based on the exploitation of solar light.
In recent years, several studies have been targeting the utilization of microorganisms for plastic bioremediation. Among the different phyla, the employment of wild-type or engineered cyanobacteria may represent an interesting, environmentally friendly, and sustainable option (e.g., mismanaged plastics as source of carbons for their cultivation: the connection between their simultaneous utilization for biofuels or chemicals production and microplastics consumption on the surface of basins).
- plastic accumulation
- cyanobacteria
- bioremediation
- microalgae
- plastic degradation
- microplastics
- nanoplastics
- plastics
The entry is excerpted from your paper. They are editable. It will better spread your research and findings. We'll help to correct the format after your submission.
1. Introduction
The use of plastic materials has advantages and disadvantages, as huge societal benefits have arisen from its utilization, especially due to their ease of production, low cost, and wide applicability. However, the ‘plastic age’ has negatively impacted the natural landscape and the lifetime of several wild species. One issue of emerging concern is the aggregation of plastics in the aquatic environment. Nowadays, numerous data documenting massive plastic accumulation in water are easily available. Plastic fragments diametrically smaller than 5 mm are generally referred to as microplastics, and as nanoplastics if the diameters are in the nanoscale[1][2] [3,4]. No analytical methods exist for the identification and quantification of nanoplastics in food, and generally, there is little information[2] [4].
Small-scale fragments are of special concern due to their entrance into the food chain and being able to absorb contaminants. The average size of plastic particles in the environment seems to have decreased, and the abundance and global distribution of microplastic fragments have increased significantly over the last few decades. Population size and the quality of country waste management systems largely determine the mass of uncaptured waste available to become marine plastic debris. Plastics typically constitute approximately 10% of discarded waste, but the impact is very severe through the accumulation of debris in terrestrial environments and open oceans[3] [5]. Plastics have been considered a key geological indicator of the Anthropocene, as a peculiar constituent[4] [6]. Studies about microplastics in various environments have highlighted the ubiquity of synthetic fibers[5][6] [7,8]. Without waste management infrastructure improvements, the cumulative quantity of plastic waste available to enter the ocean from land has been predicted to increase from about 8.8 million metric tons in 2010 to 88 million metric tons by 2025[7] [9].
2. Past and Present Applications Involving Microalgae and Cyanobacteria
Microalgae have been largely investigated for biotechnological applications in the last decades, especially to produce biofuels[8][9][10] [32,33,34]. C. reinhardtii has been considered as a model microorganism for investigations of mechanisms related to microalgal physiology, and also as a platform for industrial biotechnology[11][12] [26,35]. Cyanobacteria, prokaryotic microorganisms with oxygenic photosynthesis, have also been under close attention for utilizations based on the exploitation of the photosynthetic machinery. These Gram-negative bacteria have the capacity to convert solar energy, CO2, and water into chemical energy while releasing O2 into the atmosphere. Chlorophyll a and phycobiliproteins are the primary photosynthetic pigments[13] [36]. Some strains are able to fix N2 into ammonia. Synechococcus and Prochlorococcus are two examples of abundant cyanobacteria in seawater[14] [37]. The outer and plasma membranes are two of the three membranes present in cyanobacteria, constituting the cell envelope and delimiting the periplasmic space. The third barrier is the thylakoid membrane system, localized inside the cell and containing the complexes involved in the photosynthetic electron transfer chain (e.g., photosystem I and photosystem II)[15][16] [38,39]. Compared with other oxygenic photosynthetic organisms, cyanobacteria possess the highest solar energy capturing efficiency with corresponding adequate CO2-concentrating mechanisms and CO2 fixation. These prokaryotes have a long tradition and history of use as a source for human food and as a fertilizer in rice fields. They were also recognized early as an excellent source of proteins and vitamins, and therefore, they were used to produce health food products. Biologically active compounds with antiviral, antibacterial, antifungal, and anticancer activities can be derived from these photosynthetic microorganisms[17][18][19][20][21][22] [40,41,42,43,44,45]. The role of cyanobacteria in the carbon cycle and for other nutrients (e.g., nitrogen) in different environments, their evolution, their adaptation to climate change, and issues related to some toxic blooms are also topics of investigation[14] [37]. These are also able to produce biopolymers, such as polyhydroxyalkanoates (PHAs), and polyhydroxybutyrate (PHB) [23][24][25][26][46,47,48,49]. PHAs are polyesters, which accumulate in different microorganisms. They are recognized as alternatives for the most daily utilized plastics[27] [50]. The physiological function of PHAs in the oxygen-producing photosynthetic prokaryotes is still not fully elucidated. PHB has been considered as an intracellular storage of carbon and energy in the model cyanobacterium Synechocystis sp. PCC 6803 [28][29][51,52]. In addition, cyanobacteria have great potential as a source of renewable and carbon-neutral chemicals, including biofertilizers and biofuels. Specifically, the native capacities to produce the energy carrier hydrogen (H2) from solar energy and water were earlier recognized and have been discussed for a long time[30] [53]. Large scale research initiatives are starting to address cyanobacteria as green cell factories. This can be exemplified by the so-called “CyanoFactory” approach, which includes, for example, the development of needed genetic tools, improved photosynthetic efficiencies, analyses and understanding of the modified metabolism with its further modeling, design and development of efficient photobioreactor systems, and biosafety aspects[31] [54]. These photosynthetic micro-factories are increasingly considered important biocatalysts for the production of renewable and carbon-neutral chemicals and fuels from carbon dioxide and solar energy. As a proof-of-concept, strains have been engineered to produce numerous compounds. However, very few products show titers comparable to those achieved in heterotrophic organisms. One recent example is the systematic modular engineering of Synechocystis sp. PCC 6803, which resulted in a cumulative production titer of 4.8 g photosynthetic 1-butanol per liter with a maximal rate of 302 mg per liter and day[32] [55]. This is the highest productivity of 1-butanol from CO2 reported so far, and the strategy outlined can be seen as a blueprint for future systematic engineering in photosynthetic microorganisms. A combination of efficient genetic engineering and metabolic modeling will accelerate the development of solar chemical and fuel production in these bacteria. From a broader perspective, a recent review addressed diverse approaches to further enhance the production of all products derived from acetyl-CoA[33] [56]. All these studies collectively demonstrate that cyanobacteria, as photosynthetic microorganisms, have a real potential to function as dedicated green cell factories for a truly carbon-neutral production of renewable chemicals and fuels. Moreover, with the use of advanced genetic engineering and synthetic biology in combination with improved modeling, they might be modified to address additional societal challenges, specifically for the bioremediation of plastics-based fragments, which is an important topic in the present review.
3. The Potential of Microalgae and Cyanobacteria for Plastics Biodegradation
Towards bioremediation, the metabolism-dependent processes for biosorption involves generally two steps: (i) the attachment of the pollutant on the surface of the cell, or vice-versa, depending on the size ratio, and (ii) the active or passive transportation of the pollutant into the cell[34] [31]. The biomass can be immobilized or combined with membrane separation in order to improve the uptake of plastic particles[34] [31]. Biodegradation can be summarized in four essential steps, which have been described in detail in previous works[35][36][37] [72,73,74].
(i) Bio-deterioration is the initiation of biodegradation, indicated by superficial degradation attacking mechanical and chemical properties of the macromolecular structure, generally through abiotic parameters (mechanical, light, thermal, and chemical), for example, air turbulences, sunlight, and atmospheric pollutants[36] [73]. Soon after, a biofilm grows on the plastic particle and microbial communities produce extracellular polymeric substances, which enter the pores of the plastic and cause cracks. A probable release of acid compounds, like nitrous acid by chemolithotrophic bacteria, would weaken the structure further[35] [72].
(ii) Bio-fragmentation mainly destabilizes long carbon chains, which microorganisms accomplish by enzymes called oxygenases (mono- and di-oxygenases) to form, for instance, alcohols, by adding oxygen to the carbon chain, or by hydrolases (such as proteases and lipases). It is supported by environmental influences like UV radiation, mechanical, and/or chemical factors[35][36] [72,73].
(iii) Assimilation by microorganisms can only occur when specific carriers (e.g., receptors) are used to cross the cytoplasmic membrane[36] [73]. Once inside, building blocks are oxidized via catabolic pathways (aerobic respiration, anaerobic respiration, and fermentation) for energy and biomass production.
(iv) Mineralization refers to complete degradation, as assimilation results in secondary metabolites by the biodegrading organism, remains that might be used by other organisms. The final products are oxidized metabolites (CO2, N2, CH4, H2O)[36] [73].
Degradation of plastic fragments via living photosynthetic microorganisms should be considered as a future strategy (Figure 1).
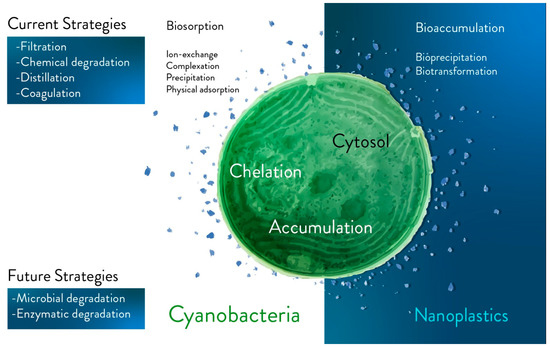
Figure 1. Main current and future strategies for plastics bioremediation. The main current (e.g., filtration, chemical degradation, distillation, and coagulation) and future (e.g., in vivo degradation via microorganisms, and enzyme-based degradation) strategies are separated into two different blocks in the figure. Future strategies are based on biological tools and potentially on in vivo approaches. Biosorption (e.g., ion-exchange, complexation, precipitation, and physical absorption), intracellular bioaccumulation (e.g., bioprecipitation and biotransformation) and extracellular mechanisms are approaches to be further investigated with the aim of capturing fragments of plastic comparable in size to photosynthetic microorganisms (e.g., the dimensions of nanoplastics and cyanobacteria).
The consideration of plastics as sources of nutrients for microorganisms depends on the presence of oxygen and the metabolic pathways of the species involved. Colonies of bacteria are present on the surface of most investigated plastic litter found on the surface of oceans or freshwater basins. Among them, cyanobacteria are included[38] [75]. Bacteroidetes and cyanobacteria dominate in many plastics communities and may play an important role in the ecological process occurring in microbial films on plastics[38] [75]. The performance of nanoplastic remediation in nature based on cyanobacteria has still not been taken into consideration. On the downside, the rates of degradation of conventional plastic by microorganisms are extremely low, even in optimized laboratory conditions[39] [76]. On the upside, chemically sensitive polymers are more suitable for microbial growth[40] [77]. We still lack information about the cyanobacterial potential for plastic degradation.
3.1. Plastics Biodegradation via Photosynthetic Microorganisms in Freshwater and Marine Basins
Plastics Biodegradation via Photosynthetic Microorganisms in Freshwater and Marine Basins
Cyanobacteria and green algae are a fundamental part of phytoplankton, organisms at the base of the trophic chain in freshwaters. They may have a role in plastic degradation[41][42] [29,30]. The time scale of their action does not reduce the amount of plastic in a contaminated basin as quickly as desired. ROS formation has been a common response to nanoparticles, which affects lipids and proteins, eventually leading to the damage of the cytoplasmic membrane [43][44][23,24]. Furthermore, the existence of microplastics in groundwater has been confirmed, albeit in small amounts[44] [78]. One example of the bioremediation of plastics in freshwater has recently been shown[45] [79]. The researchers collected PE bags found in suburban water bodies (three sites along the same river in Chennai City, India) and isolated green algae, cyanobacteria, and diatoms. The most common organisms were Scenedesmus dimorphus, Anabaena spiroides, and Navicula pupula; they have been studied for their potency on the deterioration of PE[45] [79]. The surfaces of LDPE sheets were found to be more readily colonized by the algae than HDPE sheets, with A. spiroides being the most efficient microorganism. While biological degradation of LDPE was observed for all three mentioned algae, A. spiroides caused a loss of 8.18% of mass per month on average (the test set being sheets of 1 cm2 of PE bags, held at ~27 °C), followed by N. pupula at 4.4% loss of mass per month on average. Microorganisms with enhanced capacity to produce oxidative and ligninolytic enzymes are more efficient in the biodegradation of PE[46] [80]. Due to the fact that cyanobacteria need light sources for survival, it is generally believed that degradation via these bacteria might be limited to surface-accessible particles[37] [74]. In another study, two cyanobacterial species, Phormidium lucidum and Oscillatoria subbrevis (found in domestic sewage water) were investigated for their potential in the biodegradation of PE[47] [81]. It is also assumed that cyanobacterial interaction enhances the surface hydrophilicity of PE by the formation of carbonyl, a key marker of biodegradation. PE strips (1 cm2, with 20 μm thickness) treated with the aforementioned cyanobacteria had an increased carbonyl index, and the crystallinity of these strips decreased up to 62%. Both algae utilized about 3 to 4% of the carbon present within a period of 6 weeks. A loss of weight of these pieces was reported to be 30% after 42 days[47] [81]. Though the main focus of research regarding microplastics is on marine environments and the pollution of the oceans, some articles deal with plastic pollution of soil[48][49][50] [82,83,84]. Microplastic particles, for example, PE beads, can be transported below the soil surface via earthworms and potentially reach groundwater[51] [85]. The freshwater microalgae C. reinhardtii was investigated within solutions of PS microplastic powder at concentrations of 0 to 100 mg/L in ultrapure water[52] [86]. The authors observed decreased chlorophyll a fluorescence yields and diminished photosynthetic activity, which started to recover after approximately 10 days[52] [86]. Plastic particles were attached to the surface of this microalgae, damaging the cell membrane. On the other hand, this might also indicate a potential cell-based approach to reduce PS concentration in the water. This hints at a tolerance of PS by photosynthetic bacteria, such as cyanobacteria. In another study, the effects of plastic intake by Daphnia magna were investigated[53] [87]. The main diet of this zooplankton is based on green algae, which attach to plastic particles and thus, microplastics are present in its digestive system. Over a duration of three weeks, D. magna were exposed to microplastics in moderately hard water. The researchers found that an organism of D. magna accumulated after 5 days an average amount of 0.44, 1.56 and 1.75 microplastic particles for concentrations of 25, 50, and 100 mg/L of microplastics in the solution, respectively. These numbers increased about eight-fold after 21 days. Interestingly, the researchers did not observe a significant alteration of survival and reproduction rates of D. magna[53] [87].
A complete study of the biodegradation of a polymer at sea must combine several monitoring parameters (e.g., the research network initiative, “Polymers & Oceans”), and especially be confirmed in the field with experiments in situ [37][74]. Among the microorganisms colonizing plastic fragments, cyanobacteria are most certainly present[37] [74]. An outlook on the possible degradation of floating fragments is illustrated in Figure 2. A recent study in seawater showed that plastic can stimulate the activity of heterotrophic microbes, due to the release of dissolved organic carbon[54] [88].
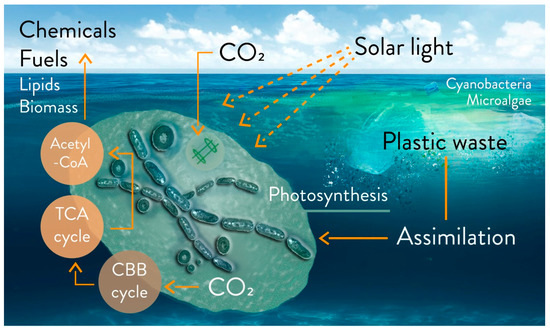
Figure 2. Outlook on the possible biodegradation and bioremediation of floating plastic fragments from accumulated plastic waste in marine water with microalgae and cyanobacteria, via metabolic uptake or biosorption. Biofuel (e.g., H2 and isobutanol), biochemicals, lipids, and biomass can be products based on photosynthetic microbial growth on floating plastic wastes. Engineered strains can be utilized on a laboratory scale, utilizing macro-, micro- and nanoplastics as sources of carbons. CBB cycle: Calvin–Benson–Bassham cycle; TCA cycle: tricarboxylic acid cycle; acetyl-CoA: acetyl coenzyme A.
Several plastics of purely petrochemical origin are not biodegradable; consequently, biodegradable plastics were developed, for example, poly(lactic acid) (PLA) and poly(ε-caprolactone) (PCL). Some potential enzymes that might play a role in the biodegradation of these compounds include PHA-, PHB-, PLA- and PCL-depolymerases, esterases, proteinases, cutinases, ureases, and dehydratases[55] [89]. The finding of cold-adapted bacterial strains with lipase activity is potentially important in view of the biodegradation process[56] [90]. Furthermore, it can be expected that other enzymes secreted by bacteria isolated from cold environments will show biodegradable activity. Extracellular lipase activity was also detected in microbial strains isolated from Arctic sea ice of the Canada Basin[57] [91]. These were identified as belonging to heterotrophic microorganisms from the genera Colwellia, Marinomonas, Pseudoalteromonas, Pseudomonas, and Shewanella. A recent review on microbial ecotoxicology contains precise observations on the interplay between pollutants and microbial communities, related to plastic pollution in the oceans[37] [74]. Microbial ecotoxicology focuses on the mutual influence of pollutants and microbial communities. Plastic pollutants in the ocean, for instance, are affected by various communities, depending if the debris is floating or has sunk to the seafloor. Cyanobacteria of the genera Phormidium and Rivularia are most certainly found in biofilms near the water surface, while the dominant microbiomes of the seafloor seem to be Bacteroidetes (Flavobacteriaceae) and Proteobacteria (Rhodobacteraceae and Alcanivoracaceae)—information on the composition of microbial communities from plastic at the seafloor is still sparse[37] [74]. Biofilm growing on plastic fragments, able to support the additional weight, shows differences in bacterial distributions between non-biodegradable and biodegradable plastics. While many factors influence the composition of the biofilm, studies in the North Pacific indicate that biogeographic origins play a major role, more important than the polymer type[37] [74]. Complex microbial communities, rather than single species, are necessary to degrade plastic. Still, a representative test of biodegradation in oceans is missing, as current standards propose measurements of CO2 emissions that might be misleading; additional methods should be applied, and guidelines formulated[37] [74].
Further research of native pathways and related enzymes is necessary for the applicability of cyanobacteria on this topic. Species identified with roles in plastic degradation (e.g., Pseudomonas, Streptomyces, Corynebacterium, Arthrobacter, Micrococcus, and Rhodococcus) give information for key metabolic routes. These are the foundations to state any native potential of photosynthetic microorganisms. The diversity of synthetic polymers in commerce results in very heterogeneous metabolic pathways of biodegradation. In these regards, the focus should be channeled into model compounds (e.g., PE, PET, PS, and PHA) for conventional and biodegradable plastics [37[74]. Metabolic pathways directly involved in the degradation of, for example, PE, PET, PS, and PHAs have been identified[37] [74]. However, PE is, so far, difficult to degrade biologically, due to its balanced charges in the long chains of carbons and hydrogen. The possible transformation via the tricarboxylic acid (TCA) cycle, in the case of specific release products by capable wild-type or engineered microorganisms, also generates adenosine triphosphate (ATP), which is a key factor for biomass production and cellular growth[37] [74]. Due to the difficulty of dealing with natural conditions, and based on the information from previously published investigations, we suggest that the initial studies regarding metabolic engineered cyanobacteria for plastic biodegradation should be performed using a culture-based approach[42][47] [79,81].
Reference (we'll rearrange the references after you submitted it)
- Steensgaard, I.M.; Syberg, K.; Rist, S.; Hartmann, N.B.; Boldrin, A.; Hansen, S.F. From macro- to microplastics—Analysis of EU regulation along the life cycle of plastic bags. Environ. Pollut. 2017, 224, 289–299.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, 30.
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998.
- Zalasiewicz, J.; Waters, C.N.; Sul, J.A.I.D.; Corcoran, P.L.; Barnosky, A.D.; Cearreta, A.; Edgeworth, M.; Gałuszka, A.; Jeandel, C.; Leinfelder, R.; et al. The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 2016, 13, 4–17.
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211.
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458.
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771.
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Eede, G.V.D. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673.
- Tang, K.H.D. Ecotoxicological Impacts of Micro and Nanoplastics on Marine Fauna. Exam. Mar. Biol. Oceanogr. 2020, 3.
- Ta, A.T.; Babel, S. Microplastic contamination on the lower Chao Phraya: Abundance, characteristic and interaction with heavy metals. Chemosphere 2020, 257, 127234.
- Corti, A.; Vinciguerra, V.; Iannilli, V.; Pietrelli, L.; Manariti, A.; Bianchi, S.; Petri, A.; Cifelli, M.; Domenici, V.; Castelvetro, V. Thorough Multianalytical Characterization and Quantification of Micro- and Nanoplastics from Bracciano Lake’s Sediments. Sustainability 2020, 12, 878.
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231.
- Dong, M.; Luo, Z.; Jiang, Q.; Xing, X.; Zhang, Q.; Sun, Y. The rapid increases in microplastics in urban lake sediments. Sci. Rep. 2020, 10, 1–10.
- Ásmundsdóttir, Á.M.; Gomiero, A.; Øysæd, K.B. Microplastics and Nanoplastics Occurrence and Composition in Drinking Water from Akureyri Urban Area, Iceland. In Proceedings of the 2nd International Conference on Microplastic Pollution in the Mediterranean Sea, Capri, Italy, 15–18 September 2019; Springer: Cham, Switzerland, 2020; pp. 106–111.
- Zhang, M.; Li, J.; Ding, H.; Ding, J.; Jiang, F.; Ding, N.X.; Sun, C. Distribution Characteristics and Influencing Factors of Microplastics in Urban Tap Water and Water Sources in Qingdao, China. Anal. Lett. 2019, 53, 1312–1327.
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and identification of microplastics in tap water from China. Chemosphere 2020, 252, 126493.
- Lam, T.W.; Ho, H.T.; Ma, A.T.; Fok, L. Microplastic Contamination of Surface Water-Sourced Tap Water in Hong Kong—A Preliminary Study. Appl. Sci. 2020, 10, 3463.
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 1–9.
- Vallero, D.A.; Gunsch, C.K. Applications and Implications of Emerging Biotechnologies in Environmental Engineering. J. Environ. Eng. 2020, 146, 03120005.
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ. 2020, 709, 135895.
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical Adsorption of Charged Plastic Nanoparticles Affects Algal Photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561.
- Nolte, T.M.; Hartmann, N.B.; Kleijn, J.M.; Garnæs, J.; Van De Meent, D.; Hendriks, A.J.; Baun, A. The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat. Toxicol. 2017, 183, 11–20.
- González-Pleiter, M.; Tamayo-Belda, M.; Pulido-Reyes, G.; Amariei, G.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments. Environ. Sci. Nano 2019, 6, 1382–1392.
- Tran, N.T.; Kaldenhoff, R. Achievements and challenges of genetic engineering of the model green alga Chlamydomonas reinhardtii. Algal Res. 2020, 50, 101986.
- Harris, E.H. The Chlamydomonas Sourcebook. Introduction to Chlamydomonas and Its Laboratory Use, 2nd ed.; Academic Press: Oxford, UK, 2008; ISBN 9780123708748.
- Eberhard, S.; Valuchova, S.; Ravat, J.; Fulneček, J.; Jolivet, P.; Bujaldon, S.; Lemaire, S.D.; Wollman, F.-A.; Teixeira, M.T.; Riha, K.; et al. Molecular characterization of Chlamydomonas reinhardtii telomeres and telomerase mutants. Life Sci. Alliance 2019, 2, 201900315.
- Kim, J.W.; Park, S.-B.; Tran, Q.-G.; Cho, D.-H.; Choi, D.-Y.; Lee, Y.J.; Kim, H.-S. Functional expression of polyethylene terephthalate-degrading enzyme (PETase) in green microalgae. Microb. Cell Fact. 2020, 19, 1–9.
- Moog, D.; Schmitt, J.; Senger, J.; Zarzycki, J.; Rexer, K.-H.; Linne, U.; Erb, T.; Maier, U.G. Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microb. Cell Fact. 2019, 18, 1–15.
- Wenten, I.G.; Khoiruddin, K.; Harimawan, A.; Ting, Y.P.; Boopathy, R. Membrane Biosorption: Recent Advances and Challenges. Curr. Pollut. Rep. 2020, 6, 152–172.
- Shanmugam, S.; Hari, A.; Kumar, D.; Rajendran, K.; Mathimani, T.; Atabani, A.; Brindhadevi, K.; Pugazhendhi, A. Recent developments and strategies in genome engineering and integrated fermentation approaches for biobutanol production from microalgae. Fuel 2021, 285, 119052.
- Brindhadevi, K.; Mathimani, T.; Rene, E.R.; Shanmugam, S.; Chi, N.T.L.; Pugazhendhi, A. Impact of cultivation conditions on the biomass and lipid in microalgae with an emphasis on biodiesel. Fuel 2021, 284, 284.
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; Fargione, J.E.; Hill, J.D.; Schenk, P.M. Microalgal biofuel production at national scales: Reducing conflicts with agricultural lands and biodiversity within countries. Energy 2021, 215.
- Perozeni, F.; Cazzaniga, S.; Baier, T.; Zanoni, F.; Zoccatelli, G.; Lauersen, K.J.; Wobbe, L.; Ballottari, M. Turning a green alga red: Engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol. J. 2020, 18, 2053–2067.
- Waterbury, J.B. The Cyanobacteria—Isolation, Purification and Identification. Prokaryotes 2006, 4, 1053–1073.
- Bullerjahn, G.S.; Post, A.F. Physiology and molecular biology of aquatic cyanobacteria. Front. Microbiol. 2014, 5, 359.
- Rengstl, B.; Oster, U.; Stengel, A.; Nickelsen, J. An Intermediate Membrane Subfraction in Cyanobacteria Is Involved in an Assembly Network for Photosystem II Biogenesis. J. Biol. Chem. 2011, 286, 21944–21951.
- Vermaas, W.F.J. Encyclopedia of Life Sciences; Nature Publishing Group: London, UK, 2001; pp. 245–251.
- Angermayr, S.A.; Rovira, A.G.; Hellingwerf, K. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 2015, 33, 352–361.
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13.
- Carroll, A.L.; Case, A.E.; Zhang, A.; Atsumi, S. Metabolic engineering tools in model cyanobacteria. Metab. Eng. 2018, 50, 47–56.
- Zhou, J.; Meng, H.; Zhang, W.; Li, Y. Production of Industrial Chemicals from CO2 by Engineering Cyanobacteria. Adv. Exp. Med. Biol. 2018, 1080, 97–116.
- Ni, J.; Tao, F.; Xu, P.; Yang, C. Engineering Cyanobacteria for Photosynthetic Production of C3 Platform Chemicals and Terpenoids from CO2. Results Probl. Cell Differ. 2018, 1080, 239–259.
- Behler, J.; Vijay, D.; Hess, W.R.; Akhtar, M.K. CRISPR-Based Technologies for Metabolic Engineering in Cyanobacteria. Trends Biotechnol. 2018, 36, 996–1010.
- Koch, M.; Berendzen, K.W.; Forchhammer, K. On the Role and Production of Polyhydroxybutyrate (PHB) in the Cyanobacterium Synechocystis sp. PCC 6803. Life 2020, 10, 47.
- Ansari, S.; Fatma, T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, Optimization and Characterization. PLoS ONE 2016, 11, e0158168.
- Markl, E.; Grünbichler, H.; Lackner, M. Cyanobacteria for PHB Bioplastics Production: A Review. In Algae; IntechOpen: London, UK, 2019.
- Miyake, M.; Takase, K.; Narato, M.; Khatipov, E.-A.; Schnackenberg, J.; Shirai, M.; Kurane, R.; Asada, Y. Polyhydroxybutyrate Production from Carbon Dioxide by Cyanobacteria. Appl. Biochem. Biotechnol. 2000, 991–1002.
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53.
- Klotz, A.; Georg, J.; Bučinská, L.; Watanabe, S.; Reimann, V.; Januszewski, W.; Sobotka, R.; Jendrossek, D.; Hess, W.R.; Forchhammer, K. Awakening of a Dormant Cyanobacterium from Nitrogen Chlorosis Reveals a Genetically Determined Program. Curr. Biol. 2016, 26, 2862–2872.
- Wu, G.; Wu, Q.; Shen, Z. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001, 76, 85–90.
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production—A review. Bioresour. Technol. 2017, 243, 1194–1206.
- Lindblad, P.; Fuente, D.; Borbe, F.; Cicchi, B.; Conejero, J.A.; Couto, N.; Čelešnik, H.; Diano, M.M.; Dolinar, M.; Esposito, S.; et al. CyanoFactory, a European consortium to develop technologies needed to advance cyanobacteria as chassis for production of chemicals and fuels. Algal Res. 2019, 41, 101510.
- Liu, X.; Miao, R.; Lindberg, P.; Lindblad, P. Modular engineering for efficient photosynthetic biosynthesis of 1-butanol from CO2 in cyanobacteria. Energy Environ. Sci. 2019, 12, 2765–2777.
- Miao, R.; Xie, H.; Liu, X.; Lindberg, P.; Lindblad, P. Current processes and future challenges of photoautotrophic production of acetyl-CoA-derived solar fuels and chemicals in cyanobacteria. Curr. Opin. Chem. Biol. 2020, 59, 69–76.
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647.
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386.
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; p. 615.
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509.
- Rubio, L.; Marcos, R.; Hernández, A. Potential adverse health effects of ingested micro- and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. J. Toxicol. Environ. Health Part B 2019, 23, 51–68.
- Alexy, P.; Anklam, E.; Emans, T.; Furfari, A.; Galgani, F.; Hanke, G.; Koelmans, A.; Pant, R.; Saveyn, H.; Kluettgen, B.S. Managing the analytical challenges related to micro- and nanoplastics in the environment and food: Filling the knowledge gaps. Food Addit. Contam. Part A 2019, 37, 1–10.
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212.
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348.
- Hantoro, I.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Widianarko, B.; Ragas, A.M.J. Microplastics in coastal areas and seafood: Implications for food safety. Food Addit. Contam. Part A 2019, 36, 674–711.
- Rist, S.; Almroth, B.M.C.; Hartmann, N.B.; Karlsson, T. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726.
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Meera, M.M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci. Rep. 2019, 9, 1–15.
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 1–7.
- Shruti, V.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks—Future research and environmental considerations. Sci. Total Environ. 2020, 726, 138580.
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310.
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074.
- Dussud, C.; Ghiglione, J.F. Bacterial Degradation of Synthetic Plastics. CIESM Workshop Monogr. 2014, 46, 43–48.
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer biodegradation: Mechanisms and estimation techniques—A review. Chemosphere 2008, 73, 429–442.
- Jacquin, J.; Cheng, J.; Odobel, C.; Pandin, C.; Conan, P.; Pujo-Pay, M.; Barbe, V.; Meistertzheim, A.-L.; Ghiglione, J.-F. Microbial Ecotoxicology of Marine Plastic Debris: A Review on Colonization and Biodegradation by the “Plastisphere”. Front. Microbiol. 2019, 10, 865.
- Oberbeckmann, S.; Loeder, M.G.; Gerdts, G.; Osborn, A.M. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol. Ecol. 2014, 90, 478–492.
- Krueger, M.C.; Harms, H.; Schlosser, D. Prospects for microbiological solutions to environmental pollution with plastics. Appl. Microbiol. Biotechnol. 2015, 99, 8857–8874.
- Pathak, V.M. Navneet Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15.
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635.
- Kumar, R.V.; Kanna, G.R.; Elumalai, S. Biodegradation of Polyethylene by Green Photosynthetic Microalgae. J. Bioremed. Biodegrad. 2017, 8, 1–8.
- Nayak, P.; Tiwari, A. Biodegradation of polythene and plastic by the help of microbial tools: A recent approach. Int. J. Biomed. Adv. Res. 2011, 2, 344–355.
- Sarmah, P.; Rout, J. Efficient biodegradation of low-density polyethylene by cyanobacteria isolated from submerged polyethylene surface in domestic sewage water. Environ. Sci. Pollut. Res. 2018, 25, 33508–33520.
